Abstract
OBJECTIVE
To examine longitudinal changes in total and appendicular lean body mass in older men with impaired fasting glucose (IFG) or diabetes and to determine whether these changes differ by diabetes treatment.
RESEARCH DESIGN AND METHODS
A total of 3,752 ambulatory men aged ≥65 years at baseline participated in a multicenter longitudinal cohort study. Baseline glycemic status was categorized as normoglycemia, IFG, undiagnosed/untreated diabetes, or treated diabetes. Insulin sensitizer medication use (metformin and/or thiazolidinediones) was assessed by prescription medication inventory. The change in total lean and appendicular lean mass was derived from dual X-ray absorptiometry scans taken at baseline and 3.5 ± 0.7 years later.
RESULTS
This male cohort included 1,853 individuals with normoglycemia, 1,403 with IFG, 234 with untreated diabetes, 151 with diabetes treated with insulin sensitizers, and 111 with diabetes treated without insulin sensitizers. Men with untreated diabetes, diabetes treated without insulin sensitizers, or IFG had greater percentage loss in total or appendicular lean mass (P ≤ 0.05 in comparison to normoglycemic men). There remained a significantly greater percentage loss in appendicular lean mass for these groups even after adjustment for medical comorbidities or lifestyle factors. In contrast, the percentage loss in total or appendicular lean mass in men with diabetes treated with insulin sensitizers was significantly less than that in normoglycemic men in minimally and fully adjusted models.
CONCLUSIONS
Skeletal muscle loss was accelerated in men with IFG and diabetes, except when the latter was treated with insulin sensitizers. These findings suggest that insulin sensitizers may attenuate muscle loss.
Aging is associated with adverse changes in body composition. The term “sarcopenia” refers to the age-related loss of skeletal muscle mass and function (1). Low muscle mass in the legs is associated with muscle weakness, poor lower-extremity performance, and mobility loss in older adults (2,3). Previous research has shown that older adults with type 2 diabetes have accelerated loss in muscle mass and strength compared with adults without diabetes (4,5). Moreover, we previously showed that older nondiabetic men with insulin resistance also have greater muscle mass loss than insulin-sensitive men (6). Although these studies do not establish causality, they suggest that insulin resistance may play a role in the development of sarcopenia.
Insulin resistance is present in people with impaired fasting glucose (IFG), impaired glucose tolerance, and type 2 diabetes. Because the prevalence of IFG and impaired glucose tolerance exceeds 37% and the prevalence of diabetes (predominantly type 2 diabetes) is ∼29% in U.S. adults over the age of 60 years, over half of older adults have a condition of underlying insulin resistance (7). Understanding potential consequences of insulin resistance is of vital importance, since these conditions are so common among older adults.
If insulin resistance has a role in the development of sarcopenia, one would expect increased muscle loss for older adults with IFG or type 2 diabetes and potential preventive effects of insulin sensitizers against muscle loss in adults with diabetes. We tested these hypotheses by examining the associations between varying states of insulin resistance and the effect of pharmacological treatment of insulin resistance on change in muscle mass in a large cohort of older men.
RESEARCH DESIGN AND METHODS
Study population and design
The Osteoporotic Fractures in Men (MrOS) study is a longitudinal observational study performed at six clinical sites in the U.S. for the primary aim of studying fracture determinants in older men. At baseline, 5,994 community-dwelling ambulatory men aged ≥65 years were enrolled using mass mailing targeted to age-eligible men, as described previously (8,9). The protocol was approved by each study site institutional review board, and written informed consent was obtained from all participants. The initial study visits occurred between March 2000 and April 2002, and participants returned for a second clinic visit between March 2005 and May 2006. A total of 12% of participants died before the second clinic visit, 9.3% were alive but terminated the study before the second visit, and 1.5% responded to postcard questions instead of attending the second visit. Participants who did not participate in the second clinic visit were on average older and had poorer self-rated health and a higher prevalence of cardiac disease, diabetes, and hypertension than participants in our analytic cohort. Compared with normoglycemic men, the age-adjusted risk of mortality did not differ significantly for men with IFG (hazard ratio 0.99, 95% CI 0.70–1.41) or for men with diabetes (1.44, 0.98–2.12). The cohort in the present analysis comprised 3,752 participants with complete body composition measurements from dual-energy absorptiometry (DXA) at baseline and visit 2 and complete measures for fasting glucose, triglycerides, HDL cholesterol levels, systolic blood pressure, and medication use for diabetes, hypertension, and dyslipidemia at baseline.
Baseline study visit measurements
Weight was taken with balance beam or digital scales, and height was measured with wall-mounted stadiometers. BMI was calculated as weight (kg)/height (m2). Mean systolic blood pressure was calculated as the mean of blood pressure measured twice using a mercury sphygmomanometer. Questionnaires and interviews were used to obtain demographic, lifestyle, and health data including age, race (white or other), education (greater than or equal to a college degree versus less than a college degree), clinic site, alcohol use (moderate/high defined as ≥7 vs. <7 drinks/week), current smoking status, physical activity levels using the Physical Activity Scale for the Elderly (PASE) score (10), self-reported health status (excellent/good vs. fair/poor/very poor), and medical conditions (self-reported physician diagnosis of hypertension, diabetes, cancer, stroke, myocardial infarction, congestive heart failure, and angina). Participants were categorized as having hypertension if their mean systolic blood pressure was ≥140 mmHg or they reported a physician diagnosis of hypertension. Men were considered to have cardiac disease if they reported a physician diagnosis of a myocardial infarction, congestive heart failure, or angina. The presence of dyslipidemia was characterized by HDL cholesterol <40 mg/dL, triglycerides ≥200 mg/dL, or the use of one or more lipid-lowering medication(s). Participants were instructed to bring in all prescription medications taken in the past 30 days to their clinic visit, and study coordinators recorded the medications. The Iowa Drug Information Service Drug Vocabulary (College of Pharmacy, University of Iowa, Iowa City, IA) was used to identify ingredient(s) in the medications, and these data were stored in an electronic medications inventory database (San Francisco Coordinating Center, San Francisco, CA) (11).
Biochemical measurements
Baseline fasting morning serum was collected and stored at −70°C. Glucose was measured using a hexokinase method from previously unthawed serum (Northwest Lipid Metabolism and Diabetes Research Laboratories, Seattle, WA). The interassay CV for glucose based on blind duplicates was <3%. Thawed and refrozen serum from the baseline visit was assayed for triglyceride and HDL cholesterol levels using a Roche COBAS Integra 800 automated analyzer (Roche Diagnostics, Indianapolis, IN) at the Veteran’s Administration Clinical Laboratory in Portland, Oregon. Interassay CVs for triglycerides and HDL cholesterol were 3.0 and 2.4%, respectively.
Body composition measurements
Total lean, appendicular lean, and total fat mass were derived from whole-body DXA scans (QDR 4500W; Hologic, Bedford, MA) at baseline and visit 2. The Hologic reading provided values for total bone mineral content, fat mass, and fat-free mass for each whole-body DXA scan. Total lean mass was calculated by subtracting total whole-body bone mineral content from total fat-free mass. For all clinic sites, the CVs were <1.7% for measurements of fat mass and <1.5% for measurements of lean mass. The DXA operators adjusted the cut lines on the whole-body DXA scans to define regions according to standard Hologic procedures. Cut lines were drawn between the head of the humerus and the scapula at the glenoid fossa for delineation of the arm and across the midpoint of the femoral neck for delineation of the leg, and appendicular lean mass was the sum of lean mass in the arms and legs. A Hologic whole-body phantom was scanned three times weekly at each clinic site for the calculation and application of correction factors to adjust for longitudinal drift in DXA measures. Reproducibility was ensured by certifying DXA operators, using standardized scanning procedures and using a central quality control laboratory (San Francisco Coordinating Center).
Glycemic status
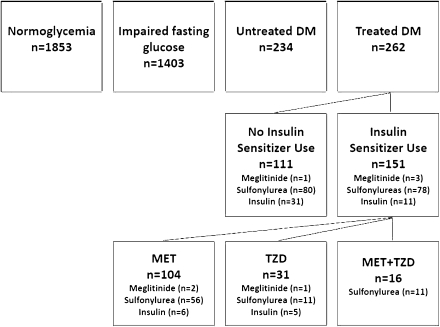
Categories of baseline glycemic status were created using fasting glucose levels, self-reported diagnosis of diabetes, and the medication inventory (Fig. 1). Men without self-reported diabetes or use of diabetes medications were considered to have normoglycemia if their fasting glucose level was <100 mg/dL and IFG if their fasting glucose level was 100–125 mg/dL. Men with a fasting glucose ≥126 mg/dL and/or self-reported diabetes were considered to have undiagnosed/untreated diabetes if they did not use medications to treat diabetes and treated diabetes if they used medication(s) to treat diabetes. Men with treated diabetes were further characterized as using insulin sensitizers (metformin and/or thiazolidinediones) at baseline or not, since studies using hyperinsulinemic-euglycemic clamps demonstrated improved peripheral insulin sensitivity with these medications (12–15). Additional diabetes medications taken by participants included insulin (n = 42), sulfonylureas (n = 158), and meglitinides (n = 4). There were no participants on α-glucosidase inhibitors.
Figure 1.
Number of men in glycemic and diabetes treatment categories. DM, diabetes mellitus; MET, metformin; TZD, thiazolidinedione.
Statistical analysis
Differences in baseline characteristics by glycemic and diabetes treatment categories were assessed using the χ2 test for categorical variables and ANOVA for continuous variables. Median absolute and percent changes in weight, total body fat mass, total body lean mass, and appendicular lean mass between baseline and visit 2 were calculated for these categories.
Linear regression models were used to determine differences in percentage change in total lean and appendicular lean mass for men in glycemic and diabetes treatment categories (IFG, untreated diabetes, diabetes treated with insulin sensitizers, and diabetes treated without insulin sensitizers) using normoglycemic men as the referent group. Covariates that were significant in the baseline table and also significantly associated with the percentage change in total or appendicular lean mass (P < 0.05) using ANOVA for categorical variables or Spearman correlation for continuous variables were considered confounders and were included in the multivariable regression model. Fasting glucose was not included in the multivariable model, since it was used to generate our glycemic and diabetes treatment categories. Because of collinearity with other baseline body composition measurements, BMI was not included in the multivariable model. Postestimation comparisons using the Wald test were performed to compare changes in lean mass for diabetic men on insulin sensitizers with untreated diabetes and diabetes treated without insulin sensitizers. Adjusted least-squares mean (LSM) percentage changes in total lean and appendicular lean mass for each category were calculated from the multivariable models. These analyses were repeated for glycemic and diabetes treatment categories restricted to participants who remained in consistent categories of normoglycemia, IFG, untreated diabetes, diabetes treated with insulin sensitizers, and diabetes treated without insulin sensitizers at the follow-up visit. Statistical analyses were performed using STATA/IC 11.0 (STATACorp LP, College Station, TX).
RESULTS
At baseline, this cohort of 3,752 men had a mean age of 72.7 years (range 65–92 years) and a BMI of 27.4 kg/m2. When separated into glycemic categories, 1,853 men had normoglycemia, 1,403 had IFG, 234 had untreated diabetes, 151 had diabetes treated with insulin sensitizers, and 111 had diabetes treated without insulin sensitizers. These groups differed significantly at baseline by race, education, alcohol use, self-rated health, cardiovascular disease, hypertension, dyslipidemia, β-blocker and ACE inhibitor use, and total body lean and fat mass (Table 1).
Table 1.
Baseline characteristics by glycemic and diabetes treatment categories
| Normoglycemia | IFG | Untreated diabetes | Diabetes treated with insulin sensitizers | Diabetes treated with no insulin sensitizers | P | |
|---|---|---|---|---|---|---|
| n | 1,853 | 1,403 | 234 | 151 | 111 | |
| Age (years) | 72.7 (5.5) | 72.7 (5.3) | 72.9 (5.0) | 71.8 (5.0) | 72.9 (5.2) | 0.34 |
| Smoking (%) | 2.9 | 2.9 | 3.0 | 0.7 | 0.9 | 0.38 |
| Race; white (%) | 92.9 | 91.0 | 92.3 | 78.8 | 79.3 | <0.01 |
| College degree or higher (%) | 60.4 | 52.2 | 47.4 | 45.0 | 47.8 | <0.01 |
| Alcohol (≥7 drinks/week) (%) | 26.5 | 28.9 | 23.9 | 14.6 | 21.6 | <0.01 |
| PASE | 153.9 (67.0) | 153.4 (68.6) | 144.2 (62.1) | 149.3 (63.2) | 145.9 (68.9) | 0.20 |
| Excellent/good self-rated health (%) | 91.9 | 90.0 | 85.9 | 76.8 | 73.0 | <0.01 |
| Cancer (%) | 29.0 | 27.5 | 23.9 | 23.2 | 26.1 | 0.29 |
| Cardiac disease (%) | 18.4 | 18.6 | 26.1 | 32.5 | 39.6 | <0.01 |
| Stroke (%) | 4.0 | 3.9 | 4.7 | 6.0 | 6.3 | 0.53 |
| Hypertension (%) | 54.1 | 63.7 | 73.9 | 72.9 | 72.1 | <0.01 |
| Dyslipidemia (%) | 44.3 | 55.4 | 67.5 | 77.5 | 71.2 | <0.01 |
| β-Blocker use (%) | 14.8 | 18.6 | 21.8 | 27.8 | 19.8 | <0.01 |
| ACE inhibitor use (%) | 14.4 | 17.2 | 19.2 | 50.3 | 50.5 | <0.01 |
| Total fat mass (kg) | 20.0 (6.4) | 23.0 (7.0) | 25.0 (7.5) | 24.9 (7.2) | 23.7 (8.0) | <0.01 |
| Total lean mass (kg) | 56.2 (6.6) | 57.9 (7.2) | 59.4 (7.4) | 60.4 (7.9) | 59.8 (6.8) | <0.01 |
| BMI (kg/m2) | 26.4 (3.3) | 28.0 (3.7) | 29.2 (4.2) | 29.4 (4.0) | 29.1 (4.0) | <0.01 |
| Fasting glucose (mg/dL) | 92.2 (5.3) | 107.8 (6.4) | 140.6 (29.5) | 158.6 (48.7) | 147.6 (51.8) | <0.01 |
Data are proportions or means (SD). P values are provided for ANOVA or χ2 test.
Over a mean duration of 3.5 ± 0.7 years of follow-up, men lost on average 1.5% in total body weight, gained 1.6% total body fat, lost 2.0% total body lean mass, and lost 3.2% appendicular lean mass. Men with untreated diabetes or diabetes treated without insulin sensitizers had the greatest median absolute and percentage loss in weight and total fat, total lean, and appendicular lean mass (Table 2). Men who were taking insulin sensitizers to treat diabetes had the least amount of total and appendicular lean mass loss.
Table 2.
Median (interquartile range) absolute and percent change (Δ) in body weight, total fat, total lean, and appendicular lean mass between visits 1 and 2 by glycemic and diabetes treatment categories over 3.5 years
| Normoglycemia | IFG | Untreated diabetes | Diabetes treated with insulin sensitizers | Diabetes treated with no insulin sensitizers | |
|---|---|---|---|---|---|
| Total body weight | |||||
| Δ (kg) | −1.0 (−3.2 to 1.4) | −1.3 (−4.0 to 1.4) | −2.5 (−5.1 to 0.8) | 0 (−3.1 to 3.1) | −2.1 (−5.9 to 0.3) |
| Δ (%) | −1.2 (−4.0 to 1.8) | −1.5 (−4.8 to 1.6) | −2.8 (−6.0 to 0.9) | 0 (−3.5 to 3.5) | −2.5 (−6.8 to 0.4) |
| Total fat | |||||
| Δ (kg) | 0.2 (−1.3 to 1.9) | 0.2 (−1.6 to 1.9) | −0.4 (−2.5 to 1.7) | 1.0 (−1.1 to 3.4) | −0.1 (−2.4 to 2.0) |
| Δ (%) | 1.2 (−6.9 to 10.4) | 0.8 (−7.1 to 8.6) | −1.9 (−10.9 to 7.4) | 3.4 (−4.8 to 14.2) | −0.4 (−9.9 to 9.9) |
| Total lean | |||||
| Δ (kg) | −1.0 (−2.2 to 0.3) | −1.1 (−2.6 to 0.2) | −1.6 (−3.3 to −0.3) | −0.9 (−2.5 to 0.7) | −1.9 (−3.8 to −0.2) |
| Δ (%) | −1.7 (−3.9 to 0.5) | −1.9 (−4.5 to 0.4) | −2.9 (−5.5 to −0.4) | −1.4 (−4.2 to 1.1) | −3.0 (−6.6 to −0.4) |
| Appendicular lean | |||||
| Δ (kg) | −0.6 (−1.4 to 0.0) | −0.8 (−1.6 to −0.1) | −1.1 (−2.0 to −0.2) | −0.6 (−1.4 to 0.2) | −1.2 (−2.1 to −0.3) |
| Δ (%) | −2.7 (−5.7 to 0.1) | −3.1 (−6.3 to −0.3) | −4.3 (−7.8 to −1.0) | −2.4 (−5.2 to 0.7) | −4.9 (−8.2 to −1.2) |
In linear regression models adjusted for age, race, and clinic site, individuals with IFG, untreated diabetes, or diabetes treated without insulin sensitizers had a greater percentage loss in total or appendicular lean mass than normoglycemic men (Table 3). In models further adjusted for medical comorbidities and physical and lifestyle characteristics, the percentage loss in appendicular lean mass in these categories still exceeded that of normoglycemic men. In contrast, the percentage of total or appendicular lean mass lost in men with diabetes treated with insulin sensitizers was significantly less than that of normoglycemic men in both models. When compared with men who had untreated diabetes or diabetes treated without insulin sensitizers, men with diabetes treated with insulin sensitizers also had a lower percentage loss in total lean mass (P < 0.001) and appendicular lean mass (P < 0.001) for these Wald test comparisons using model 1. Although the frequency of meglitinide use did not differ significantly between groups of insulin sensitizer treatment, there was a higher frequency of insulin and sulfonylurea use among men with diabetes who were not taking insulin sensitizers. Neither adjustment for sulfonylurea nor insulin use in these multivariate models altered our results significantly (results not shown).
Table 3.
Adjusted LSM percentage change (95% CI) in total lean and appendicular lean mass by glycemic and diabetes treatment categories over 3.5 years
| Normoglycemia | IFG | Untreated diabetes | Diabetes treated with insulin sensitizers | Diabetes treated with no insulin sensitizers | |
|---|---|---|---|---|---|
| %Δ Total lean | |||||
| Model 1 | −1.7 (−1.9 to −1.6) | −2.1 (−2.3 to −1.9)* | −2.8 (−3.2 to −2.3)* | −1.4 (−2.0 to −0.8) | −3.2 (−4.0 to −2.5)* |
| Model 2 | −1.9 (−2.0 to −1.7) | −2.1 (−2.3 to −1.9) | −2.5 (−3.0 to −2.0)* | −1.1 (−1.7 to −0.5)* | −2.9 (−3.6 to −2.2)* |
| %Δ Appendicular lean | |||||
| Model 1 | −2.9 (−3.1 to −2.7) | −3.4 (−3.7 to −3.2)* | −4.5 (−5.1 to −3.8)* | −2.2 (−3.0 to −1.5) | −4.8 (−5.7 to −3.9)* |
| Model 2 | −3.0 (−3.3 to −2.8) | −3.4 (−3.6 to −3.1)* | −4.2 (−4.8 to −3.6)* | −1.8 (−2.6 to −1.1)* | −4.4 (−5.3 to −3.5)* |
Model 1 is adjusted for age, race, and clinic site. Model 2 includes model 1 covariates + self-rated health, cardiac disease, hypertension, dyslipidemia, education, PASE, baseline total fat mass, and total lean mass.
*P ≤ 0.05 compared with the normoglycemic group.
Additional investigation of the lean mass changes associated with specific insulin sensitizers was performed as post hoc analyses in models adjusted for age, race, and clinic site. Men with diabetes treated with thiazolidinediones (n = 31) did not differ significantly from normoglycemic men in the amount of total lean mass loss (β = –0.62, P = 0.37, LSM change −2.4% [95% CI −3.7 to −1.0]) or appendicular lean mass loss (β = –0.15, P = 0.86, LSM change −3.1% [−4.8 to −1.4]). Although men with diabetes treated with metformin (n = 104) lost less total lean (LSM change −1.5% [−2.2 to −0.7]) and appendicular lean mass (LSM change –2.4% [−3.4 to −1.5]) than normoglycemic men, the differences were not statistically significant (total lean: β = 0.27, P = 0.49, and appendicular lean: β = 0.49, P = 0.32). Men with diabetes treated with metformin + thiazolidinedione (n = 16) had a significant gain in total lean mass (β = 2.59, P < 0.01, LSM change +0.8% [−1.0 to 2.7]) and appendicular lean mass (β = 3.44, P < 0.01, LSM change = +0.5% [−1.8 to 2.9]) compared with normoglycemic men. Men treated with metformin or metformin + thiazolidinedione had significantly less total or appendicular lean mass percentage loss than men with untreated diabetes (P < 0.005) or men with diabetes treated without insulin sensitizers (P < 0.001) for these Wald test comparisons in age-, race-, and clinic site–adjusted models.
When the above analyses were repeated after restricting to men remaining in consistent glycemic and diabetes treatment categories at visit 2, all of the results above remained unchanged. However, while the percentage loss in appendicular lean mass for men with diabetes treated with metformin + thiazolidinedione (LSM change –0.2% [95% CI −3.0 to 2.6]) was still less than for normoglycemic men (LSM change –2.9% [−3.1 to −2.7]), the findings were no longer significantly different (metformin + thiazolidinedione: β = 2.72, P = 0.06).
CONCLUSIONS
Our study confirms the greater loss in total and appendicular lean mass in older men with untreated diabetes compared with normoglycemic men that has been described in a slightly older cohort of men and women (4). The study also provides additional evidence of greater total and appendicular lean mass loss in older men with IFG or with diabetes treated without insulin sensitizers compared with normoglycemic men. However, the loss in total or appendicular lean mass for men who had diabetes treated with insulin sensitizers was significantly less than that for normoglycemic men. Furthermore, men with diabetes using insulin sensitizers lost significantly less total or appendicular lean mass than men with untreated diabetes or diabetes treated without insulin sensitizers. These data show that conditions with underlying insulin resistance are associated with greater muscle loss and suggest that such loss may be prevented with pharmacological treatment of insulin resistance.
Metformin and thiazolidinediones are among the most frequent therapies used to treat type 2 diabetes, and both drugs can improve insulin sensitivity (12,13). Because greater insulin resistance is associated with greater losses in total and appendicular lean mass (6), one could hypothesize that the reduction of insulin resistance with insulin sensitizer treatment in men with type 2 diabetes would attenuate this loss. Indeed, our findings revealed that diabetic men treated with insulin sensitizers had significantly less appendicular lean mass loss than diabetic men who were untreated or using other therapies. Whereas it is possible that glycemic control differs between these categories, it seems unlikely that these findings are explained by differences in dysglycemia, since the loss in lean mass was less for men with diabetes treated with insulin sensitizers and more for men with diabetes treated without insulin sensitizers when compared with normoglycemic men, despite higher levels of fasting glucose in men with diabetes. Further analysis of specific insulin sensitizers revealed that diabetic men on metformin or metformin + thiazolidinediones had significantly less total and appendicular lean mass loss than men with untreated diabetes or diabetes treated without insulin sensitizers. It is possible that additional differences for men taking thiazolidinediones alone were not detected because of the small sample size of this group.
The mechanisms responsible for these findings are uncertain. In contrast to sulfonylureas that act on the pancreas to stimulate insulin secretion, insulin sensitizers can act peripherally on muscle, similar to the effects of exercise on muscle, to activate 5′-adenosine monophosphate–activated protein kinase (AMPK) and stimulate gene expression of peroxisome proliferator–activated receptor-γ coactivator 1-α (PGC1α) to upregulate the transcription of genes to enhance fatty acid oxidation for reduced muscle lipid accumulation, stimulate angiogenesis, increase mitochondrial biogenesis, and switch muscle fiber types from glycolytic to more oxidative fatigue-resistant fibers (16–20). Further evidence from basic studies demonstrates that muscle-specific overexpression of PGC1α in transgenic mice increased muscle mass and reduced the number of falls (21). In addition, treatment of mice with aminoimidazole carboxamide ribonucleotide (AICAR), an AMPK agonist, increased phosphorylation of AMPK in the quadriceps muscle, induced expression of PGC1α in myoblasts, improved endurance, and extended running distance (22). More translational studies are needed to further understand the effects of insulin sensitizers on muscle in humans.
These findings are provocative, but this study has limitations. This is a cohort of older predominantly white men who were ambulatory and healthy enough to participate in follow-up visits; therefore, our results may not be generalized to ailing men, minorities, older women, or younger populations. Because this is an observational study without randomization to different diabetes treatments, confounding by indication cannot be excluded. Although adjustment for between-group differences did not materially change the significant associations among diabetes treatment groups, residual confounding may remain. Data on treatment adherence were not available, but results were similar when information derived from patient report was used to restrict analyses to men consistently reporting diabetes diagnosis and medication use at visit 2. Misclassification of men with untreated diabetes was possible, since oral glucose tolerance testing was not performed in the MrOS study; therefore, the loss in lean mass in men with IFG may be due to inclusion of men with untreated diabetes. It is possible that some men who were categorized as having treated diabetes had insulin-sensitive type 1 diabetes rather than insulin-resistant type 2 diabetes. However, analyses repeated excluding the 42 participants on insulin did not materially change our results (data not shown).
In summary, muscle loss was accelerated in individuals with IFG, untreated diabetes, or diabetes treated without insulin sensitizer medications. However, the loss in muscle mass was markedly attenuated in diabetic men using insulin sensitizers. These results suggest that insulin resistance may be causally linked to muscle loss in age-related sarcopenia. Furthermore, they raise the need for randomized clinical trials of insulin sensitizers to understand their potential effects on muscle loss and possible preventative or therapeutic applications in sarcopenia.
Acknowledgments
The MrOS study was supported by funding from the National Institutes of Health. The following institutes provided support: the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the National Institute on Aging (NIA), the National Center for Research Resources (NCRR), and the National Institutes of Health Roadmap for Medical Research under the following grant numbers: U01-AR45580, U01-AR45614, U01-AR45632, U01-AR45647, U01-AR45654, U01-AR45583, U01-AG18197, U01-AG027810, and UL1-RR024140. C.G.L. received additional support from the Building Interdisciplinary Careers in Women's Health Program (grant number K12-HD043488-09). Additional support was provided by the American Diabetes Association (grant number 1-04-JF-46) to E.S.S. I.M. was supported by the Mentored Research Scientist Development Award from the National Institute of Diabetes and Digestive and Kidney Diseases (K01-DK083029). VA Puget Sound Health Care System provided support for participation of E.J.B. in this research.
P.M.C. is a consultant for Amgen. No other potential conflicts of interest relevant to this article were reported.
C.G.L. researched data and wrote the manuscript. E.J.B. and E.S.O. researched data, reviewed and edited the manuscript, and contributed to discussion. E.B.-C., S.A.E.-R., P.M.C., and E.S.S. reviewed and edited the manuscript and contributed to discussion. I.M., A.R.H., and C.E.L. reviewed and edited the manuscript.
References
- 1.Fielding RA, Vellas B, Evans WJ, et al. Sarcopenia: an undiagnosed condition in older adults: current consensus definition: prevalence, etiology, and consequences: International Working Group on Sarcopenia. J Am Med Dir Assoc 2011;12:249–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Visser M, Kritchevsky SB, Goodpaster BH, et al. Leg muscle mass and composition in relation to lower extremity performance in men and women aged 70 to 79: the health, aging and body composition study. J Am Geriatr Soc 2002;50:897–904 [DOI] [PubMed] [Google Scholar]
- 3.Visser M, Goodpaster BH, Kritchevsky SB, et al. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J Gerontol A Biol Sci Med Sci 2005;60:324–333 [DOI] [PubMed] [Google Scholar]
- 4.Park SW, Goodpaster BH, Lee JS, et al. Excessive loss of skeletal muscle mass in older adults with type 2 diabetes. Diabetes Care 2009;32:1993–1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park SW, Goodpaster BH, Strotmeyer ES, et al. Accelerated loss of skeletal muscle strength in older adults with type 2 diabetes: the health, aging, and body composition study. Diabetes Care 2007;30:1507–1512 [DOI] [PubMed] [Google Scholar]
- 6.Lee CG, Boyko EJ, Strotmeyer ES, et al. Association between insulin resistance and lean mass loss and fat mass gain in older men without diabetes mellitus. J Am Geriatr Soc 2011;59:1217–1224 [DOI] [PMC free article] [PubMed]
- 7.Cowie CC, Rust KF, Ford ES, et al. Full accounting of diabetes and pre-diabetes in the U.S. population in 1988–1994 and 2005–2006. Diabetes Care 2009;32:287–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blank JB, Cawthon PM, Carrion-Petersen ML, et al. Overview of recruitment for the osteoporotic fractures in men study (MrOS). Contemp Clin Trials 2005;26:557–568 [DOI] [PubMed] [Google Scholar]
- 9.Orwoll E, Blank JB, Barrett-Connor E, et al. Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study: a large observational study of the determinants of fracture in older men. Contemp Clin Trials 2005;26:569–585 [DOI] [PubMed] [Google Scholar]
- 10.Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol 1993;46:153–162 [DOI] [PubMed] [Google Scholar]
- 11.Pahor M, Chrischilles EA, Guralnik JM, Brown SL, Wallace RB, Carbonin P. Drug data coding and analysis in epidemiologic studies. Eur J Epidemiol 1994;10:405–411 [DOI] [PubMed] [Google Scholar]
- 12.Maggs DG, Buchanan TA, Burant CF, et al. Metabolic effects of troglitazone monotherapy in type 2 diabetes mellitus: a randomized, double-blind, placebo-controlled trial. Ann Intern Med 1998;128:176–185 [DOI] [PubMed] [Google Scholar]
- 13.Widén EI, Eriksson JG, Groop LC. Metformin normalizes nonoxidative glucose metabolism in insulin-resistant normoglycemic first-degree relatives of patients with NIDDM. Diabetes 1992;41:354–358 [DOI] [PubMed] [Google Scholar]
- 14.Tamura Y, Watada H, Sato F, et al. Effects of metformin on peripheral insulin sensitivity and intracellular lipid contents in muscle and liver of overweight Japanese subjects. Diabetes Obes Metab 2008;10:733–738 [DOI] [PubMed] [Google Scholar]
- 15.Levin K, Hother-Nielsen O, Henriksen JE, Beck-Nielsen H. Effects of troglitazone in young first-degree relatives of patients with type 2 diabetes. Diabetes Care 2004;27:148–154 [DOI] [PubMed] [Google Scholar]
- 16.Suwa M, Egashira T, Nakano H, Sasaki H, Kumagai S. Metformin increases the PGC-1alpha protein and oxidative enzyme activities possibly via AMPK phosphorylation in skeletal muscle in vivo. J Appl Physiol 2006;101:1685–1692 [DOI] [PubMed] [Google Scholar]
- 17.Lira VA, Benton CR, Yan Z, Bonen A. PGC-1alpha regulation by exercise training and its influences on muscle function and insulin sensitivity. Am J Physiol Endocrinol Metab 2010;299:E145–E161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stephens TJ, Chen ZP, Canny BJ, Michell BJ, Kemp BE, McConell GK. Progressive increase in human skeletal muscle AMPKalpha2 activity and ACC phosphorylation during exercise. Am J Physiol Endocrinol Metab 2002;282:E688–E694 [DOI] [PubMed] [Google Scholar]
- 19.Coletta DK, Sriwijitkamol A, Wajcberg E, et al. Pioglitazone stimulates AMP-activated protein kinase signalling and increases the expression of genes involved in adiponectin signalling, mitochondrial function and fat oxidation in human skeletal muscle in vivo: a randomised trial. Diabetologia 2009;52:723–732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Musi N, Hirshman MF, Nygren J, et al. Metformin increases AMP-activated protein kinase activity in skeletal muscle of subjects with type 2 diabetes. Diabetes 2002;51:2074–2081 [DOI] [PubMed] [Google Scholar]
- 21.Wenz T, Rossi SG, Rotundo RL, Spiegelman BM, Moraes CT. Increased muscle PGC-1alpha expression protects from sarcopenia and metabolic disease during aging. Proc Natl Acad Sci U S A 2009;106:20405–20410 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22.Narkar VA, Downes M, Yu RT, et al. AMPK and PPARdelta agonists are exercise mimetics. Cell 2008;134:405–415 [DOI] [PMC free article] [PubMed] [Google Scholar]