Abstract
OBJECTIVE
To evaluate the performance of the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation to estimate glomerular filtration rate (GFR) in type 2 diabetic patients with GFR >60 mL/min/1.73 m2.
RESEARCH DESIGN AND METHODS
This was a cross-sectional study including 105 type 2 diabetic patients. GFR was measured by 51Cr-EDTA method and estimated by the MDRD and CKD-EPI equations. Serum creatinine was measured by the traceable Jaffe method. Bland-Altman plots were used. Bias, accuracy (P30), and precision were evaluated.
RESULTS
The mean age of patients was 57 ± 8 years; 53 (50%) were men and 90 (86%) were white. Forty-six (44%) patients had microalbuminuria, and 14 (13%) had macroalbuminuria. 51Cr-EDTA GFR was 103 ± 23, CKD-EPI GFR was 83 ± 15, and MDRD-GFR was 78 ± 17 mL/min/1.73 m2 (P < 0.001). Accuracy (95% CI) was 67% (58–74) for CKD-EPI and 64% (56–75) for MDRD. Precision was 21 and 22, respectively.
CONCLUSIONS
The CKD-EPI and MDRD equations pronouncedly underestimated GFR in type 2 diabetic patients.
The importance of estimating glomerular filtration rate (GFR) in addition to measuring urinary albumin excretion (UAE) has been recently recognized in individuals with or without diabetes because these two parameters are independent predictors of cardiovascular and renal outcomes and require specific approaches (1–3). However, the accuracy of creatinine-based equations to estimate GFR, including the new Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation, has been questioned for patients with diabetes (4,5). Recently, Rognant et al. (5) demonstrated that CKD-EPI presented a poor performance in diabetic patients with a wide range of renal function, working even worse than MDRD equation. The current study evaluated the performance of the CKD-EPI equation to estimate GFR in individuals with type 2 diabetes and GFR >60 mL/min/1.73 m2.
RESEARCH DESIGN AND METHODS
This study was approved by the Ethics Committee of Hospital de Clínicas de Porto Alegre, Rio Grande do Sul, Brazil. Inclusion criteria were plasma glucose <200 mg/dL and GFR >60 mL/min/1.73 m2. GFR was measured by the 51Cr-EDTA single-injection method after a single intravenous dose of 150 μCi. Serum creatinine was measured by a Jaffe reaction (Modular P; Roche Diagnostics, Mannheim, Germany) traceable to isotope dilution mass spectrometry. The CKD-EPI equation was calculated as GFR (mL/min/1.73 m2) = 141 × min (serum creatinine/k, 1)α × max (serum creatinine/k, 1)−1.209 × 0.993Age × 1.018 (if female) × 1.159 (if black), where k is 0.7 for females and 0.9 for males, α is −0.329 for females and −0.411 for males, min indicates minimum serum creatinine/k or 1, and max indicates maximum serum creatinine/k or 1 (6). The MDRD equation was calculated as GFR (mL/min/1.73 m2) = 175 × (serum creatinine)−1.154 × (age)−0.203 × 0.742 (if female) × 1.210 (if black). A1C and UAE were measured by immunoturbidimetry.
Statistical analysis
Bias was calculated as the mean difference between measured and estimated GFR. Accuracy was calculated as the percentage of estimates within 30% (P30) of measured GFR. Precision was measured as 1 SD of bias. The agreement between measured GFR and equations was evaluated using Bland-Altman plots, with the calculation of agreement limits (bias ± 2 SD) and CI (7). According to Bland-Altman, 100 individuals are enough to estimate bias and limits of agreement with a 95% CI of about 34% of SD (8).
RESULTS
This cross-sectional study included 105 individuals with type 2 diabetes. Their mean age was 57 ± 8 years (42–86); 53 (50%) were men, and 90 (86%) were white. Forty-six (44%) had microalbuminuria, and 14 (13%) had macroalbuminuria. Plasma glucose was 148 ± 28 mg/dL, and A1C was 8.3 ± 0.3%.
51Cr-EDTA GFR was 103 ± 23, CKD-EPI GFR was 83 ± 15, and MDRD GFR was 78 ± 17 mL/min/1.73 m2 (P < 0.001). According to the Kidney Disease: Improving Global Outcomes GFR classification, 75% of the patients in our study were stage 1 (GFR >90 mL/min), and 25% were stage 2 (GFR = 60–89 mL/min) as measured by 51Cr-EDTA GFR. Misclassification to stage 3a (GFR = 45–59 mL/min) occurred in 7.6 and 9.5% of the cases using the CKD-EPI and MDRD equations, respectively.
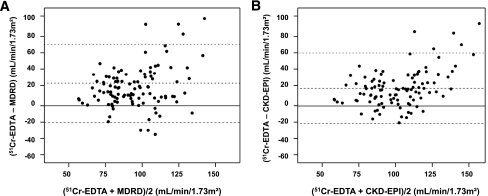
Figure 1 shows the plots of measured and estimated GFR values. The CKD-EPI and MDRD equations systematically underestimated measured GFR. Bias was 20 and 24 mL/min/1.73 m2 for CKD-EPI and MDRD, respectively (P = 0.26). Bias was significantly greater in subjects with GFR above versus below the median value (30 vs. 9 for CKD-EPI and 34 vs. 14 mL/min/1.73 m2 for MDRD [P < 0.001 for each pair of comparisons]). Accuracy P30 (95% CI) was 67% (58–74) for CKD-EPI and 64% (56–75) for MDRD. Precision was 21 and 22, respectively.
Figure 1.
Plots of average estimated and measured GFR vs. difference between them in the 105 patients with type 2 diabetes for MDRD (A) and CKD-EPI (B) equations (P < 0.001 for all comparisons). Limits of agreement, calculated as bias ± 2 SD (CI) as follows: CKD-EPI −21 (−28 to −14) to 61 (54–68) mL/min/1.73 m2, and MDRD −18 (−26 to −11) to 67 (60–74) mL/min/1.73 m2.
CONCLUSIONS
The CKD-EPI and MDRD equations significantly underestimated measured GFR in individuals with type 2 diabetes, especially at higher GFR values.
The Kidney Disease: Improving Global Outcomes, a global nonprofit foundation, has recently proposed a new chronic kidney disease staging system (2). This was based on the findings of a remarkable meta-analysis that included 45 cohorts and a total of 1,555,332 participants that confirmed that both GFR and UAE are independent predictors of cardiovascular and renal outcomes (1). Therefore, a reliable estimation of GFR and UAE is the cornerstone to predict patients’ prognosis.
The American Diabetes Association and the National Kidney Foundation recommend annual UAE measurement and GFR estimation using equations that include serum creatinine, such as MDRD and CKD-EPI. However, according to recent studies, these equations have a poor performance for patients with diabetes and markedly underestimate GFR (4,5). This disappointing performance seems to be associated with specific characteristics of the patients with diabetes, such as hyperglycemia, glomerular hyperfiltration, and obesity, which probably highlight the limitations of creatinine itself as a GFR marker. Hyperglycemia may interfere in two ways. First, it has long been known that glucose levels above 300 mg/dL may affect the performance of the Jaffe reaction to measure creatinine (9). Indeed, in a previous study we have found that patients with type 2 diabetes presented higher serum creatinine than healthy individuals, despite similar GFR values (4). Another possible explanation could be the hyperglycemia-induced glomerular hyperfiltration and the inability of creatinine to detect this typical phenomenon of diabetes (10). This was clearly shown in our hyperfiltering patients, for whom the CKD-EPI and MDRD equations unacceptably underestimated GFR. The poor performance of the formulas in our study was further expressed in the chronic kidney disease misclassification of patients in 8 and 10% of the cases when using the CKD-EPI and MDRD equations, respectively. A recent French study that included a similar number of patients with GFRs >60 mL/min/1.73 m2 also observed a bad performance of CKD-EPI equation, which also applied for GFRs <60 mL/min/1.73 m2 (5). These data stress the need of developing alternative, more precise tools to estimate GFR in individuals with diabetes.
The strengths of our study were the use of a GFR reference method, which ensured a more precise interpretation of the equation results, and a sample size tailored for the specific Bland-Altman statistical analysis. One limitation was that our results were deliberately suited for patients with GFRs >60 mL/min/1.73 m2.
In conclusion, both CKD-EPI and MDRD equations present a poor performance to estimate GFR in individuals with diabetes, especially for high-normal GFRs, with a pronounced underestimation.
Acknowledgments
No potential conflicts of interest relevant to this article were reported.
S.P.S. and E.G.C. contributed to research, wrote the manuscript, contributed to discussion, and reviewed the manuscript. G.N.A., M.N.F., F.D.S.S., and H.M.Y. contributed to research.
We would like to thank the Fundo de Incentivo à Pesquisa (FIPE) of Hospital de Clínicas de Porto Alegre and the Programa de Apoio a Núcleos de Excelência (PRONEX) for the financial support.
References
- 1.Matsushita K, van der Velde M, Astor BC, et al. ; Chronic Kidney Disease Prognosis Consortium. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet 2010;375:2073–2081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levey AS, de Jong PE, Coresh J, et al. The definition, classification, and prognosis of chronic kidney disease: a KDIGO Controversies Conference report. Kidney Int 2011;80:17–28 [DOI] [PubMed] [Google Scholar]
- 3.American Diabetes Association. Standards of medical care in diabetes--2011 (Position Statement). Diabetes Care 2011;34:S11–S61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Camargo EG, Soares AA, Detanico AB, et al. The Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation is less accurate in patients with type 2 diabetes when compared with healthy individuals. Diabet Med 2011;28:90–95 [DOI] [PubMed] [Google Scholar]
- 5.Rognant N, Lemoine S, Laville M, Hadj-Aïssa A, Dubourg L. Performance of the chronic kidney disease epidemiology collaboration equation to estimate glomerular filtration rate in diabetic patients. Diabetes Care 2011;34:1320–1322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levey AS, Stevens LA, Schmid CH, et al. ; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bland JM, Altman DG. Measuring agreement in method comparison studies. Stat Methods Med Res 1999;8:135–160 [DOI] [PubMed] [Google Scholar]
- 8.Bland M. Sample size for a study of agreement between two methods of measurement [Internet]. Available from http://www-users.york.ac.uk/~mb55/meas/sizemeth.htm. Accessed 5 June 2011
- 9.Husdan H, Rapoport A. Estimation of creatinine by the Jaffe reaction. A comparison of three methods. Clin Chem 1968;14:222–238 [PubMed] [Google Scholar]
- 10.Silveiro SP, Friedman R, Gross JL. Glomerular hyperfiltration in NIDDM patients without overt proteinuria. Diabetes Care 1993;16:115–119 [DOI] [PubMed] [Google Scholar]