A recent report in PNAS by Asahina et al. (1) addresses the fascinating question of tissue repair in plants. According to recent suggestions, plants and animals might share cellular mechanisms that allow regeneration of tissues after damage (2). However, plants and animals differ greatly in their mode of development and their ability to respond to damage-inducing environmental factors (3). Terrestrial plants cannot move their whole body in response to environmental cues, and, because of their cell walls, they also lack cellular mobility within the plant. This means that plants must regenerate damaged tissue through cellular regeneration at the point of damage. Traditionally, this regeneration was considered to occur by dedifferentiation of existing mature cells followed by cell division to form callus and differentiation to form the cellular constituents of the new tissue, although details of this process have been questioned recently (4).
Plants experience many types of tissue damage, including that caused by herbivory and other forms of physical wounding (e.g., breakage because of wind or ice or trampling by animals). They have developed elaborate responses to this damage. For example, herbivory results in a suite of responses; some are fast-acting and local, whereas others may be quite long-lived and systemic in nature, allowing the plant to develop a response at the whole-plant level to attack by particular animal species (5).
One of the simplest forms of damage to plants is the splitting or laceration of tissue. This type of wounding is frequent under both natural and agronomic conditions. It is also common with some of our well-established horticultural and research techniques (e.g., grafting). Indeed, grafting and the subsequent tissue repair have been vital for the identification of two new plant hormones over the last 5 years: the strigolactones for branching (6) and the floral stimulus or florigen for flowering (7). However, the molecular basis of tissue repair has remained largely unknown. The paper by Asahina et al. (1) provides some welcome insights into the repair process, since it shows that two plant-specific transcription factors (TFs), ANAC071 and RAP2.6L, are strongly up-regulated on the upper (ANAC071) and lower (RAP2.6L) sides of an incision in the infloresence stem. When the expression of these TFs is down-regulated using chimeric repressor silencing technology (8), repair of the wound is inhibited, indicating the importance of the TFs for the repair process.
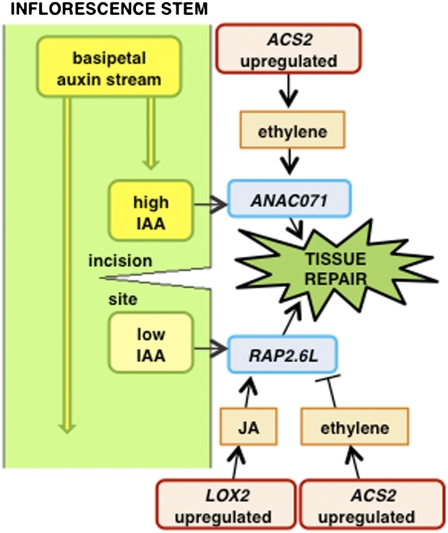
Importantly, the work by Asahina et al. (1) provides evidence that the TFs are regulated by plant hormones, with a focus on auxin. This hormone also regulates TFs involved in root tissue repair after damage by laser ablation (9). In the present case (1), the possible involvement of auxin implies communication between other parts of the plant and the repair site. Auxin is a mobile hormone, moving down the stem in a polar transport stream, and molecular evidence is presented that auxin accumulates on the upper (acropetal) side of the incision and depletes on the lower (basipetal) side. This information is used to develop a model in which auxin regulates TFs, which then initiate cell division in the pith and ultimately repair the wound (Fig. 1).
Fig. 1.
Model of tissue repair in the Arabidopsis inflorescence stem based on the work by Asahina et al. (1). The differential control of the TFs ANACO71 and RAP2.6L in the upper and lower sides, respectively, of an incision and their suggested regulation by the plant hormones auxin, ethylene, and jasmonic acid (JA) are shown along with associated synthesis genes.
Roles for other plant hormones are also suggested (1). The ethylene-insensitive ein2 mutant does not undergo the same repair as WT plants because of a lack of cell division in the pith. The expression of ANAC071 is reduced in ein2 plants suggesting that wound-induced ethylene may enhance the auxin response. Ethylene levels are not directly measured but are inferred from the expression of the ACS2 gene, one of a family of aminocyclopropane carboxylic acid synthase genes that regulates the rate-limiting step in ethylene biosynthesis.
Jasmonic acid (JA), another plant hormone, may also play a role (1). JA is a known regulator of plant responses to both biotic and abiotic stresses (10), and genes involved in its biosynthesis are up-regulated after incision, which is shown by microarray and quantitative RT-PCR analyses (1). One such gene, the lipoxygenase gene LOX2, is up-regulated below the incision in a similar pattern to RAP2.6L, and application of methyl jasmonate up-regulates RAP2.6L expression. Although studies using the expression of biosynthesis genes to infer hormone levels need to be treated with extreme caution (11), it seems that wounding up-regulates this gene independently of auxin (1).
Overall, these results provide a testable model of some of the early molecular steps involved in tissue repair. Understanding the molecular targets of the TFs and moving beyond correlations to show the direct regulation of the TFs by the hormones implicated will go a long way to elucidating the control of this essential plant response. Thus far, the effects of directly applying hormones on the expression of the TF genes are less impressive than the effects of stem incision (cutting) or decapitation (removal of material at the top of the stem, including flowers) (1). For example, in cut stems, decapitation dramatically reduces ANAC071 expression, but applying auxin to the decapitation site does not significantly reverse that effect (figure 3 in ref. 1). Possibly, the dose used (1 mM auxin in lanolin paste) is inadequate to restore the auxin content of stems. In previous research, a similar dose did not fully restore the auxin level to the level of intact stems, although the hormone was applied repeatedly (12). Similarly, applying 2 mM methyl jasmonate has only a moderate effect on RAP2.6L expression compared with cutting (figure 5 in ref. 1).
The possible roles of other hormones in tissue repair also require examination. In an earlier paper, Asahina et al. (13) noted the importance of another growth-promoting hormone, gibberellin (GA), in the repair process. In that case, hypocotyls of tomato and cucumber were studied. In considering the issue of auxin vs. GA, it should be borne in mind that high auxin content can lead to high GA content, because auxin promotes GA synthesis and inhibits its deactivation (14, 15). However, in the tomato hypocotyl, the pattern of gene expression after cotyledon removal is not consistent with an auxin-mediated effect on GA levels (16), indicating the importance of GA per se. It is suggested that, in hypocotyls, GA is a key factor in the reunion of cortical cells whereas, in the pith cells of inflorescence stems, auxin is a major player (1). In this context, it is worth noting that GA-deficient pea mutants can be easily grafted epicotyl to epicotyl and epicotyl to stem (17), indicating that GA is not essential for tissue reunion in that system.
It is also possible that different TFs regulate the repair response in hypocotyls and inflorescence stems because, earlier this year, Iwase et al. (18) reported that another recently discovered TF gene, WIND1, is up-regulated in wounded Arabidopsis hypocotyls. This gene was suggested to act as a master regulator of dedifferentiation during wound repair (18). Like RAP2.6L, WIND1 belongs
Overall, these results provide a testable model of some of the early molecular steps involved in tissue repair.
to the apetela2/ethylene response factor TF family. Interestingly, WIND1 is not included in a list of genes up-regulated by the wounding of Arabidopsis stems (1). Consistent with evidence that auxin may not be the key factor in hypocotyl repair, WIND1 (unlike RAP2.6L) is apparently not responsive to auxin (18). The work by Iwase et al. (18) implicated another hormone, cytokinin, in TF-mediated repair, but this time the hormone seemed to act downstream and not upstream of WIND1.
Different plant organs are affected in different ways by both physical damage and predation, and these differences may explain the occurrence of different repair mechanisms. For example, leaves and flowers are determinate in growth and do not directly prevent the growth of other organs, and, therefore, repair is not essential, although protection from additional damage/invasion is advantageous to the plant. However, the stem is essential for subsequent organ development (e.g., leaves, roots, flowers, and seeds) because of its critical role in connectivity, support, and nutrient transport. Although new shoots may arise from axillary buds if the upper stem is damaged, the consequences for the plant may be much greater than if an individual determinate organ is damaged.
The involvement of cell division in the repair process has been known or assumed for a long time, and implicating plant hormones in the reformation of tissues, particularly vascular tissues, is likewise not new (19). The contribution by Asahina et al. (1) is the characterization of specific TFs, which, according to their model, form a molecular link between plant hormones and the cell division response in the pith. Their work (1) provides a foundation for determining whether tissue repair is controlled by similar TFs and plant hormones in different tissues and different plant taxa.
Acknowledgments
We thank Laura Quittenden for preparing Fig. 1.
Footnotes
The authors declare no conflict of interest.
See companion article on page 16128 of issue 38 in volume 108.
References
- 1.Asahina M, et al. Spatially selective hormonal control of RAP2.6L and ANAC071 transcription factors involved in tissue reunion in Arabidopsis. Proc Natl Acad Sci USA. 2011;108:16128–16132. doi: 10.1073/pnas.1110443108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Birnbaum KD, Sánchez Alvarado A. Slicing across kingdoms: Regeneration in plants and animals. Cell. 2008;132:697–710. doi: 10.1016/j.cell.2008.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tör M, Lotze MT, Holton N. Receptor-mediated signalling in plants: Molecular patterns and programmes. J Exp Bot. 2009;60:3645–3654. doi: 10.1093/jxb/erp233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sugimoto K, Gordon SP, Meyerowitz EM. Regeneration in plants and animals: Dedifferentiation, transdifferentiation, or just differentiation? Trends Cell Biol. 2011;21:212–218. doi: 10.1016/j.tcb.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 5.León J, Rojo E, Sánchez-Serrano JJ. Wound signalling in plants. J Exp Bot. 2001;52:1–9. doi: 10.1093/jexbot/52.354.1. [DOI] [PubMed] [Google Scholar]
- 6.Beveridge CA, Dun EA, Rameau C. Pea has its tendrils in branching discoveries spanning a century from auxin to strigolactones. Plant Physiol. 2009;151:985–990. doi: 10.1104/pp.109.143909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hecht V, et al. The pea GIGAS gene is a FLOWERING LOCUS T homolog necessary for graft-transmissible specification of flowering but not for responsiveness to photoperiod. Plant Cell. 2011;23:147–161. doi: 10.1105/tpc.110.081042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hiratsu K, Matsui K, Koyama T, Ohme-Takagi M. Dominant repression of target genes by chimeric repressors that include the EAR motif, a repression domain, in Arabidopsis. Plant J. 2003;34:733–739. doi: 10.1046/j.1365-313x.2003.01759.x. [DOI] [PubMed] [Google Scholar]
- 9.Xu J, et al. A molecular framework for plant regeneration. Science. 2006;311:385–388. doi: 10.1126/science.1121790. [DOI] [PubMed] [Google Scholar]
- 10.Dombrecht B, et al. MYC2 differentially modulates diverse jasmonate-dependent functions in Arabidopsis. Plant Cell. 2007;19:2225–2245. doi: 10.1105/tpc.106.048017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Symons GM, et al. The hormonal regulation of de-etiolation. Planta. 2008;227:1115–1125. doi: 10.1007/s00425-007-0685-x. [DOI] [PubMed] [Google Scholar]
- 12.Ross JJ, O'Neill DP, Wolbang CM, Symons GM, Reid JB. Auxin-gibberellin interactions and their role in plant growth. J Plant Growth Regul. 2001;20:336–353. doi: 10.1007/s003440010034. [DOI] [PubMed] [Google Scholar]
- 13.Asahina M, et al. Gibberellin produced in the cotyledon is required for cell division during tissue reunion in the cortex of cut cucumber and tomato hypocotyls. Plant Physiol. 2002;129:201–210. doi: 10.1104/pp.010886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ross JJ, O'Neill DP, Smith JJ, Kerckhoffs LH, Elliott RC. Evidence that auxin promotes gibberellin A1 biosynthesis in pea. Plant J. 2000;21:547–552. doi: 10.1046/j.1365-313x.2000.00702.x. [DOI] [PubMed] [Google Scholar]
- 15.O'Neill DP, et al. Regulation of the gibberellin pathway by auxin and DELLA proteins. Planta. 2010;232:1141–1149. doi: 10.1007/s00425-010-1248-0. [DOI] [PubMed] [Google Scholar]
- 16.Asahina M, et al. Effects of the removal of cotyledons on endogenous gibberellin levels in hypocotyls of young cucumber and tomato seedlings. Plant Biotechnol. 2007;24:99–106. [Google Scholar]
- 17.Reid JB, Murfet IC, Potts WC. Internode length in Pisum. II. Additional information on the relationship and action of loci Le, La, Cry, Na and Lm. J Exp Bot. 1983;34:349–364. [Google Scholar]
- 18.Iwase A, et al. The AP2/ERF transcription factor WIND1 controls cell dedifferentiation in Arabidopsis. Curr Biol. 2011;21:508–514. doi: 10.1016/j.cub.2011.02.020. [DOI] [PubMed] [Google Scholar]
- 19.Sachs T. Integrating cellular and organismic aspects of vascular differentiation. Plant Cell Physiol. 2000;41:649–656. doi: 10.1093/pcp/41.6.649. [DOI] [PubMed] [Google Scholar]