Abstract
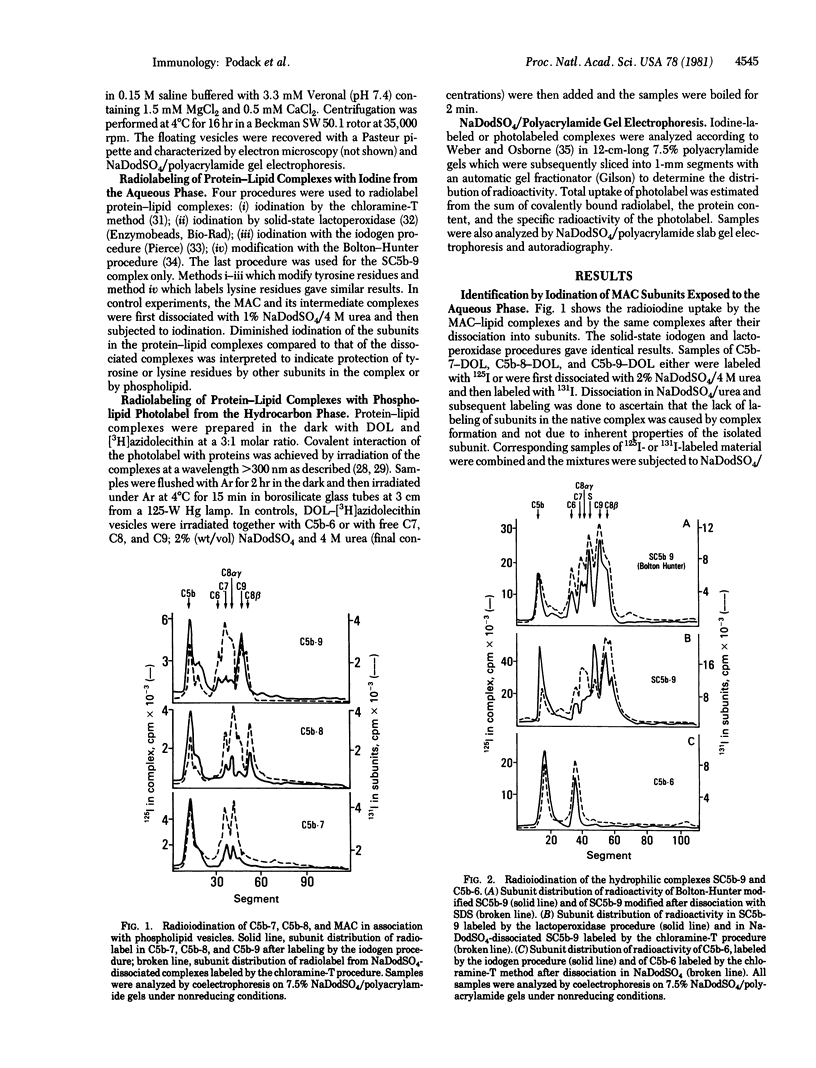
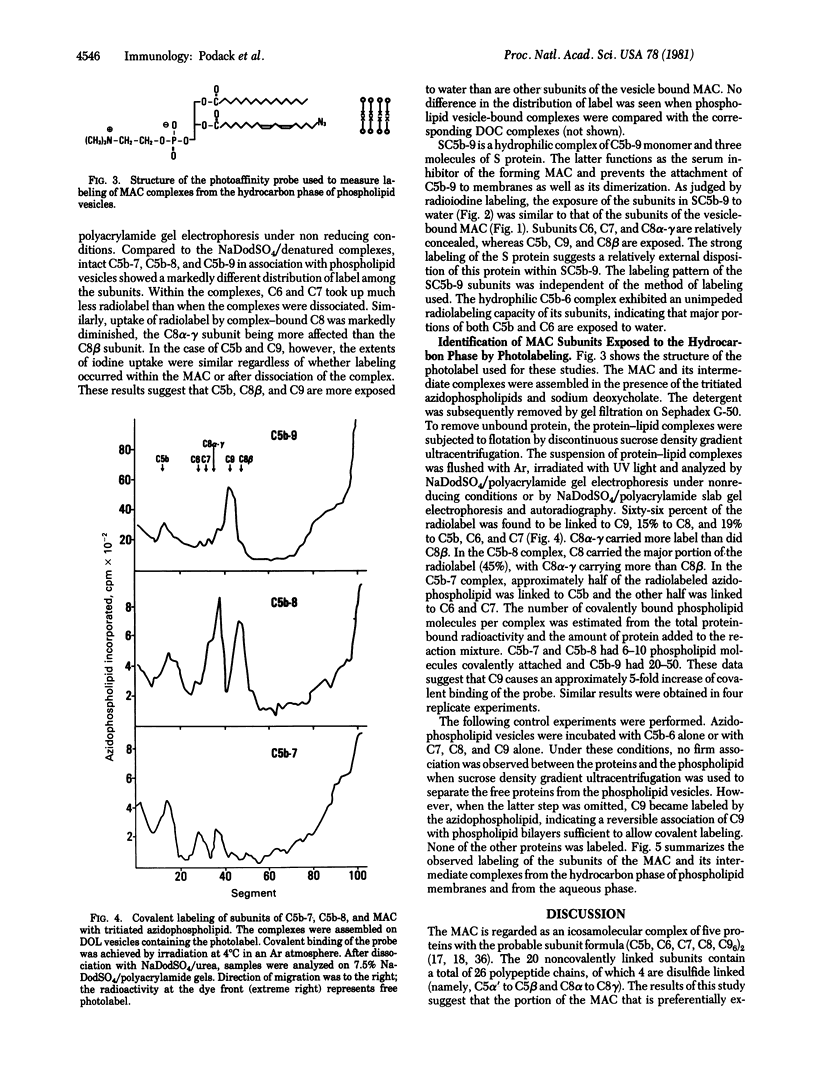
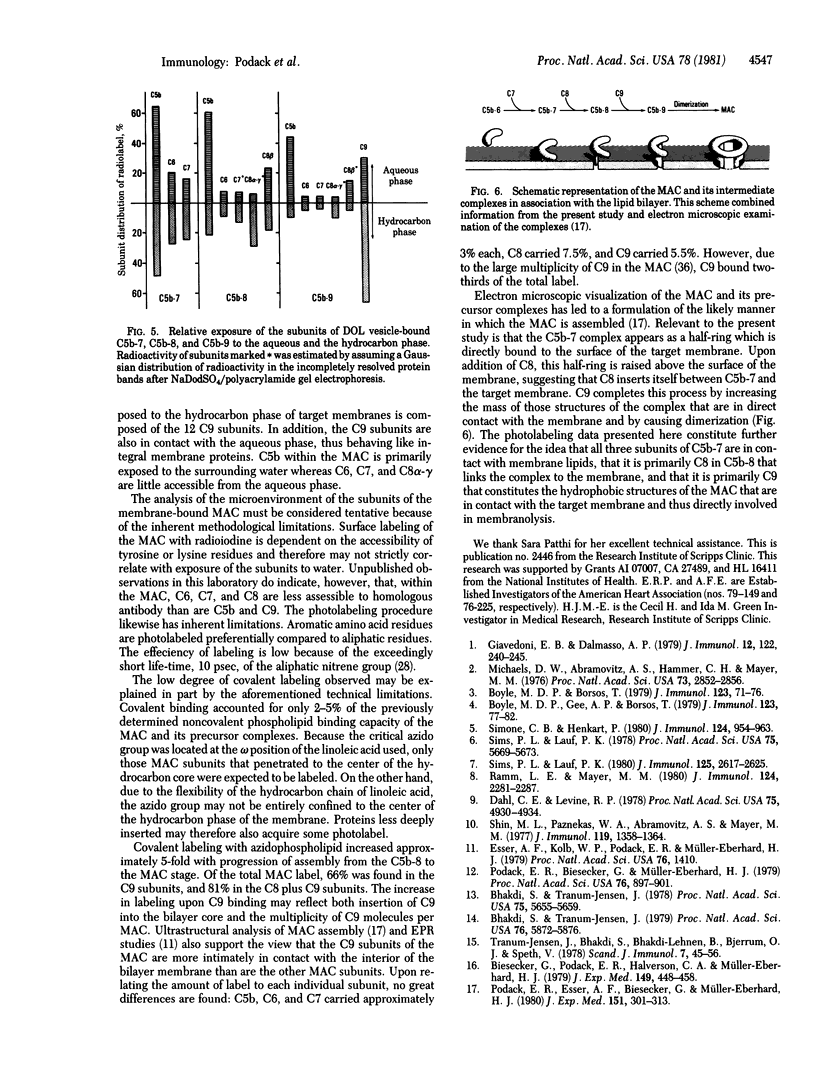
Membrane destruction by complement is effected by the membrane attack complex (MAC) which is the dimer of a fusion product of the complement proteins C5b, C6, C7, C8, and C9. Phospholipid bilayer vesicles were used as target membranes for the MAC and its intermediate complexes. The subunits of these membrane-bound complexes were explored as to their relative exposure to the hydrocarbon phase of the lipid bilayer and to water surrounding the lipid vesicles. Protein exposed to the aqueous phase was labeled with 125I; protein exposed to the hydrocarbon phase was labeled by using tritiated azido phospholipids and irradiation. Analysis of the membrane-bound MAC showed that subunits C5b, C8 beta, and C9 were exposed to the aqueous phase. The subunits C8 alpha-gamma and C9 were primarily in contact with the hydrocarbon phase. C6 and C7 were little exposed to either phase, suggesting that these proteins are inaccessible within the MAC. Analysis of the intermediate complexes showed that C5b was the subunit most exposed to water in membrane-bound C5b-7, and C5b and C8 beta were the water-exposed subunits in C5b-8. Subunit exposure to the hydrocarbon phase of the lipid bilayer changed during MAC assembly. Whereas all three subunits of C5b-7 carried the phospholipid photolabel; most of the label was bound to the C8 subunit in C5b-8 and to C9 in the MAC. It is proposed that contact with the hydrocarbon core of membranes is established by C5b-7 through each of its subunits, by C5b-8 through C8, and by the MAC through C8 and, particularly, C9.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhakdi S., Tranum-Jensen J. Evidence for a two-domain structure of the terminal membrane C5b-9 complex of human complement. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5872–5876. doi: 10.1073/pnas.76.11.5872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakdi S., Tranum-Jensen J. Molecular nature of the complement lesion. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5655–5659. doi: 10.1073/pnas.75.11.5655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biesecker G., Müller-Eberhard H. J. The ninth component of human complement: purification and physicochemical characterization. J Immunol. 1980 Mar;124(3):1291–1296. [PubMed] [Google Scholar]
- Biesecker G., Podack E. R., Halverson C. A., Müller-Eberhard H. J. C5b-9 dimer: isolation from complement lysed cells and ultrastructural identification with complement-dependent membrane lesions. J Exp Med. 1979 Feb 1;149(2):448–458. doi: 10.1084/jem.149.2.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton A. E., Hunter W. M. The labelling of proteins to high specific radioactivities by conjugation to a 125I-containing acylating agent. Biochem J. 1973 Jul;133(3):529–539. doi: 10.1042/bj1330529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle M. D., Borsos T. Studies on the terminal stages of immune hemolysis. V. Evidence that not all complement-produced transmembrane channels are equal. J Immunol. 1979 Jul;123(1):71–76. [PubMed] [Google Scholar]
- Boyle M. D., Gee A. P., Borsos T. Studies on the terminal stages of immune hemolysis. VI. Osmotic blockers of differing Stokes' radii detect complement-induced transmembrane channels of differing size. J Immunol. 1979 Jul;123(1):77–82. [PubMed] [Google Scholar]
- Brunner J., Skrabal P., Hauser H. Single bilayer vesicles prepared without sonication. Physico-chemical properties. Biochim Biophys Acta. 1976 Dec 2;455(2):322–331. doi: 10.1016/0005-2736(76)90308-4. [DOI] [PubMed] [Google Scholar]
- Costrini N. V., Bradshaw R. A. Binding characteristics and apparent molecular size of detergent solubilized nerve growth factor receptor of sympathetic ganglia. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3242–3245. doi: 10.1073/pnas.76.7.3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl C. E., Levine R. P. Electron spin resonance studies on interaction of complement proteins with erythrocyte membranes. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4930–4934. doi: 10.1073/pnas.75.10.4930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dourmashkin R. R. The structural events associated with the attachment of complement components to cell membranes in reactive lysis. Immunology. 1978 Aug;35(2):205–212. [PMC free article] [PubMed] [Google Scholar]
- Esser A. F., Kolb W. P., Podack E. R., Müller-Eberhard H. J. Molecular reorganization of lipid bilayers by complement: a possible mechanism for membranolysis. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1410–1414. doi: 10.1073/pnas.76.3.1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Giavedoni E. B., Chow Y. M., Dalmasso A. P. The functional size of the primary complement lesion in resealed erythrocyte membrane ghosts. J Immunol. 1979 Jan;122(1):240–245. [PubMed] [Google Scholar]
- Klob W. P., Müller-Eberhard H. J. The membrane attack mechanism of complement: the three polypeptide chain structure of the eigth component (C8). J Exp Med. 1976 May 1;143(5):1131–1139. doi: 10.1084/jem.143.5.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb W. P., Haxby J. A., Arroyave C. M., Müller-Eberhard H. J. The membrane attack mechanism of complement. Reversible interactions among the five native components in free solution. J Exp Med. 1973 Aug 1;138(2):428–437. doi: 10.1084/jem.138.2.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb W. P., Muller-Eberhard H. J. The membrane attack mechanism of complement. Isolation and subunit composition of the C5b-9 complex. J Exp Med. 1975 Apr 1;141(4):724–735. [PMC free article] [PubMed] [Google Scholar]
- Kolb W. P., Müller-Eberhard H. J. Mode of action of human C9: adsorption of multiple C9 molecules to cell-bound C8. J Immunol. 1974 Aug;113(2):479–488. [PubMed] [Google Scholar]
- McConahey P. J., Dixon F. J. A method of trace iodination of proteins for immunologic studies. Int Arch Allergy Appl Immunol. 1966;29(2):185–189. doi: 10.1159/000229699. [DOI] [PubMed] [Google Scholar]
- Michaels D. W., Abramovitz A. S., Hammer C. H., Mayer M. M. Increased ion permeability of planar lipid bilayer membranes after treatment with the C5b-9 cytolytic attack mechanism of complement. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2852–2856. doi: 10.1073/pnas.73.8.2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podack E. R., Biesecker G., Müller-Eberhard H. J. Membrane attack complex of complement: generation of high-affinity phospholipid binding sites by fusion of five hydrophilic plasma proteins. Proc Natl Acad Sci U S A. 1979 Feb;76(2):897–901. doi: 10.1073/pnas.76.2.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podack E. R., Esser A. F., Biesecker G., Müller-Eberhard H. J. Membrane attack complex of complement: a structural analysis of its assembly. J Exp Med. 1980 Feb 1;151(2):301–313. doi: 10.1084/jem.151.2.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podack E. R., Kolb W. P., Esser A. F., Müller-Eberhard H. J. Structural similarities between C6 and C7 of human complement. J Immunol. 1979 Sep;123(3):1071–1077. [PubMed] [Google Scholar]
- Podack E. R., Kolb W. P., Müller-Eberhard H. J. The C5b-6 complex: formation, isolation, and inhibition of its activity by lipoprotein and the S-protein of human serum. J Immunol. 1978 Jun;120(6):1841–1848. [PubMed] [Google Scholar]
- Podack E. R., Müller-Eberhard H. J. Membrane attack complex of complement. Evidence for its dimeric structure based on hybrid formation. J Biol Chem. 1981 Apr 10;256(7):3145–3148. [PubMed] [Google Scholar]
- Ramm L. E., Mayer M. M. Life-span and size of the trans-membrane channel formed by large doses of complement. J Immunol. 1980 May;124(5):2281–2287. [PubMed] [Google Scholar]
- Shin M. L., Paznekas W. A., Abramovitz A. S., Mayer M. M. On the mechanism of membrane damage by C: exposure of hydrophobic sites on activated C proteins. J Immunol. 1977 Oct;119(4):1358–1364. [PubMed] [Google Scholar]
- Simone C. B., Henkart P. Permeability changes induced in erthrocyte ghost targets by antibody-dependent cytotoxic effector cells: evidence for membrane pores. J Immunol. 1980 Feb;124(2):954–963. [PubMed] [Google Scholar]
- Sims P. J., Lauf P. K. Analysis of solute diffusion across the C5b-9 membrane lesion of complement: evidence that individual C5b-9 complexes do not function as discrete, uniform pores. J Immunol. 1980 Dec;125(6):2617–2625. [PubMed] [Google Scholar]
- Sims P. J., Lauf P. K. Steady-state analysis of tracer exchange across the C5b-9 complement lesion in a biological membrane. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5669–5673. doi: 10.1073/pnas.75.11.5669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoffel W., Schreiber C., Scheefers H. Lipids with photosensitive groups as chemical probes for the structural analysis of biological membranes. On the localization of the G- and M-protein of vesicular stomatitis virus. Hoppe Seylers Z Physiol Chem. 1978 Aug;359(8):923–931. doi: 10.1515/bchm2.1978.359.2.923. [DOI] [PubMed] [Google Scholar]
- Tranum-Jensen J., Bhakdi S., Bhakdi-Lehnen B., Bjerrum O. J., Speth V. Complement lysis: the ultrastructure and orientation of the C5b-9 complex on target sheep erythrocyte membranes. Scand J Immunol. 1978;7(1):45–46. doi: 10.1111/j.1365-3083.1978.tb00425.x. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]



