Abstract
Autoimmune responses against posttranslationally modified antigens are a hallmark of several autoimmune diseases. For example, antibodies against citrullinated protein antigens (ACPA) have shown their relevance for the prognosis and diagnosis of rheumatoid arthritis (RA), and have been implicated in disease pathogenesis. It is conceivable that other autoantibody systems, recognizing other posttranslationally modified proteins, are also present in RA. Here, we describe the presence of an autoantibody system that discriminates between citrulline- and homocitrulline-containing antigens in the sera of RA-patients. IgG antibodies recognizing carbamylated (homocitrulline-containing) antigens were present in sera of over 45% of RA-patients. Likewise, anticarbamylated protein (anti-CarP) IgA antibodies were observed in 43% of RA-sera. ACPA and anti-CarP antibodies are distinct autoantibodies because, in selected double-positive patients, the anti-CarP antibody binding to carbamylated antigens could be inhibited by carbamylated antigens, but not by control or citrullinated antigens. Similarly, ACPA-binding to citrullinated antigens could only be inhibited by citrullinated antigens. In line with this observation, 16% of ACPA-negative RA-patients, as measured by a standard ACPA assay, harbored IgG anti-CarP antibodies, whereas 30% of these patients tested positive for IgA anti-CarP antibodies. The presence of anti-CarP antibodies was predictive for a more severe disease course in ACPA-negative patients as measured by radiological progression. Taken together, these data show the presence of a unique autoantibody system recognizing carbamylated, but not citrullinated, protein antigens. These antibodies are predictive for a more severe clinical course in ACPA-negative RA-patients, indicating that anti-CarP antibodies are a unique and relevant serological marker for ACPA-negative RA.
The identification of anticitrullinated protein antibodies (ACPA) has contributed significantly to the understanding of rheumatoid arthritis (RA) (1). Significant differences between ACPA-positive and -negative disease have been reported with respect to the contribution of genetic and environmental risk factors, as well as disease progression and remission (2–5). Over the past few years important insight has been gained into the occurrence and etiophathology of ACPA-positive RA. However, less information is available on ACPA-negative RA. This lack of information is partly because of the absence of robust biomarkers characterizing this manifestation of RA.
The posttranslational modification of arginine into citrulline by peptidyl arginine deiminase (PAD) enzymes is essential for the generation of citrullinated antigens that are recognized by ACPA (1). Under physiological circumstances, citrullination is involved in tissues like hair and skin because of its role in terminal epithelial differentiation (6). In the nucleus citrullination plays a role in epigenetic regulation (7) and condensation of chromatin, and has been reported to be involved in translation (6) and the host defense against pathogens (8). Under pathological conditions where cell death may overwhelm the phagocytic capacity of phagocytes, necrotic cells may release PAD into the extracellular space, where higher calcium concentrations now also allow the citrullination of other proteins located outside the cell (6). These proteins may be targeted by ACPA, possibly leading to inflammation and arthritis.
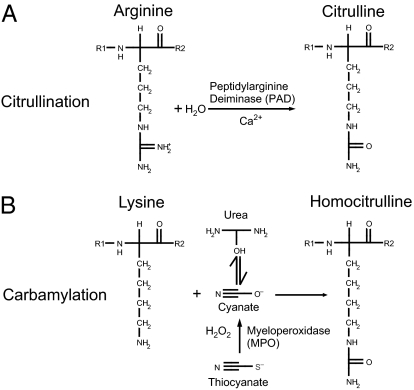
Citrulline highly resembles homocitrulline (Fig. 1), another posttranslationally modified amino acid (9). Homocitrulline is one methylene group longer, but similar in structure (9). Homocitrulline is generated from a lysine residue following a reaction of cyanate, which is present in the body in equilibrium with urea. Under physiological conditions the urea concentration may be too low to allow extensive carbamylation but the conversion process leading to the formation of homocitrulline from lysine in proteins does occur in vivo. In conditions of renal failure, the urea concentration increases and carbamylation of many proteins can be readily detected. However, most carbamylation is believed to take place during inflammation when myeloperoxidase is released from neutrophils (10). This enzyme converts thiocyanate to cyanate, now allowing more carbamylation to occur (11). It has been shown recently that homocitrulline-containing proteins are present in the RA joint and that they may affect T-cell triggering and possibly autoantibody formation in rodents (9, 12). Although highly similar, carbamylation differs from citrullination as, next to their structural difference, lysine is modified instead of arginine. Therefore, homocitrulline will, by definition, be located at other positions in proteins than citrulline. Because of the similarity between citrulline and homocitrulline, we set out to analyze whether autoantibodies against carbamylated proteins are present in RA and whether these antibodies differ from ACPA with respect to antigen binding and clinical associations.
Fig. 1.
Illustration of citrullination and carbamylation. Citrullination (A) and carbamylation (B) occur on different amino acids via different mechanisms, but yield similar end-products.
Results
Anticarbamylated Protein Antibodies and ACPA Are Different Antibody Families.
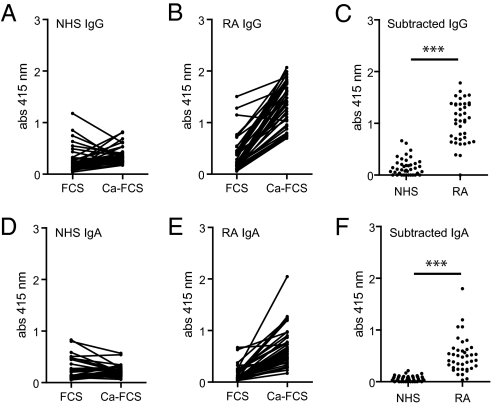
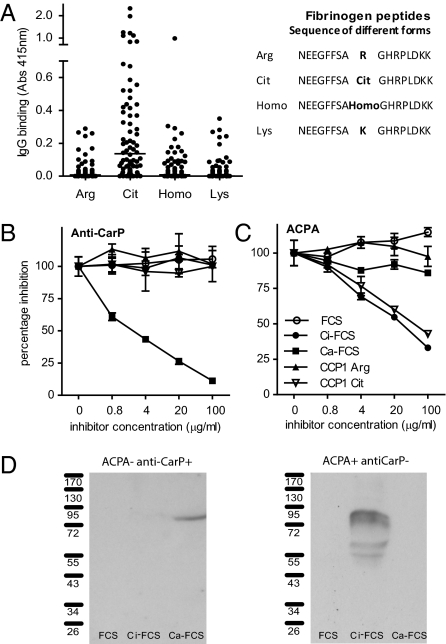
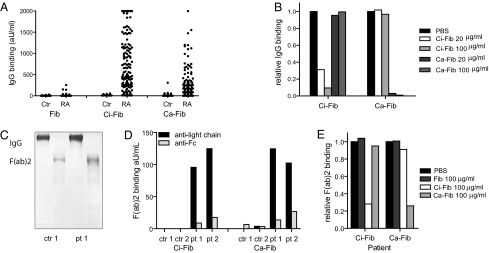
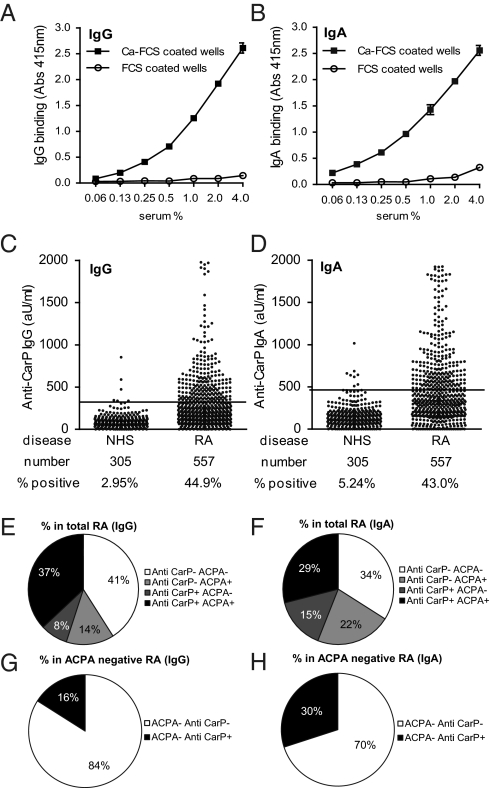
To detect antibodies against carbamylated proteins (anti-CarP antibodies), we developed an ELISA using carbamylated FCS (Ca-FCS) and nonmodified FCS as antigens. Analyzing sera of 40 RA patients and 40 controls, we observed that sera of RA-patients reacted with Ca-FCS compared with sera obtained from healthy subjects with both IgG (Fig. 2 A and B) and IgA (Fig. 2 D and E) reactivity. The enhanced reactivity of RA sera to Ca-FCS is further emphasized after subtraction of the reactivity against unmodified FCS (Fig. 2 C and F). Because citrulline and homocitrulline are two rather similar amino acids (Fig. 1), we next wished to determine whether ACPA also recognizes homocitrulline when located at the same position as citrulline in a peptide. For this purpose we performed ELISAs using a citrullinated Fibrinogen (Fib) peptide known to be recognized by ACPA (13). Within this peptide backbone, a citrulline, an arginine, a homocitrulline, or a lysine residue was introduced for further analysis. Analyzing a set of 76 RA sera, we observed that ACPA only recognized the citrullinated peptide, but not the arginine-containing or the homocitrulline-containing peptide (Fig. 3A). These data indicate that ACPA can discriminate between citrulline and homocitrulline presence within the same peptide backbone. Next, we wished to analyze whether there is cross-reactivity between anti-CarP antibodies and ACPA for binding to posttranslationally modified proteins. Therefore, we performed inhibition studies using sera that were reactive to both citrullinated and carbamylated antigens. We analyzed the binding of anti-CarP antibodies to Ca-FCS–coated plates following preincubation with Ca-FCS, citrullinated FCS (Ci-FCS), native FCS, or by citrullinated peptides used to detect ACPA (cyclic citrullinated peptide-1, CCP1). Following preincubation, we observed that anti-CarP antibody binding to Ca-FCS can only be inhibited by Ca-FCS but not by Ci-FCS, native FCS, or by peptides used to detect ACPA (Fig. 3B). We also performed the reverse inhibition experiment where we analyzed the binding of ACPA to plates coated with Ci-FCS following the same preincubation procedure. We observed that ACPA binding to Ci-FCS could only be inhibited by Ci-FCS and the citrullinated peptide but not by Ca-FCS, nonmodified FCS, or the arginine form of the peptide (Fig. 3C). Taken together, these data indicate that anti-CarP antibodies and ACPA are not, or only limited, cross-reactive and specifically directed against homocitrulline or citrulline-containing antigens, respectively. Because all observations described above were made using ELISA, we also wished to confirm our findings using a different technique. For this reason we performed a Western blot-analysis using FCS, Ca-FCS, and Ci-FCS on reduced gels, followed by Western blotting. The different blots were incubated with sera of individuals that were either anti-CarP–positive and ACPA-negative or anti-CarP–negative and ACPA-positive. We observed a positive staining of the anti-CarP–positive sample only on Ca-FCS but not on Ci-FCS or FCS (Fig. 3D). In contrast, the anti-CarP–negative, ACPA-positive sample reacted to Ci-FCS, but not to Ca-FCS and FCS (Fig. 3D). To confirm the presence of anti-CarP antibodies we repeated these experiments using a more defined protein, human Fib, as a target antigen. Fib was citrullinated by PAD (Ci-Fib) or carbamylated by cyanate (Ca-Fib). The nonmodified form (Fib), Ci-Fib, and Ca-Fib were used as antigens in ELISA. Similar to the observations for FCS, we observed significant binding of antibodies to the Ci-Fib and the Ca-Fib but not to the Fib-coated wells (Fig. 4A). This finding was largely restricted to the RA sera and not the controls (P ≤ 0.0001). To analyze cross-reactivity we also performed inhibition studies, as described above. ELISA analyses confirmed that ACPA and anti-CarP antibodies are largely noncross-reactive (Fig 4B). To ensure that reactivity toward carbamylated proteins is mediated by the antigen-binding part of the antibodies, we generated F(ab′)2. As expected, F(ab′)2, generated from anti-CarP IgG-positive samples but not from negative samples display anti-CarP reactivity (Fig. 4 C and D). As observed using intact antibodies, F(ab′)2-reactivity toward Ca-Fib could also be inhibited specifically by Ca-Fib, whereas F(ab′)2-reactivity toward Ci-Fib could only be inhibited specifically by Ci-Fib (Fig. 4E).
Fig. 2.
Antibodies against carbamylated proteins are present in sera of RA patients. The reactivity of IgG (A and B) and IgA (D and E) from sera of healthy controls (NHS) or RA patients (RA) to wells coated with nonmodified FCS (FCS) or carbamylated FCS (Ca-FCS) is depicted. Data expressed as absorbance at 415 nm. (C and F) Absorbance units of FCS were subtracted from the absorbance units of Ca-FCS, representing the specific anticarbamylated protein response. ***P < 0.0001 for a t test comparing NHS and RA.
Fig. 3.
Anti-CarP antibodies and ACPA are two separate autoantibody systems. (A) IgG reactivity of 76 sera from RA patients, toward several forms of a Fib peptide is depicted. (B and C) Antibody binding to Ca-FCS or Ci-FCS was inhibited using preincubations with fluid-phase inhibitors. (D) FCS, Ca-FCS, and Ci-FCS were separated by SDS-PAGE gels and blotted. The presence of antibodies reactive to proteins on the blots was analyzed by incubating these blots with either anti-CarP–positive ACPA-negative and anti-CarP–negative ACPA-positive sera.
Fig. 4.
Anti-CarP antibodies bind to Ca-Fib via variable domains. (A) IgG reactivity against Fib, Ci-Fib, and Ca-Fib of 54 healthy controls and 214 RA patients was analyzed by ELISA. (B) Specificity of anti–Ca-Fib reactivity was confirmed using inhibition studies. One sample is shown, where data are expressed relative to inhibition with PBS. (C) The molecular nature of purified IgG and F(ab′)2 was confimed by Coomassie-stained SDS-PAGE gel. (D) F(ab′)2 fragments were generated from purified IgG of 2 anti-CarP–positive patients and two negative controls. Only F(ab′)2 from patients reacted with Ci-Fib and Ca-Fib. (E) Inhibition experiments confirm that also F(ab′)2 are not necessarily cross-reactive between Ci-Fib and Ca-Fib.
Collectively, these data indicate that anti-CarP antibodies and ACPA recognize different antigens, one recognizing citrullinated proteins (ACPA) and the other carbamylated proteins (anti-CarP). Likewise, these data indicate that antigen-recognition is most likely mediated via the variable domains present in the F(ab′)2 fragments.
Anti-CarP Antibodies Are Present in RA.
Following the identification of anti-CarP antibodies as an autoantibody family separate from ACPA, we wished to quantify the presence of these anti-CarP antibodies in a large population of RA patients and controls. For this reason, we first generated a standard, comprising of a pool of anti-CarP antibody-positive sera. This standard displayed a specific, dose-dependent binding of both IgG and IgA to Ca-FCS but no binding to unmodified FCS (Fig. 5 A and B). For this analysis, we again used the FCS-based assay in an attempt to capture as many anti-CarP reactivities as possible. We established a cutoff for positivity using sera of 305 healthy individuals, as described in Materials and Methods. Using this approach, we observed that 45% of the sera of RA patients analyzed are positive for IgG anti-CarP antibodies (Fig. 5C). Likewise, 43% of sera from RA patients tested are positive for IgA anti-CarP antibodies (Fig. 5D).
Fig. 5.
Anti-CarP IgG and IgA antibodies are present in RA sera. (A and B) Dose-response curves of the anti-CarP antibody-positive standard (IgG and IgA) on Ca-FCS and FCS in ELISA. (C and D) ELISA was performed for the detection of anti-CarP IgG and IgA in sera of healthy controls (NHS) and RA patients. A cut-off was established using the mean plus two times the SD of the healthy controls, as described in the Materials and Methods. Reactivity is depicted as arbitrary units per milliliter. The number of samples tested and the percentage of positivity is indicated below the graph. (E and F) Pie charts showing the percentage of RA patients positive and negative for anti-CCP2 and anti-CarP antibodies. (G and H) Pie charts showing the percentage of anti-CarP IgG- or IgA-positive patients negative for anti-CCP2.
Anti-CarP Antibodies Are also Present in Sera of Anti-CCP2–Negative RA Patients.
The group of RA patients analyzed in this study consisted of both ACPA-positive and ACPA-negative individuals, as measured by the CCP2 assay. Therefore, we analyzed next the association between anti-CarP antibodies and anti-CCP2 antibodies. The presence of anti-CarP antibodies and anti-CCP2 antibodies showed a limited degree of correlation when analyzing the entire RA population (r2 = 0.27, P < 0.001 for anti-CarP IgG or r2 = 0.15, P < 0.001 for IgA). However, we also identified substantial numbers of RA patients that are only positive for anti-CCP2 antibodies, as well as a group of patients that is only positive for anti-CarP antibodies (Fig. 5 E and F). We observed that ∼16% of the anti-CCP2–negative RA patients displayed anti-CarP IgG antibodies, whereas 30% of the anti-CCP2–negative RA patients tested positive for anti-CarP IgA (Fig. 5 G and H). These data indicate that the presence of anti-CarP antibodies overlaps with the occurrence of anti-CCP2 antibodies, but that this overlap is not absolute, as over 30% of the anti-CCP2–negative patients harbor anti-CarP antibodies. In total, more than 35% of all anti-CCP2–negative patients have either anti-CarP IgG or IgA antibodies.
Anti-CarP Antibodies Are Associated with More Severe Radiological Damage.
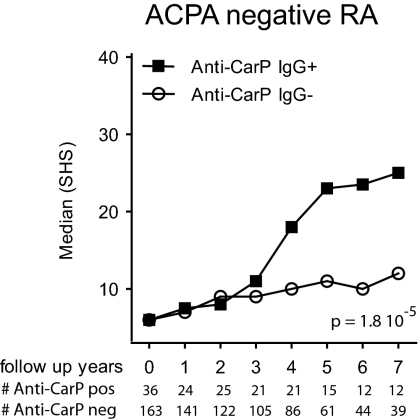
The presence of ACPA is associated with a more severe clinical disease course as measured by radiological damage. To analyze whether the presence of anti-CarP antibodies are also predictive for a more severe disease course, we compared the extent of joint damage over time between anti-CarP–positive and –negative patients participating in the Leiden Early Arthritis Clinic (EAC) cohort. This cohort is an inception cohort of patients with recent-onset arthritis where X-rays of hands and feet are taken of all RA-patients at yearly intervals to assess radiological damage using the Sharp–van der Heijde method (14). We observed that the presence of anti-CarP IgG strongly associates with a more severe disease progression. Patients positive for anti-CarP IgG had more joint destruction over 7 y than IgG-negative patients without [β = 2.01, 95% confidence interval (CI) 1.68–2.40, P = 8.68 × 10−14] or with correction of ACPA and rheumatoid factor (RF) (β = 1.41, 95% CI 1.13–1.76, P = 0.002) (Fig. S1). Anti-CarP IgA was associated with more joint destruction over 7 y than anti-CarP IgA-negative patients without correction of ACPA and RF (β = 1.21, 95% CI 1.01–1.45, P = 0.041) but not after correction (P = 0.855) (Fig. S1). As the analysis described above does not show whether anti-CarP antibodies predict radiological progression in the anti-CCP2–negative, anti-CCP2–positive, or both RA subgroups, we next performed a stratified analysis. Importantly, this analysis revealed that the presence of anti-CarP IgG is associated with a more severe joint damage in the anti-CCP2–negative subgroup (β = 1.86, 95% CI 1.41–2.66, P = 1.8·10−5) (Fig. 6). Likewise, a similar trend toward more joint damage over time was observed for anti-CCP2–negative patients who tested positive for IgA anti-CarP antibodies (β = 1.25, 95% CI 0.98–1.58, P = 0.071) (Fig. S1). In contrast, in the anti-CCP2–positive subgroup, which is already characterized by severe joint destruction, no additional increase was observed in individuals who also harbored anti-CarP antibodies (Fig. S1). Taken together, these data indicate that the detection of anti-CarP antibodies at baseline is predictive for a more destructive disease course in anti-CCP2–negative RA as measured by the Sharp–van der Heijde method.
Fig. 6.
Anti-CarP IgG antibodies are associated with a more severe radiological progression in ACPA-negative RA. The extent and rate of joint destruction were analyzed in all RA patients included, or analyzed separately, for ACPA-negative or ACPA-positive subgroups (Fig. S1). The severity of joint destruction is depicted as median Sharp–van der Heijde score (SHS) on the y axis and the follow-up years on the x axis. Below the x axis, the patient number is listed for each time point. Radiological progression for the anti-CCP2–negative RA patients is shown. The P value is derived from the analysis model, as described in Materials and Methods.
Discussion
A family of autoantibodies that recognize carbamylated proteins, anti-CarP antibodies, can be detected in sera of RA patients. Both inhibition studies and cohort studies show that anti-CarP antibodies and ACPA represent two different and independent autoantibody families, one recognizing carbamylated proteins and the other citrullinated proteins. Our data show that anti-CarP antibodies and ACPA are, by and large, noncross-reactive although we do not exclude that some cross-reactivity exists at the population level, as is also indicated in recent data obtained in rabbits after vaccination with carbamylated proteins (12). Interestingly, positivity for anti-CarP antibodies is related to clinical outcome, as individuals positive for anti-CarP IgG, but negative for anti-CCP2 antibodies, have a more destructive disease course compared with anti-CarP IgG-negative RA patients.
It is currently unknown which proteins undergo posttranslational modifications like carbamylation. Carbamylation is mediated by cyanate, which is in equilibrium with urea. Increased urea concentrations, smoking, and inflammation have been reported to shift this equilibrium toward cyanate and, hence, enhanced carbamylation (11). Because currently no in vivo relevant targets for anti-CarP antibodies are known, we used a complex protein mixture as an initial source of carbamylated protein antigens for the detection of anti-CarP antibodies. Western blot analyses indicate the recognition by anti-CarP antibodies of at least one dominant protein present in FCS after carbamylation using cyanate (representing high urea concentrations) (Fig. 3D). However, these data are likely not to represent the in vivo situation where carbamylation is a more gradual but constantly occurring process (15). In this respect, it is likely that especially long-lived proteins acquire homocitrulline residues over time, as carbamylation is nearly irreversible and thus will lead to the accumulation of homocitrulline-residues on proteins with a long half-life. Intriguingly, the joint is known for the presence of long-lived proteins, such as collagens and other cartilage-expressed proteins. Therefore, it is conceivable that such matrix-proteins will accumulate homocitrulline residues during life, especially under conditions of inflammation. Indeed, it has been shown that homocitrulline is present in the joint (9), possibly representing the long-lived nature of many joint-derived proteins. It will be interesting to know the identity of these proteins and whether these can serve as a target for anti-CarP antibodies.
The molecular nature of the antigens recognized by ACPA has been identified more than 15-y ago by describing that citrulline is an essential constituent of antigens recognized by these RA-specific antibodies (16, 17). This finding has made considerable impact, as it has opened up the way to relevant and novel insights into RA-diagnosis and etiopathology (1). For example, ACPA are now part of the new American College of Rheumatology/European League Against Rheumatism criteria for RA (18), and have been implicated in RA-pathogenesis, both in animal models (19–21) and in ex vivo human studies (22–25). Importantly, the description of ACPA has led to the realization that RA constitutes at least two clinical syndromes that share many clinical features, but differ with respect to genetic background, predisposing environmental factors and clinical progression/remission (3, 4, 26–28). Although it is clearly too early to allow any firm conclusions, it is tempting to speculate that anti-CarP antibodies also contribute to disease pathogenesis and display diagnostic value, given the similar nature of the antigens recognized and their presence in ACPA-negative disease.
The presence of anti-CarP antibodies in anti-CCP2–negative disease is highly intriguing, as it could potentially represent a unique biomarker that positively identifies at least part of this manifestation of RA. To gain further insight into this possibility, it is important to establish whether the presence of anti-CarP antibodies is specific for RA or also found in other rheumatic diseases, as well as whether their presence predict the development of (ACPA-negative) RA in patients suffering from early unclassified RA and joint complaints, such as arthralgia.
To establish a cut-off to define a positive sample, we have analyzed the presence of IgG and IgA directed against Ca-FCS and FCS in sera of healthy controls. All samples were tested for reactivity toward Ca-FCS and FCS, and absorbance values were converted into arbitrary units per milliliter using an anti-CarP antibody-positive standard present on the same plate. Because sera from several individual subjects also displayed reactivity toward nonmodified FCS, we subtracted the “FCS-reactivity” from the reactivity toward Ca-FCS using arbitrary units per milliliter as defined by the standard curve. We subsequently calculated the cut-off as the mean plus two times SD and applied the cut-off to the data of the RA patients following a similar strategy. The disadvantage of this method is that a standard is used on Ca-FCS for the determination of arbitrary units per milliliter toward FCS, another antigenic entity. However, this method did allow the calculation of a specific response to the posttranslational modification.
Every method of establishing a cut-off has advantages and limitations. Therefore, we subsequently confirmed our observations using another strategy as well by calculating the cut-off as the mean plus two times SD of the anti-Ca- FCS response in controls. This cut-off was applied to the data of the RA patients as was also used before (29). The association with radiological progression of anti-CarP IgG in ACPA-negative RA remains significant, albeit with a lower level of significance (P = 0.001).
From a clinical perspective, the detection of anti-CarP antibodies in early arthritis could be highly rewarding because they predict a more severe disease course. Because early aggressive treatment in RA has been shown to prevent future damage (30, 31), the detection of anti-CarP antibodies might be beneficial to identify anti-CCP2–negative patients at risk to develop severe disease. The identification of such patients might be important to guide treatment decisions early after onset of symptoms, especially in early arthritis patients that are difficult to classify.
In conclusion, in addition to the autoantibody system that recognizes citrullinated proteins (ACPA), an autoantibody system against carbamylated proteins (anti-CarP) is present in sera of RA patients. Detection of anti-CarP antibodies could offer new possibilities to identify patients at risk for a severe disease course.
Materials and Methods
Patient and Control Sera.
The sera analyzed were from patients participating in the Leiden EAC cohort. The Leiden EAC is an inception cohort of patients with recent-onset arthritis (symptoms duration <2 y) that was started at the Department of Rheumatology of the Leiden University Medical Center in 1993 (32). All RA patients fulfilled the American College of Rheumatology (formerly the American Rheumatism Association) 1987 revised criteria for the classification of RA (33) within 1 y of follow up. A total of 571 RA patients were involved in the analyses. Patient samples were compared with 305 healthy control samples also living in the Leiden area. The protocols were approved by the Leiden University Medical Center ethics committee and informed consent was obtained.
Detection of Anti-CarP Antibodies by ELISA.
In brief, nonmodified FCS and modified-FCS were coated on Nunc Maxisorp plates (Thermo Scientific) overnight. Following washing and blocking, the wells were incubated with serum. Bound human IgG or IgA was detected using rabbit anti-human IgG or IgA antibodies (Dako), followed by HRP-labeled goat anti-rabbit IgG antibody (Dako). Following the last washings, HRP enzyme activity was visualized using ABTS (34). A more detailed description of the protein modifications and ELISAs based on FCS and Fib, including F(ab′)2, is in SI Materials and Methods. We established the cut-off for a positive response as the mean plus two times the SD of the specific anti-CarP reactivity of the healthy controls. The methods for the detection of ACPA and Western blotting are available in SI Materials and Methods.
ELISA for Fib Peptides.
Streptavidin (Invitrogen) was coated at 2 μg/mL in 100 μL on Nunc plates at 4 °C overnight. After washing, Fib peptides containing either an arginine, citrulline, homocitruline, or lysine (Fig. 3A) (29) were incubated at 10 μg/mL in 100 μL PTB for 1 h at room temperature. Next, the reactivity of antibodies reactive to these antigens was detected as described above.
Inhibition Studies.
To determine whether anti-CarP antibodies and ACPA are cross-reactive antibodies, we performed inhibition studies in which autoantibody-positive serum samples, positive for both ACPA and anti-CarP antibodies, were preincubated with increasing concentrations of either nonmodified FCS, Ca-FCS, Ci-FCS, or the citrulline or arginine containing a form of the CCP1 peptide (17). Following preincubation at room temperature, the samples were tested for reactivity against Ca-FCS and Ci-FCS, as described above. Serum and F(ab′)2 samples positive for both Ci-Fib and Ca-Fib were preincubated with Fib, Ci-Fib, and Ca-Fib at 4 °C overnight and subsequently analyzed on the Fib ELISA (SI Materials and Methods).
Radiological Progression.
In the EAC cohort, radiographs of the hands and feet, which had been obtained in a longitudinal fashion, were scored according to the Sharp–van der Heijde method (35). Scoring and analysis have been described in detail previously (14). Data were analyzed directly, or using repeated measurement analysis to optimally make use of the longitudinal data obtained for each patient (14). More detailed information is available in SI Materials and Methods.
Supplementary Material
Acknowledgments
The authors thank G. Stoeken-Rijsbergen and S. Shi for technical support and suggestions on methods. These studies were supported by the Dutch Arthritis Foundation, The Netherlands Organization for Health Research and Development, The Netherlands Organization for Scientific Research, FP7 project Masterswitch, the Innovative Medicines Initiative-funded project BeTheCure, The Netherlands Proteomics Center, and the Center for Medical Systems Biology as part of The Netherlands Genomics Initiative.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1114465108/-/DCSupplemental.
References
- 1.Klareskog L, Rönnelid J, Lundberg K, Padyukov L, Alfredsson L. Immunity to citrullinated proteins in rheumatoid arthritis. Annu Rev Immunol. 2008;26:651–675. doi: 10.1146/annurev.immunol.26.021607.090244. [DOI] [PubMed] [Google Scholar]
- 2.Kallberg H, et al. Epidemiological Investigation of Rheumatoid Arthritis study group Gene-gene and gene-environment interactions involving HLA-DRB1, PTPN22, and smoking in two subsets of rheumatoid arthritis. Am J Hum Genet. 2007;80:867–875. doi: 10.1086/516736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van der Helm-van Mil AH, Verpoort KN, Breedveld FC, Toes RE, Huizinga TW. Antibodies to citrullinated proteins and differences in clinical progression of rheumatoid arthritis. Arthritis Res Ther. 2005;7:R949–R958. doi: 10.1186/ar1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van der Woude D, et al. Prevalence of and predictive factors for sustained disease-modifying antirheumatic drug-free remission in rheumatoid arthritis: Results from two large early arthritis cohorts. Arthritis Rheum. 2009;60:2262–2271. doi: 10.1002/art.24661. [DOI] [PubMed] [Google Scholar]
- 5.Visser K, et al. Pretreatment serum levels of anti-cyclic citrullinated peptide antibodies are associated with the response to methotrexate in recent-onset arthritis. Ann Rheum Dis. 2008;67:1194–1195. doi: 10.1136/ard.2008.088070. [DOI] [PubMed] [Google Scholar]
- 6.György B, Tóth E, Tarcsa E, Falus A, Buzás EI. Citrullination: A posttranslational modification in health and disease. Int J Biochem Cell Biol. 2006;38:1662–1677. doi: 10.1016/j.biocel.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 7.Wang Y, et al. Human PAD4 regulates histone arginine methylation levels via demethylimination. Science. 2004;306:279–283. doi: 10.1126/science.1101400. [DOI] [PubMed] [Google Scholar]
- 8.Li P, et al. PAD4 is essential for antibacterial innate immunity mediated by neutrophil extracellular traps. J Exp Med. 2010;207:1853–1862. doi: 10.1084/jem.20100239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mydel P, et al. Carbamylation-dependent activation of T cells: A novel mechanism in the pathogenesis of autoimmune arthritis. J Immunol. 2010;184:6882–6890. doi: 10.4049/jimmunol.1000075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sirpal S. Myeloperoxidase-mediated lipoprotein carbamylation as a mechanistic pathway for atherosclerotic vascular disease. Clin Sci (Lond) 2009;116:681–695. doi: 10.1042/CS20080322. [DOI] [PubMed] [Google Scholar]
- 11.Wang Z, et al. Protein carbamylation links inflammation, smoking, uremia and atherogenesis. Nat Med. 2007;13:1176–1184. doi: 10.1038/nm1637. [DOI] [PubMed] [Google Scholar]
- 12.Turunen S, Koivula MK, Risteli L, Risteli J. Anticitrulline antibodies can be caused by homocitrulline-containing proteins in rabbits. Arthritis Rheum. 2010;62:3345–3352. doi: 10.1002/art.27644. [DOI] [PubMed] [Google Scholar]
- 13.Willemze A, et al. The interaction between HLA shared epitope alleles and smoking and its contribution to autoimmunity against several citrullinated antigens. Arthritis Rheum. 2011;63:1823–1832. doi: 10.1002/art.30409. [DOI] [PubMed] [Google Scholar]
- 14.van der Linden MP, et al. Association of a single-nucleotide polymorphism in CD40 with the rate of joint destruction in rheumatoid arthritis. Arthritis Rheum. 2009;60:2242–2247. doi: 10.1002/art.24721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berlyne GM. Carbamylated proteins and peptides in health and in uremia. Nephron. 1998;79:125–130. doi: 10.1159/000045013. [DOI] [PubMed] [Google Scholar]
- 16.Masson-Bessière C, et al. The major synovial targets of the rheumatoid arthritis-specific antifilaggrin autoantibodies are deiminated forms of the alpha- and beta-chains of fibrin. J Immunol. 2001;166:4177–4184. doi: 10.4049/jimmunol.166.6.4177. [DOI] [PubMed] [Google Scholar]
- 17.Schellekens GA, de Jong BA, van den Hoogen FH, van de Putte LB, van Venrooij WJ. Citrulline is an essential constituent of antigenic determinants recognized by rheumatoid arthritis-specific autoantibodies. J Clin Invest. 1998;101:273–281. doi: 10.1172/JCI1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aletaha D, et al. The 2010 Rheumatoid Arthritis classification critereia: An American College of Rheumatology/European League Against Rheumatism collaberative initiative. Arthritis Rheum. 2010;62:2569–2581. doi: 10.1002/art.27584. [DOI] [PubMed] [Google Scholar]
- 19.Hill JA, et al. Arthritis induced by posttranslationally modified (citrullinated) fibrinogen in DR4-IE transgenic mice. J Exp Med. 2008;205:967–979. doi: 10.1084/jem.20072051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuhn KA, et al. Antibodies against citrullinated proteins enhance tissue injury in experimental autoimmune arthritis. J Clin Invest. 2006;116:961–973. doi: 10.1172/JCI25422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Uysal H, et al. Structure and pathogenicity of antibodies specific for citrullinated collagen type II in experimental arthritis. J Exp Med. 2009;206:449–462. doi: 10.1084/jem.20081862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clavel C, et al. Induction of macrophage secretion of tumor necrosis factor alpha through Fcgamma receptor IIa engagement by rheumatoid arthritis-specific autoantibodies to citrullinated proteins complexed with fibrinogen. Arthritis Rheum. 2008;58:678–688. doi: 10.1002/art.23284. [DOI] [PubMed] [Google Scholar]
- 23.Lu MC, et al. Anti-citrullinated protein antibodies bind surface-expressed citrullinated Grp78 on monocyte/macrophages and stimulate tumor necrosis factor alpha production. Arthritis Rheum. 2010;62:1213–1223. doi: 10.1002/art.27386. [DOI] [PubMed] [Google Scholar]
- 24.Schuerwegh AJM, et al. Evidence for a functional role of IgE anticitrullinated protein antibodies in rheumatoid arthritis. Proc Natl Acad Sci USA. 2010;107:2586–2591. doi: 10.1073/pnas.0913054107. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 25.Trouw LA, et al. Anti-cyclic citrullinated peptide antibodies from rheumatoid arthritis patients activate complement via both the classical and alternative pathways. Arthritis Rheum. 2009;60:1923–1931. doi: 10.1002/art.24622. [DOI] [PubMed] [Google Scholar]
- 26.Linn-Rasker SP, et al. Smoking is a risk factor for anti-CCP antibodies only in rheumatoid arthritis patients who carry HLA-DRB1 shared epitope alleles. Ann Rheum Dis. 2006;65:366–371. doi: 10.1136/ard.2005.041079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Gaalen FA, et al. Association between HLA class II genes and autoantibodies to cyclic citrullinated peptides (CCPs) influences the severity of rheumatoid arthritis. Arthritis Rheum. 2004;50:2113–2121. doi: 10.1002/art.20316. [DOI] [PubMed] [Google Scholar]
- 28.Verpoort KN, et al. Association of HLA-DR3 with anti-cyclic citrullinated peptide antibody-negative rheumatoid arthritis. Arthritis Rheum. 2005;52:3058–3062. doi: 10.1002/art.21302. [DOI] [PubMed] [Google Scholar]
- 29.Verpoort KN, et al. Fine specificity of the anti-citrullinated protein antibody response is influenced by the shared epitope alleles. Arthritis Rheum. 2007;56:3949–3952. doi: 10.1002/art.23127. [DOI] [PubMed] [Google Scholar]
- 30.van der Helm-van Mil AH, et al. A prediction rule for disease outcome in patients with recent-onset undifferentiated arthritis: How to guide individual treatment decisions. Arthritis Rheum. 2007;56:433–440. doi: 10.1002/art.22380. [DOI] [PubMed] [Google Scholar]
- 31.van Dongen H, et al. Efficacy of methotrexate treatment in patients with probable rheumatoid arthritis: A double-blind, randomized, placebo-controlled trial. Arthritis Rheum. 2007;56:1424–1432. doi: 10.1002/art.22525. [DOI] [PubMed] [Google Scholar]
- 32.de Rooy DPC, van der Linden MP, Knevel R, Huizinga TW, van der Helm-van Mil AH. Predicting arthritis outcomes—What can be learned from the Leiden Early Arthritis Clinic? Rheumatology (Oxford) 2011;50:93–100. doi: 10.1093/rheumatology/keq230. [DOI] [PubMed] [Google Scholar]
- 33.Arnett FC, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 34.Suwannalai P, et al. Anti-citrullinated protein antibodies have a low avidity compared with antibodies against recall antigens. Ann Rheum Dis. 2011;70:373–379. doi: 10.1136/ard.2010.135509. [DOI] [PubMed] [Google Scholar]
- 35.van der Heijde D. How to read radiographs according to the Sharp/van der Heijde method. J Rheumatol. 2000;27:261–263. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.