Abstract
OBJECTIVE
To examine rates of severe hypoglycemia (SH) in a large population-based cohort of children with type 1 diabetes and relationships to HbA1c.
RESEARCH DESIGN AND METHODS
Data from 1,683 children (mean [SD] age at diagnosis 10.5 [4.2]; range 1–18 years) from 2000 to 2009 were analyzed from the Western Australian Children's Diabetes Database. Rates of SH were related to HbA1c using negative binomial regression.
RESULTS
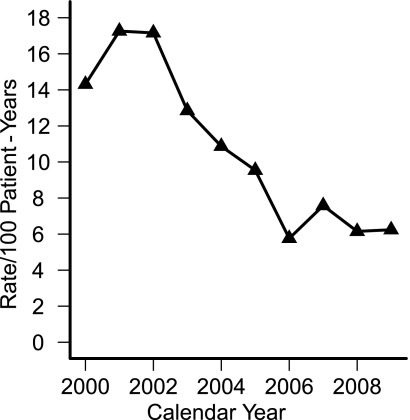
A total of 7,378 patient-years of data and 780 SH events were recorded. The rate of SH per 100 patient-years peaked at 17.3 in 2001 and then declined from 2004 to a nadir of 5.8 in 2006. HbA1c <7% was not associated with higher risk of SH (incidence rate ratio 1.2 [95% CI 0.9–1.6], P = 0.29) compared with HbA1c of 8–9%.
CONCLUSIONS
In a sample of youth with type 1 diabetes, there has been a decrease in rates of SH and a weaker relationship with glycemic control than previously observed.
The threat of hypoglycemia as a consequence of insulin treatment is a major barrier to optimizing glycemic control in type 1 diabetes (1). Previous studies have demonstrated a close relationship between glycemic control and increased rates of severe hypoglycemia (SH) (2). In the 1990s, increased emphasis on strict glycemic control was paralleled with an increase in the rate of SH, particularly in younger children (<6 years) (3,4). Therapies have changed, however, and the objectives in this study were to examine contemporary rates of SH in a large population-based cohort of pediatric type 1 diabetes and changes over the past decade and to investigate relationships with glycemic control.
RESEARCH DESIGN AND METHODS
All children with type 1 diabetes aged ≤18 years attending Princess Margaret Hospital for Children from 2000 to 2009 were prospectively included in the study. Princess Margaret Hospital for Children is the only pediatric referral center for diabetes in Western Australia, with a case ascertainment of 99.9% (5). Consent for data to be entered into the database was obtained from all parents, and the study was approved by the institution’s ethics committee.
SH was strictly defined as a hypoglycemic event leading to loss of consciousness or seizure. For each patient, SH events were counted if they were reported during any clinical visit after the 1 January 2000. Subjects exited the study upon turning 18 years, permanently leaving the state, or if 12 months had elapsed since their last clinic visit. The number of patient-years contributed by each respective clinic visit was calculated as the number of days elapsed since the previous visit.
All children attending the center are managed by a multidisciplinary diabetes care team. The children and parents were advised to keep a logbook of blood glucose levels (BGL) and insulin doses and to record all atypical events such as episodes of hypoglycemia or illness. They were taught to obtain a BGL, if possible, to confirm hypoglycemia. They were seen in the clinic every 3 months, and data on all diabetes outcomes, including hypoglycemia events and treatment types, were recorded in the Western Australian Children's Diabetes Database.
HbA1c was determined at each clinic visit by agglutination inhibition immunoassay (nondiabetic reference <6.2%; Ames DCA 2000).
Statistical analysis
Annual SH incidence rates were calculated by obtaining the total number of SH events and dividing by the total length of follow-up represented by each clinic visit recorded in that year. Initial analyses involved revisiting previously identified risk factors by applying negative binomial regression methodology, as already described (3), to the new data. This was the basis of defining the final models for effect size estimates and age by treatment interaction analysis (6). Analyses were performed using R 2.11.1 and Stata 10 software (StataCorp 2007, Stata Statistical Software: Release 10; StataCorp LP, College Station, TX).
RESULTS
The study included 1,683 children (51% boys). In total, 7,378 patient-years of data were available for analysis, and 780 SH events occurred during the decade. Of all patients, 77.4% had no episodes, 11.8% had one event, 5.2% had two events, and 5.6% had three or more episodes. The incidence rate for SH (Fig. 1), adjusted for age and sex, decreased by an average of 14% per year compared with previous year for the first 7 years (z = 5.33, P < 0.001) and did not change after that time (P = 0.446).
Figure 1.
Rates of severe hypoglycemia by calendar year.
Univariate analysis quantified the annual rate of decline in the SH incidence rate over the decade as 12.1% (95% CI 8.7–15.4, P < 0.001) per year.
The mean HbA1c level for the decade was 8.3% (SD 1.5%), with only minor fluctuations. After adjusting for age and sex, the overall trend showed a decline in mean HbA1c levels of 0.07% per year (95% CI 0.009–0.15; P = 0.03). Multivariate analysis, adjusting for age and treatment type, showed an HbA1c <7% was not significantly associated with higher risk of SH (incidence rate ratio 1.2 [0.9–1.6]; P = 0.29) compared with the reference group of HbA1c 8–9%, which was the average level in our cohort across the decade.
There was no relationship between age and risk of SH and no sex difference. Children with duration of diabetes >1 year had a significantly higher risk (incidence rate ratio 3.7 [95% CI 2.4–5.7]; P < 0.001 for 1–5 years, increasing thereafter), than those with duration of diabetes <1 year. In adolescents (>13 years), pump therapy was associated with a reduced incidence of SH (64% lower, P = 0.048).
CONCLUSIONS
The primary objective of this study was to determine incidence rates of SH in a population-based sample of pediatric type 1 diabetes during a period when there were changes to treatment regimens. This is one of the largest population-based cohorts for which clinical data have been collected prospectively, and this report follows on from rates previously reported for 1992–2002 (3) and 1992–1995 (4). The reduction in the hypoglycemia rate may have resulted from changes in clinical practice (e.g., new insulin regimens, more intensive glucose monitoring, improved management guidelines), but this remains speculative.
Despite the decrease in SH rates, glycemic control has remained relatively static since the middle of the decade. This is similar to a recent report from the Hvidoere group demonstrating no recent improvement in glycemic control (7,8). An important finding was that compared with past studies, the relationship between glycemic control and the risk of SH is now weaker, with no significant increased risk of SH associated with improved glycemic control range from 8–9% to <7%. Another change has been the reduced risk of SH in children aged <6 years (3). This is an important observation in view of concerns regarding the potential effect of hypoglycemia on the developing brain (9).
In summary, in a population-based sample of pediatric type 1 diabetes, rates of SH have decreased during the past decade, and the previously close relationship between tight glycemic control and risk of severe events is now weaker. Although the data are encouraging and suggest that the risk of SH is in part being reduced with modern therapy, the risk remains significant and fear of hypoglycemia continues to be a barrier to optimal glycemic control.
Acknowledgments
S.O'C. received a fellowship grant from Novo Nordisk Ltd. in 2010. No other potential conflicts of interest relevant to this article were reported.
S.O'C. collected and researched data and wrote the manuscript. M.N.C. performed statistical analysis, generated the figures, and reviewed and edited the manuscript. M.K.B. provided methodologic, data, and statistical support based on previous publications. E.A.D. contributed to study design, data collection, analysis, and discussion. T.W.J. contributed to study design, data collection and analysis, and reviewed and edited the manuscript.
The authors thank Nirubasini Paramalingam, Telethon Institute for Child Health Research, The University of Western Australia, for assistance with data extraction.
References
- 1.Cryer PE. Hypoglycaemia: the limiting factor in the glycaemic management of type I and type II diabetes. Diabetologia 2002;45:937–948 [DOI] [PubMed] [Google Scholar]
- 2.Nathan DM, Zinman B, Cleary PA, et al. ; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Research Group. Modern-day clinical course of type 1 diabetes mellitus after 30 years’ duration: the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications and Pittsburgh Epidemiology of Diabetes Complications experience (1983–2005). Arch Intern Med 2009;169:1307–1316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bulsara MK, Holman CD, Davis EA, Jones TW. The impact of a decade of changing treatment on rates of severe hypoglycemia in a population-based cohort of children with type 1 diabetes. Diabetes Care 2004;27:2293–2298 [DOI] [PubMed] [Google Scholar]
- 4.Davis EA, Keating B, Byrne GC, Russell M, Jones TW. Hypoglycemia: incidence and clinical predictors in a large population-based sample of children and adolescents with IDDM. Diabetes Care 1997;20:22–25 [DOI] [PubMed] [Google Scholar]
- 5.Kelly HA, Byrne GC. Incidence of IDDM in Western Australia in children aged 0–14 yr from 1985 to 1989. Diabetes Care 1992;15:515–517 [DOI] [PubMed] [Google Scholar]
- 6.Altman DG, Bland JM. Interaction revisited: the difference between two estimates. BMJ 2003;326:219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Beaufort CE, Swift PG, Skinner CT, et al. ; Hvidoere Study Group on Childhood Diabetes 2005. Continuing stability of center differences in pediatric diabetes care: do advances in diabetes treatment improve outcome? The Hvidoere Study Group on Childhood Diabetes. Diabetes Care 2007;30:2245–2250 [DOI] [PubMed] [Google Scholar]
- 8.Mortensen HB, Hougaard P; The Hvidøre Study Group on Childhood Diabetes. Comparison of metabolic control in a cross-sectional study of 2,873 children and adolescents with IDDM from 18 countries. Diabetes Care 1997;20:714–720 [DOI] [PubMed] [Google Scholar]
- 9.Silverstein J, Klingensmith G, Copeland K, et al. ; American Diabetes Association. Care of children and adolescents with type 1 diabetes: a statement of the American Diabetes Association. Diabetes Care 2005;28:186–212 [DOI] [PubMed] [Google Scholar]