Abstract
OBJECTIVE
To examine the effects of crossing over from optimized multiple daily injection (MDI) therapy to sensor-augmented pump (SAP) therapy for 6 months, and the effects of 18 months’ sustained use of SAP.
RESEARCH DESIGN AND METHODS
The 6-month, single-crossover continuation phase of Sensor-Augmented Pump Therapy for A1C Reduction (STAR 3) provided SAP therapy to 420 subjects who completed the 1-year randomized study. The primary outcome was change in A1C in the crossover group.
RESULTS
A1C values were initially lower in the continuing-SAP group than in the crossover group (7.4 vs. 8.0%, P < 0.001). A1C values remained reduced in the SAP group. After 3 months on the SAP system, A1C decreased to 7.6% in the crossover group (P < 0.001); this was a significant and sustained decrease among both adults and children (P < 0.05).
CONCLUSIONS
Switching from optimized MDI to SAP therapy allowed for rapid and safe A1C reductions. Glycemic benefits of SAP therapy persist for at least 18 months.
The sensor-augmented pump (SAP) system combines insulin pump and continuous glucose monitoring (CGM) technologies, and its efficacy was studied in the 1-year randomized phase of the Sensor-Augmented Pump Therapy for A1C Reduction (STAR 3) study (1). Compared with subjects on multiple daily injections (MDI), those on SAP experienced greater reductions in A1C levels by 3 months, and this advantage persisted for the entire study (2). An optional continuation phase allowed MDI subjects to switch to SAP therapy for 6 months (the crossover group) and allowed SAP subjects to remain on uninterrupted SAP therapy (the SAP group) for a total of 18 months. We examined the effectiveness of SAP therapy in subjects transitioning from previously optimized MDI therapy and the durability of glycemic benefits in the SAP group over 18 months.
RESEARCH DESIGN AND METHODS
STAR 3 eligibility criteria included type 1 diabetes, age 7–70 years, use of MDI with a long-acting insulin analog, A1C between 7.4 and 9.5%, and less than two severe hypoglycemic events (3) in the previous year. Subjects were randomized to receive SAP (Paradigm REAL-Time System, Medtronic MiniMed, Inc., Northridge, CA) with insulin aspart or to MDI using insulins aspart and glargine. Therapy was optimized individually, and A1C was obtained at quarterly visits. Subjects beginning SAP therapy received training for pumps, CGM sensors, and therapy management software. All subjects in the continuation phase were supplied with sensors and encouraged to wear them regularly. The primary efficacy measure was the change in A1C from 12 to 18 months in both treatment groups; the primary safety measure was the difference in the rates of severe hypoglycemia. The study Consolidated Standards of Reporting Trials (CONSORT) diagram and statistical methods are given in the Supplementary Material.
RESULTS
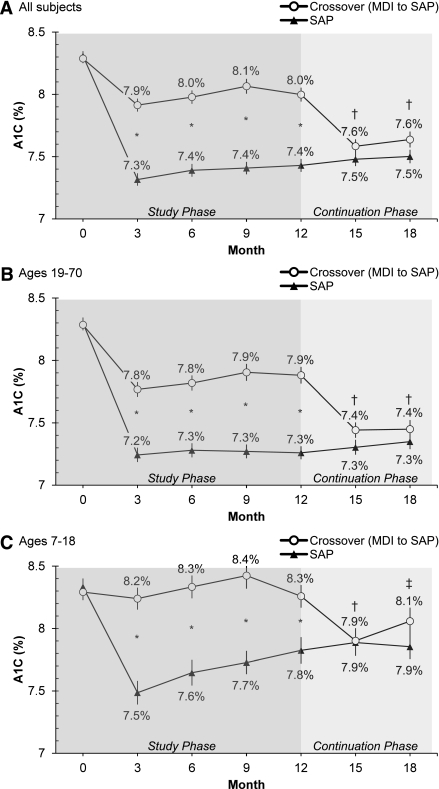
A total of 420 of 443 subjects completed the study phase and participated in the continuation phase; 204 of 216 SAP subjects (94%) and 190 of 204 crossover subjects (93%) completed both phases of the study. In the SAP group, the improvement in A1C levels seen during the study phase was maintained during the continuation phase (Fig. 1A). Overall mean (± SEM) A1C levels for these subjects at 15 and 18 months were not significantly different than the 12-month value of 7.4 ± 0.1% (P > 0.05). In contrast, patients in the crossover group realized a significant decrease in A1C from 12 months (8.0 ± 0.1%) to 15 or 18 months (7.6 ± 0.1%, P < 0.001; Fig. 1A). The significant decrease in A1C values in the crossover group was seen in adult (n = 141; Fig. 1B) and pediatric (n = 63; Fig. 1C) subjects.
Figure 1.
Mean (± SEM) A1C values of 420 subjects who entered the STAR 3 continuation phase. All subjects in the continuation phase used SAP therapy. A: All subjects (MDI, n = 204; SAP, n = 216). B: Adult subjects (MDI, n = 141; SAP, n = 151). C: Pediatric subjects (MDI, n = 63; SAP, n = 65). *P < 0.001 for between-groups comparison; †P < 0.001 and ‡P < 0.05 for within-group comparison using the crossover group’s 12-month A1C value as the comparator.
Subjects in the SAP group were able to maintain their A1C levels if CGM sensors were used >40% of the time; subjects who used sensors less frequently experienced slight deteriorations in glycemic control. In the crossover group, maximum improvements in A1C were observed with sensor wear times >60% (Supplementary Table 1). Mean sensor wear times in the continuation phase were greater among adults (61 ± 24% of the time) than among pediatric subjects (45 ± 24% of the time; P < 0.001). Rates of severe hypoglycemia were not significantly different among the SAP and crossover groups in the continuation phase (2.8 vs. 1.0%, respectively; P > 0.05; Supplementary Table 2).
CONCLUSIONS
The STAR 3 continuation phase results support and extend the findings of the study phase. In the study phase, A1C levels were lowered by ∼0.5 to 0.6% more with SAP treatment than with MDI. The current data show that a similar degree of improvement in A1C levels was achieved when subjects switched from MDI to SAP after a 12-month period of optimized MDI therapy. Maximal lowering of A1C levels was associated with CGM sensor wear times of >60% in the crossover group; this was similar to wear times associated with maximal A1C lowering in the SAP group during the randomized study phase.
The improved A1C levels achieved by the SAP group during the first 12 months of the study were maintained at 15 and 18 months. Sensor wear times of >40% were required during the continuation phase for experienced SAP users to maintain the A1C benefits achieved during the study phase.
Age-dependent patterns of response in crossover adult and pediatric subjects during the continuation phase were similar to those observed during the study phase. Pediatric patients used their sensors less frequently than adults, and lower wear times were associated with a smaller reduction in A1C levels. A separate analysis of STAR 3 data comparing children and adolescents showed additional age-dependent differences in outcomes and behaviors (4).
Participants were fully aware of the devices they were using and may have been motivated to use the SAP system appropriately. Because the study only enrolled subjects with type 1 diabetes with initial A1C values of 7.4–9.5% and only included 2 treatment arms, its generalizability may be limited. The benefits of pump therapy with or without real-time CGM have been recently compared (5). Work continues toward the integration of SAP platforms and controller algorithms that can safely reduce hypoglycemic exposure (6) and may someday provide fully closed-loop insulin delivery (7).
Supplementary Material
Acknowledgments
This study was supported by Medtronic; Novo Nordisk, which supplied all insulin aspart used in the study; and LifeScan, Bayer Healthcare, and Becton Dickinson, which supplied blood glucose meters used in the study.
R.M.B. reports that the International Diabetes Center has received consulting fees from Abbott Diabetes Care, Amylin, Bayer, Calibra, Eli Lilly, Intarcia, MannKind, Medtronic MiniMed, Inc., Novo Nordisk, Pfizer, ResMed, Roche, sanofi-aventis, and Takeda for his services; grant support from Abbott Diabetes Care, Biodel, Eli Lilly, Hygieia, Intuity, LifeScan, Medtronic MiniMed, Inc., Novo Nordisk, Pfizer, ResMed, Roche, sanofi-aventis, Takeda, UnitedHealth Group, and Valeritas; honoraria from Abbott Diabetes Care, Amylin, Bayer, Calibra, Eli Lilly, Intarcia, MannKind, Medtronic MiniMed, Inc., Novo Nordisk, Pfizer, ResMed, Roche, sanofi-aventis, and Takeda; royalties on his behalf for the Betty Crocker Diabetes Cookbook; travel and accommodation reimbursements from Abbott Diabetes Care, Amylin, Bayer, Biodel, Calibra, Eli Lilly, Hygieia, Intarcia, Intuity, LifeScan, MannKind, Medtronic MiniMed, Inc., Novo Nordisk, Pfizer, ResMed, Roche, sanofi-aventis, Takeda, UnitedHealth Group, and Valeritas; and having inherited Merck stock. W.V.T. reports receiving consulting fees from Medtronic MiniMed, Inc. as a member of the Diabetes Advisory Board. A.A. reports that Oregon Health and Science University has received consulting fees from Biodel, Novo Nordisk, and GlaxoSmithKline for his services; grant support from Amylin, Medtronic MiniMed, Inc., and Novo Nordisk; and honoraria from Amylin, Lilly, Animas, NDEI, and Medical Education Resources. J.B.B. reports that for his services the University of North Carolina School of Medicine has received consulting fees from Novo Nordisk, Amylin, Becton Dickinson, Eli Lilly, Hoffmann–La Roche, GlycoMark, Wyeth, Daiichi Sankyo, Bristol-Myers Squibb, Bayhill Therapeutics, LipoScience, MannKind, Valeritas, MicroIslet, GlaxoSmithKline, Abbott, Exsulin, and GI Dynamics; funding from Novo Nordisk for expert testimony; grant support from Amylin, Novo Nordisk, Medtronic MiniMed, Inc., Lilly, Novartis, Tolerex, Osiris, Halozyme, Pfizer, Hoffmann–La Roche, Interkrin, Merck, sanofi-aventis, Dexcom, Johnson & Johnson, Bristol-Myers Squibb, Fujisawa, and Novartis; and travel and accommodation reimbursement from Novo Nordisk, Amylin, Medtronic MiniMed, Inc., Novartis, Tolerex, Becton Dickinson, Eli Lilly, Hoffmann–La Roche, Fujisawa, GlycoMark, Wyeth, Daiichi Sankyo, Bristol-Myers Squibb, Bayhill Therapeutics, Osiris, Interkrin, Merck, sanofi-aventis, Dexcom, Johnson & Johnson, Halozyme, Pfizer, LipoScience, MannKind, Valeritas, MicroIslet, GlaxoSmithKline, Abbott, Exsulin, and GI Dynamics; he also reports having an equity interest in Insulet. G.D. reports membership in the Speakers’ Bureaus of Merck, sanofi-aventis, and BMS-Astra Zeneca; research funding from sanofi-aventis, GlaxoSmithKline, Merck, Eli Lilly, Amylin, Novo Nordisk, Pfizer, Roche, Novartis, Halozyme, Medtronic MiniMed, Inc., Boehringer Ingelheim, and MannKind; and service as a consultant for sanofi-aventis and Merck. S.N.D. reports receiving consulting fees from Amylin and sanofi-aventis. C.J. reports receiving consulting fees from Eli Lilly, sanofi-aventis, and AstraZeneca, travel reimbursement from AstraZeneca, speaking honoraria from Medtronic MiniMed, Inc., and grant support paid to the Memorial University of Newfoundland from Eli Lilly, sanofi-aventis, AstraZeneca, and Boehringer Ingelheim. B.A.P. reports receiving research support from Medtronic MiniMed, Inc. J.B.W. reports being an employee of and having an equity interest in Medtronic MiniMed, Inc. and receiving compensation for manuscript preparation. S.M.W. reports that the Children's Hospital of Philadelphia has received grant support from sanofi-aventis for his services. M.A.W. reports receiving consulting fees from Medtronic MiniMed, Inc. No other potential conflicts of interest relevant to this article were reported.
R.M.B. was responsible for patient recruitment and care and study conduct, interpreted data, drafted the manuscript, revised the manuscript for critical intellectual content, made final decisions on manuscript content, and gave final approval of the manuscript. W.V.T. was responsible for patient recruitment and care and study conduct, interpreted data, drafted the manuscript, revised the manuscript for critical intellectual content, and gave final approval of the manuscript. A.A., J.B.B., G.D., S.N.D., C.J., and B.A.P. were responsible for patient recruitment and care and study conduct, revised the manuscript for critical intellectual content, and gave final approval of the manuscript. J.B.W. interpreted data, drafted the manuscript, and gave final approval of the manuscript. S.M.W. and M.A.W. were responsible for patient recruitment and care and study conduct, revised the manuscript for critical intellectual content, and gave final approval of the manuscript.
Parts of this study were presented in poster form at the 71st Scientific Sessions of the American Diabetes Association, San Diego, California, 24–28 June 2011.
The authors thank all of the patients who participated in this study and the staff members of the participating centers.
Footnotes
Clinical trial reg. no. NCT00417989, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc11-1248/-/DC1.
A complete list of the members of the STAR 3 Study Group can be found in the Supplementary Data online.
References
- 1.Davis SN, Horton ES, Battelino T, Rubin RR, Schulman KA, Tamborlane WV. STAR 3 randomized controlled trial to compare sensor-augmented insulin pump therapy with multiple daily injections in the treatment of type 1 diabetes: research design, methods, and baseline characteristics of enrolled subjects. Diabetes Technol Ther 2010;12:249–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bergenstal RM, Tamborlane WV, Ahmann A, et al. ; STAR 3 Study Group. Effectiveness of sensor-augmented insulin-pump therapy in type 1 diabetes. N Engl J Med 2010;363:311–320 [DOI] [PubMed] [Google Scholar]
- 3.American Diabetes Association Workgroup on Hypoglycemia. Defining and reporting hypoglycemia in diabetes: a report from the American Diabetes Association Workgroup on Hypoglycemia. Diabetes Care 2005;28:1245–1249 [DOI] [PubMed] [Google Scholar]
- 4.Slover RH, Welsh JB, Criego A, et al. Effectiveness of sensor-augmented pump therapy in children and adolescents with type 1 diabetes in the STAR 3 study. Pediatr Diabetes. 3 July 2011 [Epub ahead of print] [DOI] [PubMed]
- 5.Raccah D, Sulmont V, Reznik Y, et al. Incremental value of continuous glucose monitoring when starting pump therapy in patients with poorly controlled type 1 diabetes: the RealTrend study. Diabetes Care 2009;32:2245–2250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agrawal P, Welsh JB, Kannard B, Askari S, Yang Q, Kaufman FR. Usage and effectiveness of the low glucose suspend feature of the Medtronic Paradigm Veo insulin pump. J Diabetes Sci Tech 2011;5:1137–1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kowalski AJ. Can we really close the loop and how soon? Accelerating the availability of an artificial pancreas: a roadmap to better diabetes outcomes. Diabetes Technol Ther 2009;11(Suppl. 1):S113–S119 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.