Abstract
Background: The goal of this study was to evaluate the behavior of neonatal rat calvarial osteoblast-like cells cultured on different implant surfaces and exposed once or three times to a 660-nm light-emitting diode (LED).
Methods: An LED with a 660-nm wavelength was applied once or three times to cultured cells on standard and modified sandblasted acid-etched surfaces (SLA and SLActive; Straumann, Basel, Switzerland). To analyze the effect of the LED on cell proliferation, numbers, and viability, cells were cultured on titanium discs, and measurements were taken after 72 h. Cell proliferation rates were assessed using a bromodeoxyuridine immunohistochemical technique. Cell morphologies were evaluated by scanning electron microscopy (SEM).
Results: Osteoblast-like cells proliferated on all tested surfaces, with differences among groups in cell counts and DNA synthesis values. The application of one LED treatment caused a significant increase in cell count in the SLActive group in comparison with the SLA group (p = 0.001), whereas the application of three LED treatments caused a significant decrease in cell count in the SLA group compared with the SLActive group (p < 0.001). After 72 h, the number of cells was highest in the SLActive group exposed once to the LED.
Conclusions: One LED application in the SLActive group resulted in significantly increased cell numbers. However, these findings were not exactly compatible with the SEM findings, which demonstrated fewer cells and weak attachments between cells and to the surface. Thus, further studies using different LED application times are needed to clarify the reason for the increased number of cells that are apparently incapable of attaching to the titanium surfaces after 72 h.
Keywords: Light-emitting diode, SLA and SLActive surface, primary osteoblast cell culture
Introduction
The replacement of one or more missing teeth is an important goal in modern dentistry. Dental implants are frequently the best treatment for missing teeth today. Although several restorative options are available for the treatment of missing teeth, none has proven to be as functionally effective and durable as dental implants.
Since their introduction, titanium and titanium alloys have been used widely because of their superior mechanical strength and biocompatibility 1. Currently, dental implant manufacturers use commercial pure titanium and titanium alloys with treated surfaces to optimize the osseointegration process. Several mechanical and chemical treatments have been used to modify the surface morphology and properties of titanium dental implants to promote the process of osseointegration 2. According to the conventional dental implant protocol proposed by Branemark, the healing period resulting in osseointegration can take 4-6 months 1. The clinical success of oral implants is related to their early osseointegration. Surface treatments, such as titanium plasma spraying, grit blasting, acid etching, anodization, and calcium phosphate coatings, are commonly used 3. One possible method to improve dental implant biocompatibility, leading to early osseointegration, is to increase surface roughness and/or hydrophilicity and to decrease the contact angle. To date, conventional titanium surfaces (sand-blasted and acid-etched) are initially hydrophobic, due to microstructuring and partial coverage with hydrocarbons and carbonates. Studies have indicated that microstructuring as a result of sand blasting and acid etching (SLA) enhanced the osteogenic properties of titanium 4. However, conventional titanium surfaces available currently have a low surface energy and distinct hydrophobic properties due to the microtopography and to adsorbed hydrocarbons. The undesired initial hydrophobicity presumably decreases primary interactions with the aqueous biosystem. However, investigations of osteoblast responses to titanium surface chemistry have shown that osteogenesis is enhanced in vitro by hydrophilic surfaces 5,6. Recently, a new modified SLA surface (SLActive; Insitut Straumann AG, Waldenburg, Switzerland) has been produced by rinsing under a N2 atmosphere. After acid etching, the metal is submerged in an isotonic NaCl solution to avoid contact with molecules from the atmosphere, thereby increasing hydrophilicity.
Implant osseointegration is known to depend not only on the properties of the implanted material, but also on the characteristics and regenerative capability of the host bone. For this reason, researchers involved in biomaterial evaluation now place great importance on the various methods that can positively affect the healing of bone in patients, thus increasing the success of surgical implants. Biologically based strategies have been developed over the years, including ultrasound, shockwave stimulation, low-intensity pulsed ultrasound, electric fields, electromagnetic fields, lasers, and photomodulation 7-10.
Today, lasers and photomodulation have gained popularity and photomodulation has become an attractive method to enhance wound healing 11. Light-emitting diodes (LEDs) have become a new favorite in the field of medical treatment and phototherapy. LED radiation is monochromatic red-to-near-infrared (NIR) radiation 6. Light in the NIR 630-1000 nm range, generated using LED arrays, has been shown to improve retinal function in an animal model of mitochondrial dysfunction 7. LEDs have many advantages over lasers for use in phototherapy, including a readily expandable optical footprint due to the use of LED arrays, a smaller hardware package, and lower energy density. LED differs from low-level laser (LLL) radiation in that the latter is from a laser with the characteristic of coherency, whereas LED light is not coherent 6. LED radiation can also be produced at a lower cost than LLL and it can be safely applied to a larger area of the body surface. LED photobiomodulation therapy (LPT) has been shown to stimulate the intracellular production of adenosine triphosphate (ATP), particularly in cells that are ischemic or wounded 8.
In vitro assays assessing titanium surfaces and osteoblast-like cells can provide fundamental information for the investigation of osteoblast behavior. Evaluations of cell proliferation, cell growth, viability, and morphology of osteoblast-like cells cultured on titanium surfaces are important markers for determining these responses. Although there are lots of cell culture studies about low-level laser therapy there are only a few cell culture studies about LED which have similiar characteristics.
The aim of this study was to investigate the effects of LED light on DNA synthesis, cell numbers, and cell viability using neonatal rat calvarial osteoblast-like cells cultured on SLA and SLActive (chemically modified SLA; Institute Straumann AG) titanium discs.
Methods
We prepared three subgroups of the SLA and SLActive groups: one control group and two study groups. The control group was given no treatment after incubation of the titanium discs. Study Group I was treated at the time of incubation with a 660-nm LED light (Biolux Research Ltd., Vancouver, Canada), placed 10 cm above the cell culture for a 1‑min period. Study Group II was treated with a 660‑nm LED light placed 10 cm above the cell culture for a 1‑min period at the time of incubation and again after 24 and 48 h.
Titanium discs
Titanium discs, 10 mm in diameter and 1 mm in thickness, were prepared and kindly supplied by the manufacturer (Fig. 1). The surfaces were prepared as SLA or SLActive (chemically modified SLA; Institute Straumann AG). All specimens were prepared from commercial pure Ti (grade 4; ASTM F 67) by the Institute Straumann AG. Both groups of surfaces were subjected to a procedure including acid etching and sand blasting. Then, the SLActive surfaces were further rinsed under nitrogen protection to prevent exposure to air during the procedure and were stored in a sealed glass tube containing isotonic NaCl solution. Average roughness values (Ra; ISO 4287) of the surfaces were provided by the manufacturers and were as follows: SLA 2.93 ± 0.46 µ, SLActive 1-5 µ. All discs were sterilized by gamma irradiation at 25 kGy overnight and were then ready for use.
Figure 1.
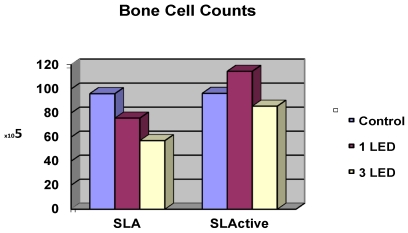
Effects of LED application on osteoblast-like cell counts
Cell culture
Primary osteoblastic cells were isolated from neonatal Sprague-Dawley rats using a method modified from previous studies 12. The calvaria of the neonatal rats were dissected under aseptic conditions and then subjected to sequential digestion in HEPES (Sigma-Aldrich Corp., St. Louis, MO, USA) balanced salt solution (pH 7.4) with 2 mg/mL collagenase for 10-min periods. Osteoblasts released in the third and fourth digests were collected and then incubated in hypotonic HEPES (Sigma-Aldrich Corp.) balanced salt solution at 4°C to lyse remaining erythrocytes. Isolated osteoblasts were cultured in Dulbecco's modified Eagle medium (DMEM-12; Gibco, Invitrogen Ltd., Paisley, UK) media supplemented with 10% fetal calf serum (Sigma-Aldrich Corp.), 100 µg/mL penicillin, and 100 µg/mL streptomycin in 75-cm2 flasks. The cells were maintained at 37°C in an incubator with a humidified 5% CO2 atmosphere. First-passage cells were then used in the following experiment. The phenotype and function of the osteoblasts were characterized by the presence of alkaline phosphatase (ALP) activity. The use of animals in this study was approved by the ethics committee of Istanbul University. The National Institutes of Health (NIH) guidelines for the care and use of laboratory animals (NIH publication #85-23, Rev. 1985) were observed.
Light-emitting diode protocol
A red light source (Biolux Research Ltd.) with a wavelength of 660 nm was used. After the titanium discs were added to the osteoblast cultures, each plate was treated with LED light at a density of 20 mW/cm2 placed 10 cm above the culture for 1 min. The LED light source was fixed at a standardized 10-cm height.
Bone cell identification
ALP activity was measured spectrophotometrically using an automatic analyzer (Prestige 24i; Tokyo Boeki Medical System Ltd., Tokyo, Japan). The rates of p-nitrophenol formation at 405 nm (ALP) are directly proportional to ALP activity.
DNA analysis
Cells were cultured on SLA and SLActive in well plates at a density of 5 × 105 cells/mL in the DMEM-12 medium described above. All experiments were performed in triplicate. The percentage of DNA-synthesizing cells was determined by a bromodeoxyuridine (BrdU) immunohistochemistry technique. Osteoblast cells were seeded on cover slips and treated with drugs as in the survival studies for 24 h and 7 d. BrdU (20 µM; Sigma-Aldrich Chemie GmbH, Steinheim, Germany) was added to the cells for the final hour in culture. The cells were then fixed in 70% cold ethanol at ‑20°C for 30 min. Endogenous peroxidase activity was quenched with 0.5% H2O2 in methanol. Double-stranded DNA was denatured with 4 N HCl at 37°C for 30 min. After washing, a non-specific blocking reagent (Ultra-V-Block; Lab Vision Co., Westinghouse, CA, USA) was used to prevent non-specific binding. Monoclonal mouse anti-BrdU (1:200, 1 h; Novocastra, Newcastle, UK) was used as the primary antibody. The secondary antibody used was biotinylated goat anti-mouse (Lab Vision Co.). After washing, peroxidase-conjugated streptavidin (Lab Vision Co.) was applied and the aminoethylcarbazole chromogen was used. Sections were counterstained with Mayer's hematoxylin to enhance nuclear staining. For negative controls, adjacent sections were processed through the same steps but with no primary antibody. Photographs were taken using a camera attached to a light microscope. All BrdU-labeled cells were observed by the same person. The BrdU labeling index (number of positively stained cells / total number of cells counted) was calculated by evaluating at least 3000 cells in multiple well-labeled high-power fields.
Plating efficiency and cell viability
Exponentially growing cells, prepared in 5 mL of DMEM-12, were plated in each well of a 6‑well plate at a density of 5 × 105 cells/well with 100% vitality. The number of cells in each well was counted and mean values were determined. Cell viability was estimated using the trypan blue exclusion test with a hemocytometer after 24 h and 7 d. The viability of the control cells was more than 90%. All samples were tested in triplicate.
Scanning electron microscopy
The morphologies of growing osteoblastic cells were examined by SEM. Discs were washed with phosphate-buffered saline (PBS) and fixed with 2.5% glutaraldehyde (Polysciences, Inc., Warrington, PA, USA) in 0.1 mol/L phosphate buffer (pH 7.3) for 30 min and 1% osmium tetroxide in 0.1 mol/L cacodylate buffer (pH 7.4) for 1 h. Discs were washed with PBS three times, dehydrated through a graded ethanol series, placed in a 100% ethanol bath, and rinsed three times (30 min × 3). Discs were critical-point dried, sputter-coated with gold palladium, and then observed by SEM (JSM 5200; Jeol Ltd., Tokyo, Japan). Photographs were taken at 10 kV using various magnifications and angles.
Statistical analysis
The NCSS 2007 software (NCSS, Kaysville, UT, USA) was used for the statistical analyses. The significance of differences between groups was evaluated using the Kruskal-Wallis test, crosschecks between subgroups were evaluated with Dunn's statistical analysis, and crosschecks between pairs of groups were evaluated with the Mann-Whitney U-test. A p-value of < 0.05 was considered to indicate statistical significance for all tests.
Results
Throughout the study, no bacterial or fungal infection was found in any culture.
Cell counts
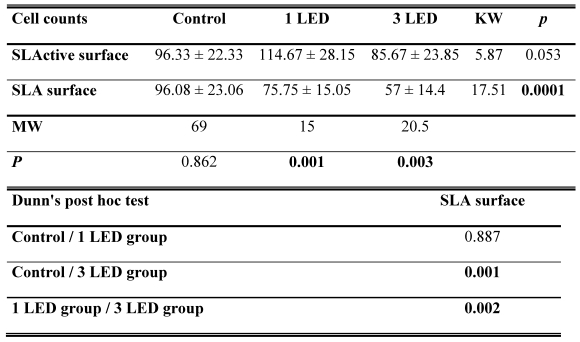
Cell counts for the control groups did not differ between the SLA and SLActive discs. The highest cell count was achieved in the SLActive group, which was treated once with the LED light. The SLA group, which was treated three times with the LED light, had the lowest cell count. The SLActive group treated once with LED had a higher cell count than the control group; other groups had lower cell counts than the control groups. While the application of one LED treatment caused a significant increase in the cell count in the SLActive group in comparison with the SLA group (p = 0.001), the application of three LED treatments caused a significant decrease in cell count in the SLA group compared with the SLActive group (p < 0.001). There were no significant differences between the cell counts of control, 1 LED, 3LED groups of SLActive surfaces. So no post tests (Dunn's Multiple Comparison Test) for SLActive group were needed (p > 0.05). (Table 1, Fig. 1).
Table 1.
Effects of LED application on osteoblast-like cell counts
Viability
The SLActive groups treated once and three times with the LED showed a significantly greater dead cell count in comparison with the SLA groups (p = 0.021). No significant difference was observed among the other groups.
BrdU
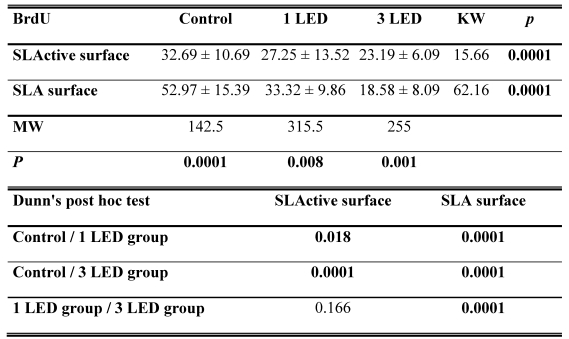
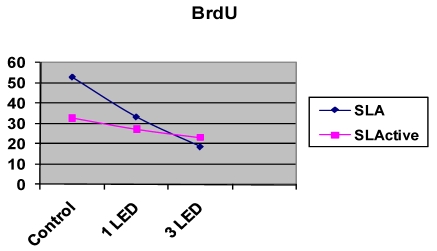
The SLA control group had the highest DNA activity of all groups (p = 0.0001). At the end of day 3, DNA activity was higher in the SLA group treated once with LED light than in the SLActive group (p = 0.008). At the end of the 3-d observation period, DNA activity was higher in the SLActive group treated three times with the LED light than in the SLA group (p = 0.001). DNA activity in the SLA group treated once with the LED light was higher than in the SLActive group (Table 2, Figs. 2, 3).
Table 2.
BrdU incorporation in osteoblast-like cells
Figure 2.
BrdU incorporation in osteoblast-like cells
Figure 3.
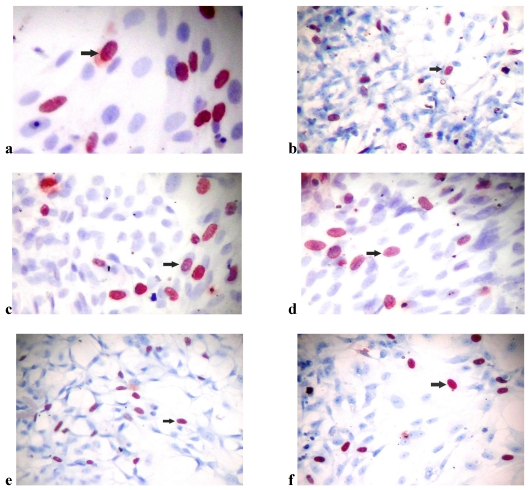
(a) Bromodexyuridine (BrdU) incorporation of osteoblast-like cells in (a) the control group of SLActive surfaces (×40), (b) the control group of SLA surfaces (×10), (c) the SLActive group exposed once to the LED (×40), (d) the SLActive group exposed three times to the LED (×40), (e) the SLA group exposed once to the LED (×10), and (f) the SLA group exposed three times to the LED (×10).
Scanning electron microscopy
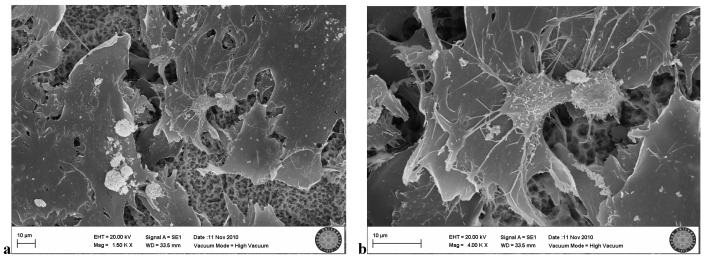
In the SLA control group, many osteoblastic cell groups were seen on the clear surface. Villi and cytoplasmic extensions were clearly visible. Cells were attached to each other and to the surface, with cytoplasmic extensions. They became flattened while adhering to the surface (Fig. 4a,b).
Figure 4.
SLA control group(no exposion to LED)
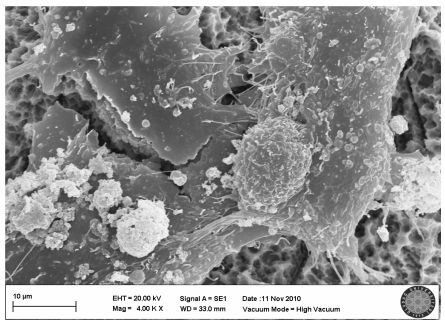
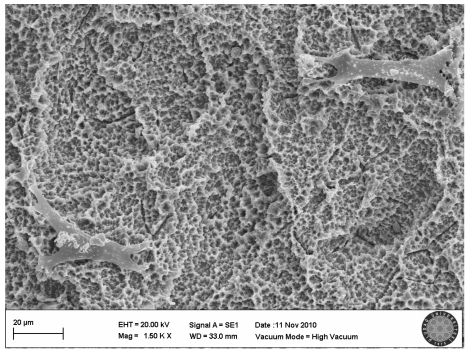
In the SLActive control group, the villi of cells were clearly seen and there were attachments between the cells (Fig. 5).
Figure 5.
SLActive control group(no explosion to LED)
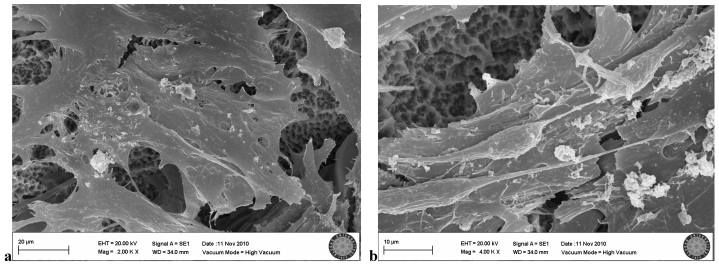
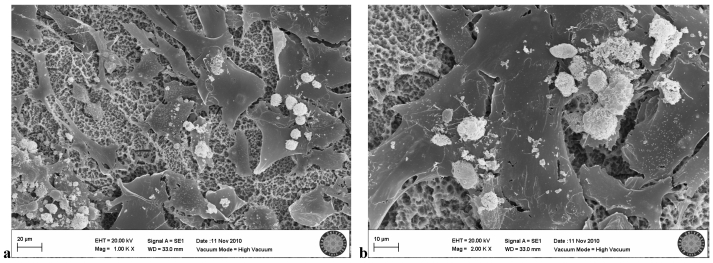
In the SLA group exposed once to the LED, flattened cells with normal shape and form were attached to the surface. Groups of cells that had completed mitosis were observed in some areas (Fig. 6a, b).
Figure 6.
SLA group exposed 1 time to LED
In the SLA group exposed three times to the LED, cell villi and cytoplasmic extensions were clearly visible, as were attachments between the cells. Many mitotic cells were observed (Fig. 7a, b).
Figure 7.
SLA group exposed 3 times to LED
In the SLActive group exposed once to the LED, few cell groups were observed on the titanium surface, and cell attachment was clearly weak (Fig. 8).
Figure 8.
SLActive group exposed 1 time to LED
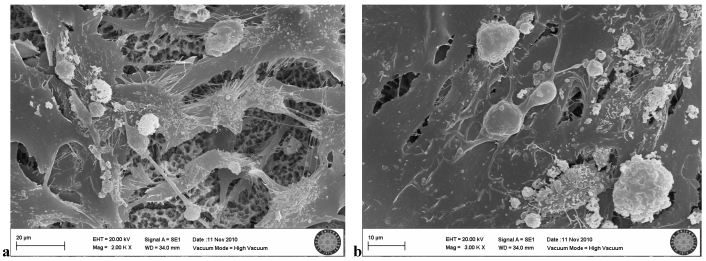
In the SLActive group exposed three times to the LED, many cells were visible, but some showed negative effects of the LED application. Loss of villi and cell damage were observed (Fig. 9a, b).
Figure 9.
SLActive group exposed 3 times to LED
Discussion
Many studies have shown that surface topography and the chemical composition of an implant surface can influence the behavior of cells in vitro, including cell attachment, differentiation, and proliferation rates. Today, there are new strategies in the field of implantology to deliberately alter these rates.
In past decades, lasers have been used for several therapeutic purposes. Many beneficial effects have been demonstrated with in vitro and in vivo test systems, including antibacterial, antiviral, antitumor, cell differentiation, immunopotentiating, and tissue repair activities 13. The rapid development of high-brightness LEDs has made feasible the use of LEDs among other light sources, such as lasers, intense pulse light, and other incoherent light systems, for medical treatment and light therapy. In particular, LLL therapy has been increasingly used to treat hard tissue injuries, promoting wound healing and reducing pain. This type of laser has been demonstrated as a noninvasive method for the stimulation of osteogenesis and the reduction of fracture consolidation time through bioenergetic, bioelectrical, biochemical, and biostimulatory effects on cells 14,15.
Li et al. 15 found that LED application in three different doses (5, 10, 15 mW/cm2) did not cause a significant difference over a 3-d period. Kim et al. 16 used an LED device with an intensity of 9.29 mW/cm2. In the present study, our LED applications had an intensity of 20 mW/cm2.
Khadra et al. 17 stated that during the early stages of wound healing the energy requirements of the cells are increased and photostimulation might play an important role in this phase. They found that irradiation on three consecutive days enhanced production of osteocalcin and TGF-β1. We also decided to have application times of LED for 0h, 24h and 48h evaluating these findings of Khadra et al.
While Stein et al. 18 applied laser from a distance of 11.5 cm, Khadra et al. 17 applied it from a distance of 9 cm. We applied LED from a distance of 10 cm due to the manufacturer's instructions which is stated to be the most effective distance.
Aybar et al. 19 investigated DNA synthesis in osteoblasts using BrdU analysis. Mustafa et al. 11 and Aybar et al. used BrdU analyses to evaluate cellular DNA activity in their studies examining the surface properties of titanium discs. We also used the BrdU method for the detection of DNA activity.
In our study, BrdU activity in the SLA group treated once with the LED was higher after 72 h than in the SLActive group, although the opposite result was observed with three LED applications. When cell count and BrdU results are taken into consideration, LED application at 0, 24, and 48 h appeared to slow down the osteoblast-like proliferation process and DNA activity. However, when LED was applied only once, cell proliferation and DNA activity in the early stages were significantly higher and a higher cell count was reached in all groups after 72 h, but DNA activity and live cell counts were found to be lower than in the SLA group. In our study, the high cell count after 72 h despite a decrease in DNA activity suggested that rapid cell proliferation occurred in the early phase. Aybar et al. 19 reached the same conclusion.
In light of these findings, it appears that the application of a single LED treatment raised early-phase DNA activity and promoted cell proliferation of osteoblast-like cells in vitro.
Kim et al. 16 reported that after LED treatment, differentiation was much faster in early-phase osteoblast cultures. They also found that early differentiation of progenitor cells was demonstrated by increased ALP activity and that this increased activity stimulated cell proliferation, resulting in a high cell count in the early phase. In our study, the result of one LED application was consistent with the findings of Kim et al. 16. We saw enhanced cell proliferation and DNA activity in the early phase of LED application.
Our SEM images showed osteoblast-like cells that tended to be elongated and squamous. This cell morphology on the surface of SLA was consistent with that observed by Aybar et al. 19. In the SEM images of the SLActive group at a high cell count, few squamous cells were seen. However, in the images of SLA group at a lower cell count, many squamous and elongated cells covered the surface, with intermittent mitosis.
Evaluations of DNA activity, cell counts, and SEM findings indicated that a significant increase in cell count occurred after 72 h due to the proliferation rate and the inhibition of cell death on the SLActive surfaces exposed once to the LED. We believe that the reduced numbers of cells on the titanium surfaces was due to the inability of cells that had undergone apoptosis to adhere to the surfaces.
In conclusion, one-time LED application in the SLActive group resulted in significantly increased cell numbers. However, these findings were not exactly compatible with the SEM findings, which demonstrated fewer cells and weak cell attachment between cells and to the surface. Thus, we suggest that further studies using different LED application intervals are needed to clarify the reason for the increased cell numbers that are apparently incapable of attaching to the titanium surfaces after 72 h.
Acknowledgments
The authors would like to thank Fusun Oncu and Seref Kurudal (Bati Dental) for their contributions to this study.
References
- 1.Brånemark PI, Adell R, Breine U. Intraosseous anchorage of dental prostheses. Scand J Plast Reconstr Surg. 1969;3:81–100. doi: 10.3109/02844316909036699. [DOI] [PubMed] [Google Scholar]
- 2.Wennerberg A, Albrektsson T. On implant surfaces: a review of current knowledge and opinions. Int J Oral Maxillofac Implants. 2010;25(1):63–74. [PubMed] [Google Scholar]
- 3.Klein MO, Bijelic A, Ziebart T. et al. Submicron scale-structured hydrophilic titanium surfaces promote early osteogenic gene response for cell adhesion and cell differentiation. Clin Implant Dent Relat Res. 2011 doi: 10.1111/j.1708-8208.2011.00339.x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 4.Zhang Y, Andrukhov O, Berner S. et al. Osteogenic properties of hydrophilic and hydrophobic titanium surfaces evaluated with osteoblast-like cells (MG63) in coculture with human umbilical vein endothelial cells (HUVEC) Dent Mater. 2010;26(11):1043–51. doi: 10.1016/j.dental.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 5.Lang NP, Salvi GE, Huynh-Ba G. et al. Early osseointegration to hydrophilic and hydrophobic implant surfaces in humans. Clin Oral Implants Res. 2011;22(4):349–56. doi: 10.1111/j.1600-0501.2011.02172.x. [DOI] [PubMed] [Google Scholar]
- 6.Romanos GE, Gutknecht N, Dieter S. et al. Laser wavelengths and oral implantology. Lasers Med Sci. 2009;24(6):961–70. doi: 10.1007/s10103-009-0676-1. [DOI] [PubMed] [Google Scholar]
- 7.Deppe H, Horch HH. Laser applications in oral surgery and implant dentistry. Lasers Med Sci. 2007;22(4):217–21. doi: 10.1007/s10103-007-0440-3. [DOI] [PubMed] [Google Scholar]
- 8.Liu Q, Liu X, Liu B. et al. The effect of low-intensity pulsed ultrasound on the osseointegration of titanium dental implants. Br J Oral Maxillofac Surg. 2011 doi: 10.1016/j.bjoms.2011.03.001. Epub. [DOI] [PubMed] [Google Scholar]
- 9.Buzzá EP, Shibli JA, Barbeiro RH. et al. Effects of electromagnetic field on bone healing around commercially pure titanium surface: histologic and mechanical study in rabbits. Implant Dent. 2003;12(2):182–7. doi: 10.1097/01.id.0000058385.23346.4d. [DOI] [PubMed] [Google Scholar]
- 10.Song JK, Cho TH, Pan H. et al. An electronic device for accelerating bone formation in tissues surrounding a dental implant. Bioelectromagnetics. 2009;30(5):374–84. doi: 10.1002/bem.20482. [DOI] [PubMed] [Google Scholar]
- 11.Mustafa K, Wennerberg A, Wroblewski J. et al. Determining optimal surface roughness of TiO(2) blasted titanium implant material for attachment, proliferation and differentiation of cells derived from human mandibular alveolar bone. Clin Oral Implants Res. 2001;12(5):515–25. doi: 10.1034/j.1600-0501.2001.120513.x. [DOI] [PubMed] [Google Scholar]
- 12.Bilir A, Ceyhan T, Altinöz MA. Culturability of osteoblast cells extracted from mature and fetal BALB/C mice calvaria. Acta Orthop Traumatol Turc. 2000;34:389–95. [Google Scholar]
- 13.Whelan HT, Smits Jr. RL, Buchman EV, et al. Effect of NASA light-emitting diode irradiation on wound healing. J Clin Laser Med Surg. 2001;19(6):305–14. doi: 10.1089/104454701753342758. [DOI] [PubMed] [Google Scholar]
- 14.Gary N, Wu YCH, Cheng TC. Light-emitting diodes: their potential in biomedical applications. Renewable and Sustainable Energy Reviews. 2010;14(8):2161–6. [Google Scholar]
- 15.Li WT, Leu YC. Effects of low level red-light irradiation on the proliferation of mesenchymal stem cells derived from rat bone marrow. Lyon, France: Proceedings of the 29th Annual International Conference of the IEEE EMBS Cité Internationale; 2007. pp. 23–6. [DOI] [PubMed] [Google Scholar]
- 16.Kim HK, Kim JH, Abbas AA. et al. Red light of 647 nm enhances osteogenic differentiation in mesenchymal stem cells. Lasers Med Sci. 2009;24(2):214–22. doi: 10.1007/s10103-008-0550-6. [DOI] [PubMed] [Google Scholar]
- 17.Khadra M, Lyngstadaas SP, Haanaes HR, Mustafa K. Effect of laser therapy on attachment, proliferation and differentiation of human osteoblast-like cells cultured on titanium implant material. Biomaterials. 2005 Jun;26(17):3503–9. doi: 10.1016/j.biomaterials.2004.09.033. [DOI] [PubMed] [Google Scholar]
- 18.Stein E, Koehn J, Sutter W, Wendtlandt G, Wanschitz F, Thurnher D, Baghestanian M, Turhani D. Initial effects of low-level laser therapy on growth and differentiation of human osteoblast-like cells. Wien Klin Wochenschr. 2008;120(3-4):112–7. doi: 10.1007/s00508-008-0932-6. [DOI] [PubMed] [Google Scholar]
- 19.Aybar B, Emes Y, Atalay B. et al. The influence of titanium surfaces in cultures of neonatal rat calvarial osteoblast-like cells: an immunohistochemical study. Implant Dent. 2009;18(1):75–85. doi: 10.1097/ID.0b013e31818c5bd8. [DOI] [PubMed] [Google Scholar]