Abstract
OBJECTIVE
To investigate the effects of inflammation on perfusion regulation and brain volumes in type 2 diabetes.
RESEARCH DESIGN AND METHODS
A total of 147 subjects (71 diabetic and 76 nondiabetic, aged 65.2 ± 8 years) were studied using 3T anatomical and continuous arterial spin labeling magnetic resonance imaging. Analysis focused on the relationship between serum soluble vascular and intercellular adhesion molecules (sVCAM and sICAM, respectively, both markers of endothelial integrity), regional vasoreactivity, and tissue volumes.
RESULTS
Diabetic subjects had greater vasoconstriction reactivity, more atrophy, depression, and slower walking. Adhesion molecules were specifically related to gray matter atrophy (P = 0.04) and altered vasoreactivity (P = 0.03) in the diabetic and control groups. Regionally, sVCAM and sICAM were linked to exaggerated vasoconstriction, blunted vasodilatation, and increased cortical atrophy in the frontal, temporal, and parietal lobes (P = 0.04–0.003). sICAM correlated with worse functionality.
CONCLUSIONS
Diabetes is associated with cortical atrophy, vasoconstriction, and worse performance. Adhesion molecules, as markers of vascular health, have been indicated to contribute to altered vasoregulation and atrophy.
Diabetes is associated with microvascular disease, white matter hyperintensities (WMHs), cerebral atrophy (1,2), and functional decline (3,4). Hyperglycemia and proatherogenic factors (5) are main causes of endothelial dysfunction (6,7) and neuronal cell damage (8,9). We hypothesized that type 2 diabetes is associated with a chronic inflammation that alters cortical vasoregulation and contributes to regional atrophy. We investigated the relationship between adhesion molecules, as markers of vascular integrity, and regional vasoreactivity, WMHs, and brain tissue volumes in older diabetic and nondiabetic adults.
RESEARCH DESIGN AND METHODS
We prospectively recruited 147 subjects aged 65.2 ± 0.7 years (mean ± SE). Of these, 71 had type 2 diabetes (40 males, diabetes duration 10.5 ± 1.0 years, 15 treated with insulin, 31 hypertensive, 47 hypercholesterolemic) and 76 were age- and sex-matched nondiabetic control subjects (33 males, 19 hypertensive, 29 hypercholesterolemic). Exclusion criteria were type 1 diabetes; recent history of stroke or myocardial infarction; dementia; significant cardiac, renal, neurologic, and kidney disorders; claustrophobia; and magnetic resonance imaging (MRI)-incompatible implants.
Protocol
Participants signed informed consent and were admitted to the Clinical Research Center at the Beth Israel Deaconess Medical Center. Antihypertensive medications were withdrawn and fasting serum samples were collected.
Markers of vascular integrity and inflammation were measured by the quantitative sandwich enzyme immunoassay technique (R&D Systems, Minneapolis, MN) and a high sensitivity C-reactive protein (hs-CRP) assay (Immulite-1000; Diagnostic Product, Los Angeles, CA). Functionality was assessed by the Mini Mental State Exam, the Behavioural Assessment of Dysexecutive Syndrome (10), the Geriatric Depression Scale, and normal walking for 12 min.
MRI
Anatomical and perfusion images were acquired on a 3T GE HDx MRI scanner using three-dimensional magnetization prepared rapid gradient echo, fluid attenuated inversion recovery, and three-dimensional continuous arterial spin labeling sequences (11). Regional cerebral vasoreactivity to CO2 challenges (CO2VR) was measured as blood flow response to hypercapnia (CO2 rebreathing with 95% air and 5% CO2) and hyperventilation (12,13). CO2VR was calculated as the slope of the regression between perfusion and CO2 during normocapnia, hypercapnia, and hypocapnia. Flow augmentation during hypercapnia (i.e., vasodilation response, CO2VR-VD) and flow reduction during hyperventilation (i.e., vasoconstriction response, CO2VR-VC) were quantified. Magnetization prepared rapid gradient echo and fluid attenuated inversion recovery images were coregistered to a standard template and segmented to calculate regional brain tissue and WMH volumes normalized for intracranial volume (SPM7, University College London, U.K.) (14) (IDL, Research Systems, Boulder, CO; MATLAB, MathWorks, Natick, MA) (12,13). Perfusion and vasoreactivity maps were coregistered with anatomical images.
Statistical analysis
We used least square models to assess the relationships between adhesion molecules and regional vasoreactivity, gray matter (GM), white matter, cerebrospinal fluid (CSF), and WMHs. Variables with significant correlations (r2 >0.1, P < 0.05) were included in the models. Overall model fit (r2) and P values were calculated separately for each region to minimize repeated measures effects. Models were adjusted for age, sex, and group or glucose. Perfusion models were also adjusted for CO2 and hematocrit. MANCOVA with Sidak adjustment was used for comparisons across regions and between groups. Demographics and laboratory values were compared by ANOVA and Wilcoxon tests.
RESULTS
Characteristics of diabetic and control groups
Compared with control subjects, diabetic subjects had elevated fasting glycemia (124.4 ± 7.4 vs. 79.3 ± 1.6 mg/dL, P < 0.0001), HbA1c (7.1 ± 0.1 vs. 5.4 ± 0.1%, P < 0.0001), BMI (28.4 ± 0.6 vs. 25.3 ± 0.6 kg/m2, P < 0.0001), and tumor necrosis factor-α (1.8 ± 0.1 vs. 1.5 ± 0.1 pg/mL, P = 0.0004). Soluble intercellular adhesion molecule (sICAM; 246.2 ± 13.9 vs. 226.5 ± 5.8 ng/mL), soluble vascular adhesion molecule (sVCAM; 758.6 ± 28.4 vs. 729.9 ± 20.7 ng/mL), endothelin-1, interleukin-6, and CRP were not different. Diabetic subjects were more depressed (6.0 ± 0.8 vs. 2.8 ± 0.9, P = 0.003) and walked slower (1.06 ± 02 vs. 1.12 ± 0.02 m/s, P = 0.02) than control subjects.
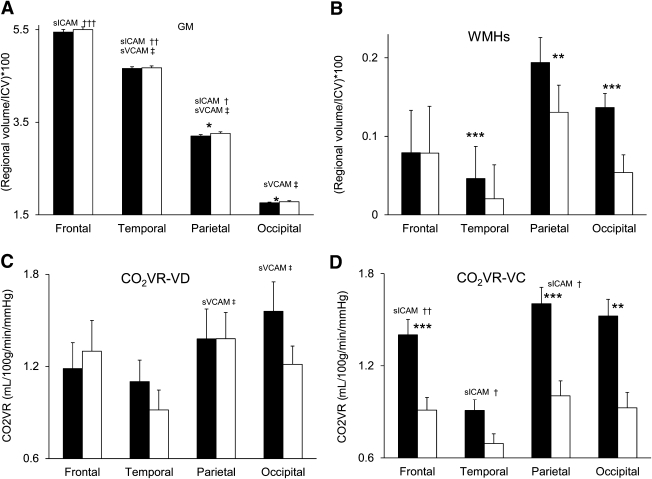
Diabetic subjects had lower GM volumes (parietal and occipital lobes and cerebellum, P < 0.02) (Fig. 1A) and greater WMH volume globally (P = 0.0004) and in the temporal, parietal, and occipital lobes (P < 0.01) (Fig. 1B). Baseline perfusion and CO2VR-VD were similar (Fig. 1C), yet the diabetic group had exaggerated CO2VR-VC in the frontal, parietal, and occipital regions (P < 0.01) (Fig. 1D).
Figure 1.
Relationships between adhesion molecules, regional brain volumes, and vasoreactivity: regional GM (A), WMHs (B), CO2VR-VD (C), and CO2VR-VC (D) in the diabetic (black bars) and control groups (white bars). The diabetic group as compared with the control group had lower regional GM volumes, greater WMH load, and exaggerated vasoconstriction reactivity. sVCAM was associated with lower GM volume in the temporal and parietal lobes (A) and decreased vasodilatation reactivity (C). sICAM was associated with lower GM volume in the frontal, temporal, and parietal lobes (A); blunted vasodilatation in the parietal and occipital lobes (C); and exaggerated vasoconstriction in the frontal, temporal, parietal, and occipital lobes (D) in the diabetic and control groups. WMHs were not related to adhesion molecules. sICAM: †P < 0.05, ††P < 0.01, †††P = 0.003. sVCAM: ‡P < 0.05. *P < 0.05, **P < 0.01, ***P < 0.0001 between group comparisons mean ± SE.
Adhesion molecules, brain tissue volumes, and vasoreactivity.
Adhesion molecules were associated with decreased GM volume, exaggerated CO2VR-VC, and blunted CO2VR-VD in both groups (Fig. 1A–D). sVCAM was linked to lower GM volumes globally (r2 = 0.24, P = 0.04) and regionally (temporal and parietal, r2 = 0.13–0.23, P = 0.02–0.006), greater CSF volumes globally (r2 = 0.12, P = 0.05) and regionally (temporal and occipital, r2 = 0.07–0.12, P = 0.03–0.007), and blunted CO2VR-VD (parietal and occipital, r2 = 0.12–16, P = 0.03). Higher sICAM and glucose levels were related to GM atrophy (frontal, temporal, and parietal, r2 = 0.14–29, P = 0.02–0.003) and blunted global CO2VR-VD (r2 = 0.09, P = 0.03) and exaggerated CO2VR-VC (frontal, temporal, and parietal, r2 = 0.12–16, P = 0.03–0.01). sICAM was linked to slower walking (r2 = 0.12, P = 0.01) and worse behavioral measures (r2 = 0.16, P = 0.03).
Within the diabetic group, the relationships between adhesion molecules and brain measures were stronger; sVCAM was associated with GM atrophy (temporal r2 = 0.13, P = 0.05; parietal r2 = 0.4, P = 0.02), and sICAM was associated with altered vasodilatation (global r2 = 0.32, P = 0.02; parietal r2 = 0.32, P = 0.02). sICAM (r2 = 0.24, P = 0.0003) and sVCAM (r2 = 0.24, P = 0.0003) correlated with glycemia but not with HbA1c, medications, diabetes duration, or hypertension. In control subjects, sVCAM correlated with greater CSF volume (r2 = 0.24, P = 0.03). sICAM was related to smaller CSF volume (r2 = 0.26, P = 0.0007) and greater GM volume (frontal, temporal, and parietal, r2 = 0.24–43, P = 0.01–0.003).
Tumor necrosis factor-α, interleukin-6, endothelin-1, and CRP were not related to brain volumes or vasoreactivity measures. WMHs were not associated with adhesion molecules, inflammation markers, or regional vasoreactivity.
CONCLUSIONS
Markers of endothelial integrity (sVCAM and sICAM) were specifically associated with altered cortical vasoreactivity and GM atrophy in multiple brain regions in both diabetic and nondiabetic participants. These relationships were independent of WMHs and were not observed for other inflammatory markers. The diabetic group exhibited exaggerated vasoconstriction, more atrophy, lower functionality, and more depression. sVCAM was associated with atrophy affecting temporal and parietal cortices. sICAM and glucose levels were related to exaggerated vasoconstriction and regional cortical atrophy. Adhesion molecules were linked to slower walking and executive and behavioral dysfunction, which are hallmarks of behavioral decline in older adults.
The relationship between adhesion molecules and vasoreactivity to CO2 challenges suggests a nitric oxide–dependent endothelial dysfunction (15). Observations of microglial activation and sVCAM expression in the cortex and subcortical areas support this notion and indicate that insulin resistance and obesity may facilitate an inflammatory process in the brain (9). A combination of altered vasoregulation and hyperglycemia may enhance neurotoxicity of chronic hyperglycemia in the aging diabetic brain.
Acknowledgments
V.N. has received grants from the National Institutes of Health (NIH)–National Institute on Aging (NIA) (1R01-AG-0287601-A2), the NIH–National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (5R21-DK-084463-02), and the American Diabetes Association (ADA) (1-03-CR-23 and 1-06-CR-25) related to this study and is also funded by NIH grants for projects unrelated to this study (NIH-NIA 4R37-AG-253705, 1R43-AT-006088-01, R21-AT-005501, and 4P30-AG-02871702). P.Z. has received grants from the NIH–NIA (1R01-AG-0287601-A2), the NIH–NIDDK, and the ADA (1-06-CR-25). B.M. has received grants from the NIH–NIA (1R01-AG-0287601-A2) and NIH (5R21-DK-084463-02) related to this study as well as NIH grants for projects unrelated to this work (R43-AT-06088-01 and R37-AG-025037). E.S. has received grants from the NIH–NIA (1R01-AG-0287601-A2) and the NIH–NIDDK (5R21-DK-084463-02). D.A. has received grants from the NIH–NIA (1R01-AG-0287601-A2) and from the ADA (1-06-CR-25). A.A. has received research support from the Ohio Department of Development. P.K.R. has received grants from the NIH–NIA (1R01-AG-0287601-A2) and the NIH–NIDDK (5R21-DK-084463-02). M.M. has received grants from the NIH (1R01-AG-0287601-A2) and the NIH–NIA (1R01-AG-0287601-A2). P.N. has received research support from the NIH (1R01-AG-0287601-A2 and 1R43-NS-064640-01A2), the NIH–NIA (1R01-AG-0287601-A2), the NIH–NIDDK (5R21-DK-084463-02), Teva Pharmaceutical Industries, Chelsea Therapeutics, the Langer Family Charitable Foundation, Chirag Foundation Investment Trust, and Baker MSA (Multiple System Atrophy) Research Fund. This study was supported as well by grants from the Harvard Clinical and Translational Science Center (UL1-RR-025758) and the National Center for Research Resources (M01-RR-01032). The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or NIH. No other potential conflicts of interest relevant to this article were reported.
V.N. designed the study and protocol and oversaw all aspects of the study conduct, experiments, and manuscript preparation. P.Z. contributed to study conduct, performed MRI image analysis and statistical analysis, and contributed to manuscript preparation. B.M. contributed to study conduct and to manuscript preparation. E.S. contributed to statistical analysis and manuscript preparation. D.A. oversaw MRI protocols and contributed to MRI analysis. A.A. contributed to MRI analysis. P.K.R. contributed to study design, oversaw statistical analysis, and contributed to manuscript preparation. M.M. contributed to study design and clinical aspects of the study. P.N. contributed to study design, oversaw clinical aspects of the study, and contributed to manuscript preparation.
Parts of this study were presented in abstract form at the 16th Annual Meeting of the Organization for Human Brain Mapping, Barcelona, Spain, 6–10 June 2010.
The authors acknowledge contributions of Clinical Research Center nursing and MRI staff.
References
- 1.van Elderen SG, de Roos A, de Craen AJ, et al. Progression of brain atrophy and cognitive decline in diabetes mellitus: a 3-year follow-up. Neurology 2010;75:997–1002 [DOI] [PubMed] [Google Scholar]
- 2.de Bresser J, Tiehuis AM, van den Berg E, et al. ; Utrecht Diabetic Encephalopathy Study Group. Progression of cerebral atrophy and white matter hyperintensities in patients with type 2 diabetes. Diabetes Care 2010;33:1309–1314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu WL, Qiu CX, Wahlin A, Winblad B, Fratiglioni L. Diabetes mellitus and risk of dementia in the Kungsholmen project: a 6-year follow-up study. Neurology 2004;63:1181–1186 [DOI] [PubMed] [Google Scholar]
- 4.Reijmer YD, van den Berg E, de Bresser J, et al. ; Utrecht Diabetic Encephalopathy Study Group. Accelerated cognitive decline in patients with type 2 diabetes: MRI correlates and risk factors. Diabetes Metab Res Rev 2011;27:195–202 [DOI] [PubMed] [Google Scholar]
- 5.Kim JA, Montagnani M, Koh KK, Quon MJ. Reciprocal relationships between insulin resistance and endothelial dysfunction: molecular and pathophysiological mechanisms. Circulation 2006;113:1888–1904 [DOI] [PubMed] [Google Scholar]
- 6.Mäkimattila S, Yki-Järvinen H. Endothelial dysfunction in human diabetes. Curr Diab Rep 2002;2:26–36 [DOI] [PubMed] [Google Scholar]
- 7.Starr JM, Wardlaw J, Ferguson K, MacLullich A, Deary IJ, Marshall I. Increased blood-brain barrier permeability in type II diabetes demonstrated by gadolinium magnetic resonance imaging. J Neurol Neurosurg Psychiatry 2003;74:70–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes 2005;54:1615–1625 [DOI] [PubMed] [Google Scholar]
- 9.Drake C, Boutin H, Jones MS, et al. Brain inflammation is induced by co-morbidities and risk factors for stroke. Brain Behav Immun 2011;25:1113–1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burgess P, Alderman N, Wilson B, Evans J, Emslie H. The Dysexecutive Questionnaire (DEX). In Behavioral Assessment of the Dysexecutive System. Wilson B, Alderman N, Burgess P, Emslie H, Evans J, Eds. Bury St. Edmunds, U.K., Thames Valley Test Company, 1996 [Google Scholar]
- 11.Alsop DC, Detre JA. Reduced transit-time sensitivity in noninvasive magnetic resonance imaging of human cerebral blood flow. J Cereb Blood Flow Metab 1996;16:1236–1249 [DOI] [PubMed] [Google Scholar]
- 12.Last D, Alsop DC, Abduljalil AM, et al. Global and regional effects of type 2 diabetes on brain tissue volumes and cerebral vasoreactivity. Diabetes Care 2007;30:1193–1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao P, Alsop D, Abduljail A, et al. Altered vasoreactivity and peri-infarct hyperintensities affect multiple territories in stroke. Neurology 2009;72:643–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.D'Agostino E, Maes F, Vandermeulen D, Suetens P. Atlas-to-image non-rigid registration by minimization of conditional entropy. Inf Process Med Imaging 2007;20:320–332 [DOI] [PubMed] [Google Scholar]
- 15.Kevil CG, Orr AW, Langston W, et al. Intercellular adhesion molecule-1 (ICAM-1) regulates endothelial cell motility through a nitric oxide-dependent pathway. J Biol Chem 2004;279:19230–19238 [DOI] [PubMed] [Google Scholar]