Abstract
A lipid extract of Perna canaliculus (New Zealand green-lipped mussel) has reportedly displayed anti-inflammatory effects in animal models and in human controlled studies. However, the anti-inflammatory lipid components have not been investigated in detail due to the instability of the lipid extract, which has made the identification of the distinct active components a formidable task. Considering the instability of the active component, we carefully fractionated a lipid extract of Perna canaliculus (Lyprinol) and detected furan fatty acids (F-acids). These naturally but rarely detected fatty acids show potent radical-scavenging ability and are essential constituents of plants and algae. Based on these data, it has been proposed that F-acids could be potential antioxidants, which may contribute to the protective properties of fish and fish oil diets against chronic inflammatory diseases. However, to date, in vivo data to support the hypothesis have not been obtained, presumably due to the limited availability of F-acids. To confirm the in vivo anti-inflammatory effect of F-acids in comparison with that of eicosapentaenoic acid (EPA), we developed a semisynthetic preparation and examined its anti-inflammatory activity in a rat model of adjuvant-induced arthritis. Indeed, the F-acid ethyl ester exhibited more potent anti-inflammatory activity than that of the EPA ethyl ester. We report on the in vivo activity of F-acids, confirming that the lipid extract of the green-lipped mussel includes an unstable fatty acid that is more effective than EPA.
Keywords: natural product, tetraalkyl-subsituted furan ring
The green-lipped mussel Perna canaliculus is native to the New Zealand coast and is a dietary staple of the indigenous Maori culture. It is distinguished from other bivalve species by the presence of a bright green stripe around the posterior ventral margin of the shell and its distinctive green lip, which is visible on the inside of the shell. The anti-inflammatory effects of the green-lipped mussel have been studied since the observation of the lower incidence of arthritis in coastal Maoris compared with its prevalence in European or inland Maori people. The development of a natural remedy for the management of arthritis that will not only replace nonsteroidal anti-inflammatories (NSAIDs) as the standard treatment for all types of arthritis but also treat the condition successfully, without any known side effects, is being eagerly pursued (1). Therefore, numerous studies have been published on the lipid composition of the extract (2–4), its complex mode of action (5–8), activity in animal models (9, 10), and efficacy in controlling osteoarthritis (11, 12) and moderate asthma in patients (13). However, the active component from the mussel is a lipid rich in polyunsaturated fatty acids (PUFAs) and is therefore prone to activation of degrading enzymes including phospholipases and lipoxygenases (14, 15) after cell damage, unless it is stored and transported properly. In the case of severe damage, this enzymatic reaction switches to a nonenzymatic one producing radicals (16). A freeze-dried powdered preparation of whole mussel (i.e., without shell) was first tested orally in rats, and showed either modest or no anti-inflammatory activity (carrageenan paw edema in rats), probably due to the remaining activity of degrading enzymes. The preparation process was subsequently improved, and a lipid-rich fraction (Lyprinol), a supercritical fluid [CO2] extraction of the freeze-dried stabilized green-lipped mussel powder, was prepared. This extraction method avoids warming the biological material, and therefore prevents activation of enzymes. The lipid-rich extract was found to contain five main lipid classes including sterol esters, triglycerides, free fatty acids, sterols, and polar lipids. A detailed analysis of the fatty acid composition revealed that omega-3 PUFAs, such as eicosapentaenoic acid (EPA; 20:5) and docosahexanoic acid (22:6), were the most abundant PUFAs among the 90 fatty acid components in this extract (3, 4). Although omega-3 PUFAs are the likely source of the anti-inflammatory activity of the green-lipped mussel, via the inhibition of both the 5-lipoxygenase and cyclooxygenase arachidonate oxygenation pathways, Whitehouse and coworkers reported that the lipid-rich oil of the green-lipped mussel showed potent anti-inflammatory activity in rat paw edema assays at a concentration two orders of magnitude lower than that of fish oil containing abundant EPA (9). More recently, Howarth and coworkers also reported that the beneficial effect is unlikely to be due to omega-3 fatty acid content (10). These data indicated the presence of another active component, in addition to the omega-3 PUFAs commonly found in fish oils. Based on these reports, we hypothesized that other unstable fatty acids remained to be identified as the active components in the lipid extract.
Results and Discussion
We fractionated the lipid extract of the green-lipped mussel and used a rat model of adjuvant-induced arthritis to identify the active components. After separation into each lipid class by silica gel chromatography, anti-inflammatory activities were usually found in the cholesterol ester and free fatty acid fractions. Because the common components for these fractions are apparently fatty acids, we focused on the further analysis of the fatty acid components. This estimation agrees with Macrides and coworkers’ report that the free fatty acid fraction, and to a lesser extent the triglyceride fraction, were the only lipid classes to exhibit strong inhibition of COX isoforms (8). However, a precise separation of the free fatty acid fraction is usually difficult for minor fatty acids in the presence of an abundance of PUFAs. Moreover, further fractionation based on the guidance of the bioassay was a formidable task, due to the concomitant decomposition of the active compounds.
There is a large body of evidence demonstrating a role for reactive oxygen species and oxidant stress in the pathogenesis of chronic inflammation such as rheumatoid arthritis (17–19). The high susceptibility of the cellular membrane to oxidation triggers lipid peroxidation and concomitant radical chain reactions, resulting in severe progression of symptoms (20). To suppress lipid peroxidation, there are many lipophilic antioxidants, such as vitamins A and E, astaxanthin as a natural product, and probucol as an antioxidant drugs (21). On the other hand, if an antioxidative fatty acid can be embedded into the lipid component, then its antioxidative effect would be more promising. Spiteller hypothesized that furan fatty acids (F-acids), which were first isolated from the northern pike in 1974 (22), are such naturally occurring antioxidative fatty acids that ameliorate the oxidative risk of the cellular membrane (23, 24). Even though F-acid concentration is usually low, these fatty acids are widely distributed in plants and aquatic organisms (25–36). The unique structural feature of F-acids is the tetraalkyl-substituted furan ring, which is a rarely observed skeleton in natural products. This electron-rich furan ring is susceptible to oxidation, and the subsequent ring opening generates dioxoene, which is highly reactive and further converted to other compounds (23, 30). Actually, F-acids readily decompose during prolonged exposure to silica gel or in neat oil. Thus, natural products bearing the tetraalkyl-substituted furan ring are relatively unstable, which is a possible reason why the tetraalkyl-substituted furan ring has rarely been found in nature. Recently, a macrolactone bearing a tetraalkyl-substituted furan ring was identified as the aggregation pheromone of leaf beetle (37) and, to the best our knowledge, this is the only example of a tetraalkyl furan ring in a natural product except for F-acids. Moreover, the activity of the green-lipped mussel is known to disappear in aqueous solution. This instability in water reasonably agrees with the features of F-acids, because F-acids were also missing after homogenization in aqueous solution (38). Considering these findings, we suspected the involvement of F-acids in the anti-inflammatory activity of the green-lipped mussel. In 2002, Sinclair and coworkers reported a detailed GC-MS analysis of the fatty acid composition of the green-lipped mussel (3). Their research focused on defining the similarities and differences in the lipid compositions between the New Zealand green-lipped mussel and the Tasmanian blue mussel. However, no fatty acids unique to the New Zealand green-lipped mussel were found. Considering the instability of F-acids in aqueous solution and on silica gel, previous studies using preparative TLC to separate the lipid classes would have allowed the F-acids to decompose before GC-MS analysis, even though over 90 component fatty acids were detected. Therefore, we expected that the F-acids might still be detected.
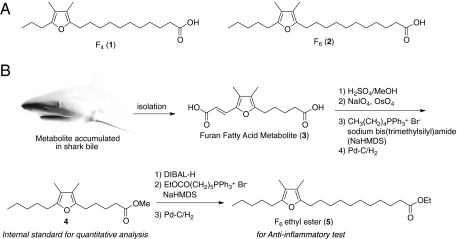
However, the separation of F-acids from other fatty acids is usually difficult and requires special workup procedures. F-acids, especially those with two β-methyl substituents, are quite susceptible to the peroxy radicals produced during lipid peroxidation, and are no longer detectable after homogenization in aqueous media. Thus, F-acids were found only occasionally, when a tissue rich in F-acids was homogenized in organic solvents. Additionally, to circumvent the autoxidation of the tetraalkyl-substituted furan ring on the surface of silica gel (30, 39), careful manipulations are required, especially in the case of lower quantities from natural sources. Previous reports used the method of Folch et al. (40) or Bligh and Dyer (41) to extract total lipids, which were further separated by silica gel into cholesteryl ester, triglycerides, and phospholipid fractions (25–35). These esters were transesterified with a methanolic sodium methoxide solution to form fatty acid methyl esters and then were investigated by GC-MS. Furthermore, Glass and coworkers enriched the F-acid methyl esters and separated them from PUFAs by hydrogenation of the methyl ester fraction, leaving the F-acids unchanged (22, 26, 27). The obtained saturated fatty acid methyl esters were removed by precipitation as urea complexes (42). It should be noted that the hydrogenation method of Glass is important for separation by silica gel chromatography, because F-acids could be separated due to different retention times in respect to the saturated acids. According to previous reports, we attempted to develop an isolation procedure based on a modification of Glass et al.'s protocol (26). To detect F-acids selectively, we tried to minimize the period during which the oil extract was exposed to the silica gel as much as possible. First, Lyprinol was treated with Na/MeOH to yield fatty acid methyl esters. The remaining free fatty acids were completely methylated with diazomethane. The resultant mixture was separated by silica gel flash chromatography to afford the PUFA methyl ester fractions. Because it was anticipated that the F-acid methyl esters would be eluted along with the PUFA methyl esters from the silica gel, the unsaturated fatty acid methyl esters were selectively transformed to saturated fatty acid methyl esters by hydrogenolysis with H2 and Pd-C, to detect the F-acid methyl esters selectively (26). Finally, the mixture was separated by silica gel flash chromatography again, to afford the fractions containing the F-acid methyl esters. A GC-MS analysis revealed that these fractions predominantly contained F4 and F6 (Fig. 1A and Fig. S1) as well as other minor F-acids identified as F2, F3, and F5. The predominant components, F4 and F6, were the most common F-acids in other organisms.
Fig. 1.
(A) Structures of the representative furan fatty acids detected in the green-lipped mussel. (B) Partial synthesis of furan fatty acid F6.
To examine the anti-inflammatory activity, a sufficient amount of pure material was required. Although several groups previously reported the synthesis of F-acid (43–49), the synthetic route required many steps. To prepare these fatty acids easily, a shark metabolite was selected as the starting material, because sharks are commercially available for consumption in the northeast area of Japan. We found that furan dicarboxylic acid 1, a structural metabolite of F-acids (50), was highly accumulated in shark bile (ca. 10% dry weight). This metabolite is relatively stable, due to the presence of an electron-withdrawing group conjugated to the tetraalkyl furan ring. Moreover, one half of the side chains have an olefinic bond, which is versatile for chemical modification. Taking advantage of the double bond, cleavage by Lemieux–Johnson oxidation generated the conjugated aldehyde, in which the alkyl side chain was selectively elongated by a Wittig reaction. After hydrogenolysis, the methyl ester terminal was transformed to the aldehyde by diisobutylaluminium hydride (DIBAL) reduction. A second Wittig reaction with the resulting aldehyde elongated the C6 ester side chain, and subsequent hydrogenolysis yielded the F6 ethyl ester 3 (Fig. 1B and Fig. S2). Concomitantly, because the intermediate 2 with a shorter carboxylic side chain had not been reported from a natural source, it was used as the internal standard of the quantitative analysis.
It was reported that F-acids were especially accumulated by salmonid fishes (22, 26). Liver and testes lipids contain high concentrations of F-acids, which vary seasonally, depending on spawning (27, 32–35). We isolated authentic F-acid methyl esters from the testes of salmon captured at Hokkaido, Japan, in September 2007. Lyophilized testes were extracted with methanol and then fractionated by the same procedure as the isolation scheme that yielded the fraction containing both the F4 and F6 methyl esters. We confirmed that the ionization ratios of these two major F-acid methyl esters were identical, and therefore the peak areas for these two methyl esters were expected to correspond to each quantity. With authentic material in hand, a quantitative analysis of the F-acid in Lyprinol was implemented by GC-MS. After the addition of an internal standard to Lyprinol, according to a similar procedure as the isolation scheme, the lipid extract was methylated and separated to yield the fatty acid methyl ester fraction before the GC-MS analysis. Consequently, the mean values for the F4 and F6 methyl esters were 1.87 mg/g and 2.17 mg/g, respectively (Table S1).
Besides the in vitro antioxidative activity of F-acids, other biological activities, particularly in vivo, and the role in the host organism remained to be investigated. Watanabe, Okada, and coworkers reported that F-acids exhibited potent radical-scavenging activity due to the electron-rich furan ring (51). The potency of F-acids as scavengers of, for example, hydroxyl radicals is further exemplified by the findings that they suppress hemolysis better than other singlet oxygen quenchers such as dimethylfuran, α-tocopherol, ascorbic acid, and uric acid (52). One tetraalkyl furan ring can trap two radicals efficiently, which is a very effective feature, as pointed out by Spiteller (23). Lipid peroxidation, which is the initial event of inflammation or oxidative stress, would be significantly inhibited by these natural fatty acids. Considering these in vitro activities, F-acids may show potent anti-inflammatory activity in vivo. However, the in vivo activity of F-acids has not been previously reported, probably due to the difficulty in obtaining the materials either in nature or by chemical synthesis. Taking advantage of the semisynthetic route from the furan dicarboxylic acid, the shark metabolite of F-acid, we tried to examine the anti-inflammatory activity of the F6 ethyl ester in comparison with that of the EPA ethyl ester.
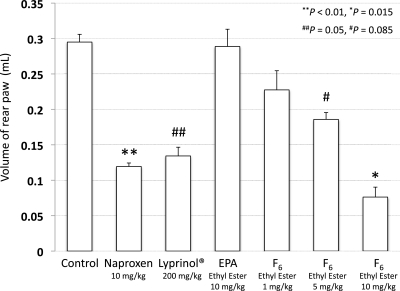
The anti-inflammatory effect of Lyprinol was previously examined in various assay systems, including a rat model of adjuvant-induced arthritis (9) and a mouse model of bowel disease (10) in vivo and leukotriene biosynthesis by human polymorphonuclear leukocytes (7) and cyclooxygenase inhibition (8) in vitro. Among them, we used the rat model with adjuvant-induced arthritis, because the potent anti-inflammatory activity of Lyprinol had previously been assessed in this model after oral administration. Whitehouse and coworkers reported that rear paw swelling in the rat model was almost completely inhibited at a dose of 20 mg/kg when administered orally for 16 d (9). According to their procedure, female Wistar rats were sensitized by injecting Mycobacterium butyricum with squalene into the right hind limb. At day 10 after arthritis induction, test materials were administered orally for 5 d. The suppressive effect was determined based on the increase in the volume of the rear left paw between the beginning and the end of dosing. In our assay, Lyprinol significantly suppressed the rear paw swelling at a dose of 200 mg/kg. Similar effects were detected by the oral administration of 5 mg/kg of a commercially available NSAID, naproxen, which were comparable to those of F6 ethyl ester (between 5 and 10 mg/kg). These data revealed that F-acids were apparently one of the anti-inflammatory components of Lyprinol. Next, to examine the activity differences between PUFAs and F-acids, the assay was performed with both EPA and F6 ethyl esters. The administration of the EPA ethyl ester did not induce significant inhibition of the rear paw swelling, even at a dose of 10 mg/kg. Furthermore, the whole-body symptoms, such as red sniff and front paw, were not cured (Fig. S3). In contrast, the suppression of paw swelling by the administration of F6 ethyl ester was observed dose-dependently. The administration of 10 mg/kg of F6 ethyl ester showed 74% suppression of paw swelling, which was a more potent effect than that of EPA at the same dose (Fig. 2).
Fig. 2.
Inhibition of adjuvant-induced arthritis in Wistar rats. The ordinate is the increase in the volume of the rear left paw between the beginning (day 10) and the end of dosing (day 15). Results are means ± SEM (n = 7–18).
We have thus revealed the potent anti-inflammatory activity of F-acids in an animal model and the presence of F-acids in the lipid extract of the New Zealand green-lipped mussel. Because fish and filter feeders, such as shellfish, would lack any enzymes to generate F-acids, the contents of F-acids are probably dependent on either the consumption of the real producer, such as algae (53), or the production by intestinal bacteria (54, 55). The New Zealand green-lipped mussel is specially regarded among the other bivalves by the New Zealand coastline, suggesting that the habitat of the mussel might be crucial for the higher concentration of F-acids exerting anti-inflammatory activity. Boselli and coworkers examined the contents of F-acids in several Adriatic fishes (56), and reported that the F6 and EPA contents were positively correlated. Furthermore, the European pilchard had the highest F-acid content (30 mg/100 g fillet) among the five species studied in their report. In general, many aquatic organisms in our diets are expected to contain F-acids to some extent, but their efficient ingestion is probably limited, due to their susceptibility to processing. The New Zealand green-lipped mussel might be a preferred food for the efficient ingestion of F-acids, by consuming raw mussels or a stabilized oil extract.
In summary, this report demonstrates that F-acids are a minor component of the fatty acids in the lipid extract of the New Zealand green-lipped mussel and exhibit more potent anti-inflammatory activity than that of EPA. Although F-acids have been overshadowed by the more abundant omega-3 PUFAs in marine oils, these studies shed light on F-acids as potential anti-inflammatory agents and pave the way for more detailed examinations of the anti-inflammatory efficacy of the New Zealand green-lipped mussel as well as other food ingredients containing F-acids.
Materials and Methods
Isolation of Furan Fatty Acids from Lyprinol.
The lipid extract of the green-lipped mussel (Lyprinol; 2.98 g) was dissolved in CH2Cl2 (80 mL). The resulting pale yellow solution was hydrogenated with Pd-C (80 mg) under an H2 atmosphere (25). After 3 h of stirring, the catalyst was removed by filtration. To the colorless solution was added freshly prepared diazomethane in diethyl ether until the yellow color was retained. The methylated solution was concentrated in vacuo, and the residue was purified by silica gel flash chromatography to afford the fraction containing F4 methyl ester (0.8 mg/g; methyl-12,15-epoxy-13,14-dimethyloctadeca-12,14-dienoate) and F6 methyl ester (1.2 mg/g; methyl-12,15-epoxy-13,14-dimethyleicosa-12,14-dienoate). Alternatively, isolation from total fatty acids in Lyprinol was performed after methanolysis with sodium methoxide as described in the following methods.
Isolation of Furan Fatty Acids from Salmon Testicle.
The salmon were captured in the Yambetsu River in Hokkaido, Japan, in late September 2007. Freeze-dried testicle (5 g) was dissolved in CHCl3 (10 mL) and MeOH (10 mL). To the solution was added freshly prepared sodium methoxide (1 M, 10 mL). After stirring for 20 min, the mixture was treated with 1 M HCl (100 mL) and extracted with CHCl3. The organic layer was washed with brine and dried over Na2SO4. The resulting solution was further methylated with a diethyl ether solution of CH2N2, until all free fatty acids were completely methylated. The resulting solution was concentrated in vacuo and purified by silica gel flash chromatography (3.0 × 10 cm), eluted with hexane, hexane:diethyl ether (99:1), hexane:diethyl ether (98:2), and hexane:diethyl ether (97:3). This solvent system separates the saturated and unsaturated fatty acid methyl esters. To remove the concomitant unsaturated fatty acid methyl esters, the fraction was hydrogenated with Pd-C (50 mg) under an H2 atmosphere for 5 min, and then purified again by silica gel flash chromatography to yield the fractions containing F4 and F6 methyl esters (12 mg).
Quantitative Analysis of Furan Fatty Acids.
Lipid extract of the New Zealand green-lipped mussel (Lyprinol; 2.0 g) was dissolved in MeOH:CHCl3 (1:1, 20 mL). The mixture was treated with freshly prepared 1 M sodium methoxide (10 mL), and the resulting solution was partitioned between 1 M HCl and CHCl3. The organic layer was washed with saturated aqueous NaCl, dried over Na2SO4, and filtered to yield a pale yellow solution. Subsequently, the remaining free fatty acids were completely methylated by a treatment with an ethereal solution of CH2N2. After concentration in vacuo, the resulting brown oil was purified by silica gel flash chromatography. The furan fatty acid methyl esters were eluted with the unsaturated fatty acid methyl ester fractions, which were combined and subjected to GC-MS analysis. GC-MS analysis was performed with a JEOL JMS-SUN200 mass spectrometer coupled to an Agilent 6890N gas chromatograph. The gas chromatograph oven temperature was increased from 120 °C to 240 °C at 24 °C/min. The peak areas of ion peaks derived from the total-ion chromatogram were subjected to quantitative analysis.
Assay for Arthritis Inhibition.
Arthritis was induced in rats by the injection of heat-killed M. butyricum (Difco) suspended in squalene (1.1 mg per rat) into the right hind paw of female Wistar rats (57). The swelling of the paw (volume) was measured daily during the experiment using a plethysmometer (MK-101P; Muromachi Kikai). The injection was given on day 1 and resulted in the onset of chronic inflammation on day 9. The therapeutic regime started on day 10 and was continued until day 14. The inhibitory activity against arthritic inflammation was measured based on the inhibition of swelling of the untreated left hind paws during days 10–15 after administration of the adjuvant.
Supplementary Material
Acknowledgments
We dedicate this paper to the memory of Mr. Masayoshi Yoshikawa, who passed away on December 25, 2009. We thank Prof. Nobuhiro Fusetani for fruitful discussions; Mr. Jim Broadband of MacLab, Australia, for the kind gift of Lyprinol; and Mr. Masayoshi Yoshikawa, Mr. Ryuki Kitamoto, and Mr. Kazuo Kitamoto for technical assistance with the salmon procurement at Shiretoko, Hokkaido, Japan. This work was financially supported by The Mochida Memorial Foundation for Medical and Pharmaceutical Research, The Mishima Kaiun Memorial Foundation, and a Grant-in-Aid for Young Scientists (B) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1110577108/-/DCSupplemental.
References
- 1.Gibson RG, Gibson SLM, Conway V, Chappell D. Perna canaliculus in the treatment of arthritis. Practitioner. 1980;224:955–960. [PubMed] [Google Scholar]
- 2.Kosuge T, Tsuji K, Ishida H, Yamaguchi T. Isolation of an anti-histaminic substance from green-lipped mussel (Perna canaliculus) Chem Pharm Bull (Tokyo) 1986;34:4825–4828. doi: 10.1248/cpb.34.4825. [DOI] [PubMed] [Google Scholar]
- 3.Murphy KJ, Mooney BD, Mann NJ, Nichols PD, Sinclair AJ. Lipid, FA, and sterol composition of New Zealand green lipped mussel (Perna canaliculus) and Tasmanian blue mussel (Mytilus edulis) Lipids. 2002;37:587–595. doi: 10.1007/s11745-002-0937-8. [DOI] [PubMed] [Google Scholar]
- 4.Wolyniak CJ, Brenna JT, Murphy KJ, Sinclair AJ. Gas chromatography-chemical ionization-mass spectrometric fatty acid analysis of a commercial supercritical carbon dioxide lipid extract from New Zealand green-lipped mussel (Perna canaliculus) Lipids. 2005;40:355–360. doi: 10.1007/s11745-006-1394-0. [DOI] [PubMed] [Google Scholar]
- 5.Halpern GM. Novel anti-inflammatory mechanism of action of Lyprinol in the AIA rat model. Prog Nutr. 2008;10:146–152. [Google Scholar]
- 6.Lee CH, Butt YKC, Wong MS, Lo SCL. Differential protein expression induced by a lipid extract of Perna canaliculus in splenocytes of rats with adjuvant-induced arthritis. Inflammopharmacology. 2008;16:188–194. doi: 10.1007/s10787-008-8010-2. [DOI] [PubMed] [Google Scholar]
- 7.Dugas B. Lyprinol inhibits LTB4 production by human monocytes. Allerg Immunol (Paris) 2000;32:284–289. [PubMed] [Google Scholar]
- 8.McPhee S, et al. Anti-cyclooxygenase effects of lipid extracts from the New Zealand green-lipped mussel, Perna canaliculus. Comp Biochem Physiol B Biochem Mol Biol. 2007;146:346–356. doi: 10.1016/j.cbpb.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 9.Whitehouse MW, et al. Anti-inflammatory activity of a lipid fraction (Lyprinol) from the NZ green-lipped mussel. Inflammopharmacology. 1997;5:237–246. doi: 10.1007/s10787-997-0002-0. [DOI] [PubMed] [Google Scholar]
- 10.Tenikoff D, Murphy KJ, Le M, Howe PR, Howarth GS. Lyprinol (stabilised lipid extract of New Zealand green-lipped mussel): A potential preventative treatment modality for inflammatory bowel disease. J Gastroenterol. 2005;40:361–365. doi: 10.1007/s00535-005-1551-x. [DOI] [PubMed] [Google Scholar]
- 11.Gibson SLM, Gibson RG. The treatment of arthritis with lipid extract of Perna canaliculus: A randomized trial. Complement Ther Med. 1998;6:122–126. [Google Scholar]
- 12.Lau CS, et al. Treatment of knee osteoarthritis with Lyprinol, lipid extract of the green-lipped mussel—A double-blind placebo-controlled study. Prog Nutr. 2004;6:17–31. [Google Scholar]
- 13.Emelyanov A, et al. Treatment of asthma with lipid extract of New Zealand green-lipped mussel: A randomised clinical trial. Eur Respir J. 2002;20:596–600. doi: 10.1183/09031936.02.02632001. [DOI] [PubMed] [Google Scholar]
- 14.Wills ED. Mechanisms of lipid peroxide formation in animal tissues. Biochem J. 1966;99:667–676. doi: 10.1042/bj0990667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galliard T. The enzymic breakdown of lipids in potato tuber by phospholipid- and galactolipid-acyl hydrolase activities and by lipoxygenase. Phytochemistry. 1970;9:1725–1734. [Google Scholar]
- 16.Spiteller G. Are lipid peroxidation processes induced by changes in the cell wall structure and how are these processes connected with diseases? Med Hypotheses. 2003;60:69–83. doi: 10.1016/s0306-9877(02)00333-x. [DOI] [PubMed] [Google Scholar]
- 17.Paredes S, et al. Antioxidant vitamins and lipid peroxidation in patients with rheumatoid arthritis: Association with inflammatory markers. J Rheumatol. 2002;29:2271–2277. [PubMed] [Google Scholar]
- 18.Darlington LG, Stone TW. Antioxidants and fatty acids in the amelioration of rheumatoid arthritis and related disorders. Br J Nutr. 2001;85:251–269. doi: 10.1079/bjn2000239. [DOI] [PubMed] [Google Scholar]
- 19.Jaswal S, Mehta HC, Sood AK, Kaur J. Antioxidant status in rheumatoid arthritis and role of antioxidant therapy. Clin Chim Acta. 2003;338:123–129. doi: 10.1016/j.cccn.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 20.Spiteller G. Peroxyl radicals are essential reagents in the oxidation steps of the Maillard reaction leading to generation of advanced glycation end products. Ann N Y Acad Sci. 2008;1126:128–133. doi: 10.1196/annals.1433.031. [DOI] [PubMed] [Google Scholar]
- 21.Adameova A, et al. Anti-atherosclerotic molecules targeting oxidative stress and inflammation. Curr Pharm Des. 2009;15:3094–3107. doi: 10.2174/138161209789058048. [DOI] [PubMed] [Google Scholar]
- 22.Glass RL, Krick TP, Echardt AE. New series of fatty acids in northern pike (Esox lucius) Lipids. 1974;9:1004–1008. doi: 10.1007/BF02533826. [DOI] [PubMed] [Google Scholar]
- 23.Spiteller G. Furan fatty acids: Occurrence, synthesis, and reactions. Are furan fatty acids responsible for the cardioprotective effects of a fish diet? Lipids. 2005;40:755–771. doi: 10.1007/s11745-005-1438-5. [DOI] [PubMed] [Google Scholar]
- 24.Spiteller G. The relation of lipid peroxidation processes with atherogenesis: A new theory on atherogenesis. Mol Nutr Food Res. 2005;49:999–1013. doi: 10.1002/mnfr.200500055. [DOI] [PubMed] [Google Scholar]
- 25.Hannemann K, et al. The common occurrence of furan fatty acids in plants. Lipids. 1989;24:296–298. doi: 10.1007/BF02535166. [DOI] [PubMed] [Google Scholar]
- 26.Glass RL, Krick TP, Sand DM, Rahn CH, Schlenk H. Furanoid fatty acids from fish lipids. Lipids. 1975;10:695–702. doi: 10.1007/BF02532763. [DOI] [PubMed] [Google Scholar]
- 27.Glass RL, Krick TP, Olson DL, Thorson RL. The occurrence and distribution of furan fatty acids in spawning male freshwater fish. Lipids. 1977;12:828–836. doi: 10.1007/BF02533272. [DOI] [PubMed] [Google Scholar]
- 28.Ishii K, Okajima H, Okada Y, Watanabe H. Studies on furan fatty acids of salmon roe phospholipids. J Biochem. 1988;103:836–839. doi: 10.1093/oxfordjournals.jbchem.a122356. [DOI] [PubMed] [Google Scholar]
- 29.Okajima H, Ishii K, Watanabe H. Studies on lipids of crayfish, Procambarus clarkii. I. Furanoid fatty acids. Chem Pharm Bull (Tokyo) 1984;32:3281–3286. doi: 10.1248/cpb.32.3281. [DOI] [PubMed] [Google Scholar]
- 30.Ishii K, Okajima H, Koyamatsu T, Okada Y, Watanabe H. The composition of furan fatty acids in the crayfish. Lipids. 1988;23:694–700. doi: 10.1007/BF02535671. [DOI] [PubMed] [Google Scholar]
- 31.Ishii K, Okajima H, Okada Y, Konishi H, Watanabe H. Fatty chain composition of phospholipids from muscle of crayfish, Procambarus clarkii. Chem Pharm Bull (Tokyo) 1989;37:1564–1567. [Google Scholar]
- 32.Ota T, Takagi T. Changes in furan fatty acids of testis lipids of chum salmon Oncorhynchus keta at spawning season. Nippon Suisan Gakkai Shi. 1990;56:153–157. [Google Scholar]
- 33.Sasaki S, Ota T, Takagi T. Composition of furan acids in the lipids of chum salmon during spawning migration. Nippon Suisan Gakkai Shi. 1989;55:2191–2197. [Google Scholar]
- 34.Ota T, Takagi T. Furan fatty acids in the lipids of the cresthead flounder. Nippon Suisan Gakkai Shi. 1992;58:721–725. [Google Scholar]
- 35.Ota T, Takagi T. Furan fatty acids of lipids from serum and sexual organs of chum salmon. Nippon Suisan Gakkai Shi. 1991;57:1565–1571. [Google Scholar]
- 36.Groweiss A, Kashman Y. A new furanoid fatty acid from the soft corals Sarcophyton glaucum and gemmatum. Experientia. 1978;34:299. [Google Scholar]
- 37.Bartelt RJ, et al. Dimethylfuran-lactone pheromone from males of Galerucella calmariensis and Galerucella pusilla. J Chem Ecol. 2006;32:693–712. doi: 10.1007/s10886-005-9026-3. [DOI] [PubMed] [Google Scholar]
- 38.Schödel R, Spiteller G. Structure elucidation of (hydroxy-oxo-cyclopentenyl)alkanoic acids, the aldol-condensation products of dioxoene acids from cattle liver. Helv Chim Acta. 1985;68:1624–1634. [Google Scholar]
- 39.Sehat N, et al. Autoxidation of the furan fatty acid ester, methyl 9,12-epoxyoctadeca-9,11-dienoate. J Am Oil Chem Soc. 1998;75:1313–1319. [Google Scholar]
- 40.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 41.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 42.Schlenk H. Crystallization of fatty acids. J Am Oil Chem Soc. 1961;38:728–736. [Google Scholar]
- 43.Rahn CH, et al. Synthesis of naturally occurring furan fatty acids. J Org Chem. 1979;44:3420–3424. [Google Scholar]
- 44.Rahn CH, Sand DM, Krick TP, Glass RL, Schlenk H. Synthesis of radioactive furan fatty acids. Lipids. 1981;16:360–364. [Google Scholar]
- 45.Lie Ken Jie MSF, Ahmad F. Conversion of linoleic and latex furanoid acid to fish C18 dimethyl furanoid isomers. J Chem Soc Chem Commun. 1981:1110–1111. [Google Scholar]
- 46.Marson CM, Harper S. Total synthesis of the naturally occurring furanoid fatty acid, F5. Tetrahedron Lett. 1998;39:333–334. [Google Scholar]
- 47.Bach T, Krüger L. Sequential Pd(0)-catalyzed reactions for the construction of multiple substituted furans. A short synthesis of the F5 furan fatty acid. Tetrahedron Lett. 1998;39:1729–1732. [Google Scholar]
- 48.Lie Ken Jie MSF, Lau MML, Lam CNW. Synthesis of novel tri- and tetrasubstituted C18 furan fatty esters. Lipids. 2003;38:1293–1297. doi: 10.1007/s11745-003-1192-8. [DOI] [PubMed] [Google Scholar]
- 49.Evans AB, Flügge S, Jones S, Knight DW, Tan WF. A new synthesis of the F5 furan fatty acid and a first synthesis of the F6 furan fatty acid. Arkivoc. 2008:95–102. [Google Scholar]
- 50.Sand DM, Schlenk H, Thoma H, Spiteller G. Catabolism of fish furan fatty acids to urofuran acids in the rat. Biochim Biophys Acta. 1983;751:455–461. doi: 10.1016/0005-2760(83)90306-5. [DOI] [PubMed] [Google Scholar]
- 51.Okada Y, et al. Antioxidant effect of naturally occurring furan fatty acids on oxidation of linoleic acid in aqueous dispersion. J Am Oil Chem Soc. 1990;67:858–862. [Google Scholar]
- 52.Okada Y, Kaneko M, Okajima H. Hydroxyl radical scavenging activity of naturally occurring furan fatty acids. Biol Pharm Bull. 1996;19:1607–1610. doi: 10.1248/bpb.19.1607. [DOI] [PubMed] [Google Scholar]
- 53.Batna A, Scheinkönig J, Spiteller G. The occurrence of furan fatty acids in Isochrysis sp. and Phaeodactylum tricornutum. Biochim Biophys Acta. 1993;1166:171–176. doi: 10.1016/0005-2760(93)90093-o. [DOI] [PubMed] [Google Scholar]
- 54.Shirasaka N, Nishi K, Shimizu S. Occurrence of a furan fatty acid in marine bacteria. Biochim Biophys Acta. 1995;1258:225–227. doi: 10.1016/0005-2760(95)00126-w. [DOI] [PubMed] [Google Scholar]
- 55.Shirasaka N, Nishi K, Shimizu S. Biosynthesis of furan fatty acids (F-acids) by a marine bacterium, Shewanella putrefaciens. Biochim Biophys Acta. 1997;1346:253–260. doi: 10.1016/s0005-2760(97)00042-8. [DOI] [PubMed] [Google Scholar]
- 56.Pacetti D, Alberti F, Boselli E, Frega NG. Characterisation of furan fatty acids in Adriatic fish. Food Chem. 2010;122:209–215. [Google Scholar]
- 57.Pearson CM, Wood FD. Studies of polyarthritis and other lesions induced in rats by injection of mycobacterial adjuvant. I. General clinical and pathologic characteristics and some modifying factors. Arthritis Rheum. 1959;2:440–449. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.