Abstract
Rab5 is a small GTPase known to regulate vesicular trafficking during interphase. Here, we show that Rab5 also plays an unexpected role during mitotic progression. RNAi-mediated silencing of Rab5 caused defects in chromosome congression and extensive prometaphase delay, and it correlated with a severe reduction in the localization of the centromere-associated protein CENP-F to kinetochores. CENP-F is a component of the nuclear matrix required for chromosome congression that, at mitotic entry, localizes to the nuclear envelope and assembles on kinetochores, contributing to the establishment of kinetochore microtubule interactions. We found that Rab5 forms a complex with a subset of CENP-F in mitotic cells and regulates the kinetics of release of CENP-F from the nuclear envelope and its accumulation on kinetochores. Simultaneous depletion of both Rab5 and CENP-F recapitulated the mitotic defects caused by silencing of either Rab5 or CENP-F alone, indicating epistatic roles for these two proteins in the pathway that orchestrates chromosome congression. These results reveal the involvement of Rab5 in the proper execution of mitotic programs whose deregulation can undermine chromosomal stability.
Keywords: endocytosis, mitosis
Rab5 is a GTPase involved in the early steps of endocytosis (1–3) and in cell motility (4, 5). In human cells, Rab5 exists in three distinct isoforms (Rab5A, Rab5B, and Rab5C) that show overlapping localization and redundant function in endocytosis (6, 7). Rab5 is mainly localized to early endosomes and controls their docking, fusion, and movement on microtubules. In its active GTP-bound form, it recruits downstream effectors that, in turn, are responsible for distinct aspects of early endosome function from signal transduction to selection and transport of cargoes (reviewed in refs. 8 and 9). In Caenorhabditis elegans, Rab5 is also required to maintain the morphology of the endoplasmic reticulum and control the kinetics of nuclear envelope disassembly (10).
Recently, many endocytic proteins have also been shown to contribute to spindle stability (11). In this regard, Rab5C has been found in an MS-based spindle inventory (12), suggesting that it might have a function at the spindle. However, the potential functional involvement of Rab5 in mitosis has not been addressed so far.
In this study, we show a role of Rab5 in chromosome congression. Our results indicate that depletion of Rab5 affects the localization of CENP-F, a transient kinetochore component required for chromosome congression, to kinetochores.
CENP-F is a protein of the nuclear matrix that accumulates during the G2 phase and is rapidly degraded after anaphase (13). Interestingly, CENP-F has no homologs in invertebrates and displays a complex pattern of localization (13, 14). At the G2/M transition, it is recruited to the nuclear envelope by the nuclear pore protein Nup133 (15). Here, CENP-F, in complex with NudE/EL, participates in centrosome tethering to the nuclear surface before nuclear envelope breakdown (15). During prophase, CENP-F assembles on maturing kinetochores, where it remains until anaphase (13, 14). At kinetochores, CENP-F contributes to the assembly of the outer plate by recruiting other kinetochore components, such as the mitotic kinesin CENP-E, and to the stability of kinetochore microtubule interactions (16–20). Several defects have been reported in mitotic cells depleted of CENP-F, including misaligned chromosomes, unstable microtubule capture at kinetochores, prometaphase delay, and aberrant chromosome segregation (16–20). In our study, all these defects were recorded to comparable levels in the Rab5-silenced cells and more importantly, also in the cells simultaneously depleted of Rab5 and CENP-F, indicating that the two proteins lie on a linear pathway. Furthermore, we found that Rab5 is in complex with CENP-F in mitosis and that it controls the kinetics of release of CENP-F from the nuclear envelope and its accumulation on kinetochores, indicating that CENP-F is a relevant target of Rab5 in chromosome congression.
Results
Rab5 Is Required for Chromosome Congression.
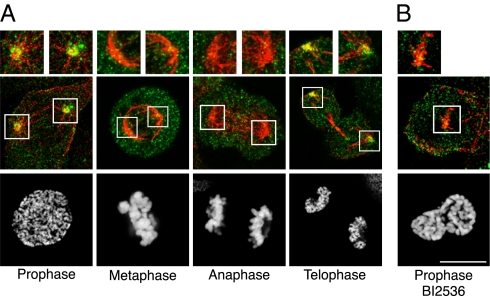
In most human cell lines, early endosomes are located beneath the plasma membrane (21). We investigated the localization of Rab5 in mitosis using the human osteosarcoma cell line U2OS stably expressing both YFP-Rab5A and mRFP–α-tubulin (to label microtubules). We noticed that Rab5-positive vesicles clustered around spindle poles at the onset of mitosis (Movie S1). The number of vesicles close to the poles abruptly diminished at prometaphase and metaphase, resuming only at late telophase (Movie S1). A similar localization was also detected for endogenous Rab5A (Fig. 1A). Accumulation of Rab5 vesicles around spindle poles in prophase might depend on the presence of astral microtubules, because treatment of cells with the Plk1 inhibitor BI2536, which abrogates prophase microtubule asters (22), prevented vesicles from clustering at poles (Fig. 1B). We also noticed that, at metaphase, some Rab5-positive vesicles moved within the spindle (Movie S2 shows a single section taken in the equatorial region of the spindle). To calculate the frequency of this event, 13 metaphase cells were video-recorded and analyzed as described in SI Materials and Methods (Movie S3 is a representative movie). The average number of Rab5 vesicles found at the spindle in an equatorial region of 3 μm was 8.6 ± 4% of their total number in the analyzed area.
Fig. 1.
Rab5 localization during mitosis. (A and B) Confocal Z projections of U2OS cells at various mitotic stages (indicated at the bottom) stained with anti-Rab5A (green), anti–α-tubulin (red) antibodies, (merged in the Middle panel), and DAPI (gray; Bottom). Enlargements of regions corresponding to spindle poles are boxed in Middle and shown in Top. (B) Prophase U2OS cell treated with 100 nM BI2536 for 2 h was stained as in A. (Scale bar: 10 μm.)
To investigate whether Rab5 could play a role in mitosis, we depleted it by siRNA-mediated silencing in U2OS cells. Inhibition of endocytosis can only be achieved by silencing all three Rab5 isoforms (7). We, therefore, simultaneously knocked down all Rab5 isoforms (Rab5-KD) in U2OS cells stably transfected with a tetracycline-inducible (TET ON) Rab5A silencing-resistant plasmid. Targeting of Rab5 by siRNAs resulted in ∼90% depletion of Rab5 proteins (Fig. 2A). This level of Rab5 ablation caused, as previously reported (7), a strong reduction in the endocytosis of the transferrin receptor (Fig. S1A). By treating the Rab5-silenced cells with doxycycline, we recovered Rab5A expression to levels comparable with endogenous Rab5A (Fig. 2A) and could rescue transferrin uptake, thereby validating our experimental model (Fig. S1A).
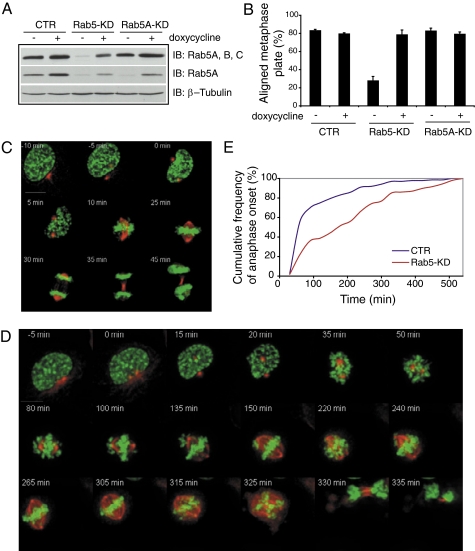
Fig. 2.
Depletion of Rab5 causes chromosome congression defects and delays mitosis. U2OS cells expressing the inducible (TET ON) Rab5A silencing-resistant plasmid were transiently transfected with control siRNA oligos (CTR), oligos for Rab5A, -B, and -C (Rab5-KD), or oligos for Rab5A alone (Rab5A-KD). Cells were treated with doxycycline (+) to express Rab5A or mock-treated (−). (A) Total cellular lysates (50 μg) were immunoblotted as indicated. (B) Cells as in A were treated with MG132 and stained with anti–α-tubulin, anti-Rab5A antibodies, and DAPI. Representative images are in Fig. S1C. The bar graph is the quantification of metaphase-arrested cells with aligned chromosomes. Mean values ± SD (n = 3, 200 cells/condition per experiment) are shown. P value for Rab5-KD vs. control is 0.0014. Statistical significance, when indicated, was ascertained by Heteroscedastic t test. (C and D) Still images from C (Movie S4; cell silenced with control oligos) and D (Movie S5; Rab5-silenced cell). Time is shown in minutes; t = 0 is defined as late prophase. (E) Cumulative frequency plot showing the time from prophase to the onset of anaphase; 139 cells in control (CTR; blue line) and 108 cells in Rab5-KD (red line) were analyzed. P value = 6.35 × 10−09.
We observed that, in mitotic Rab5-silenced cells, chromosome congression was impaired compared with control cells (Fig. S1B). To quantify this effect, we arrested the silenced cells at metaphase by treating them with the proteasome inhibitor MG132. Metaphase-arrested Rab5-depleted cells displayed severe chromosome misalignments (Fig. 2B and Fig. S1C) compared with control cells. The individual knockdown of Rab5A (Fig. 2 A and B), -B, or -C (Fig. S1 D and E) did not have any appreciable effect. Importantly, reexpression of Rab5A by doxycycline treatment rescued alignment of chromosomes on the metaphase plate, thereby excluding off-target effects (Fig. 2 A and B and Fig. S1C). These results also indicate functional redundancy among Rab5s, a phenomenon broadly reminiscent of the overlapping roles of Rab5s in endocytosis (6, 7). Of note, expression of the dominant-negative Rab5 mutant Rab5AS34N, which has preferential affinity for GDP (2), also caused chromosome uncongression, indicating that the GTPase activity of Rab5 is required for efficient chromosome alignment (Fig. S1 F–H).
To explore spindle assembly and chromosome alignment in Rab5-depleted cells, we used time-lapse videomicroscopy. We silenced the three Rab5 isoforms in U2OS cells stably expressing H2B-GFP (to label chromosomes) and mRFP–α-tubulin. Control cells assembled bipolar spindles and aligned their chromosomes on the metaphase plate. At anaphase, chromosomes segregated to the opposite poles (Fig. 2C and Movie S4).
In Rab5-silenced cells, although bipolar spindles assembled, a variable number of chromosomes remained at the poles and failed to congress (Fig. 2D and Movie S5). This finding correlated with a remarkable delay in the onset of anaphase (median = 175 min and n = 108 compared with median = 55 min and n = 139 in control cells) (Fig. 2E). However, after a variable time (Fig. 2E), cells managed to undergo anaphase and segregated their chromosomes to the two daughter cells. In some cases, it was possible to detect cells undergoing anaphase with misaligned chromosomes (an example is provided in Movie S6).
Taken together, our data indicate that Rab5 is required for chromosome congression and segregation under physiological conditions and that its functional ablation causes a significant mitotic delay.
To exclude that chromosome congression in Rab5-silenced cells could occur as a consequence of defective endocytosis, we silenced another Rab protein involved in early endocytic pathways: Rab21. Despite the fact that Rab5 and Rab21 share common effectors in endocytosis and their impairment affects endocytosis (23, 24), Rab21 depletion did not alter chromosome congression (Fig. S2), indicating that chromosome alignment is not strictly dependent on the correct execution of endocytic programs.
Reduced Tension Across Sister Kinetochores and Decreased Kinetochore Microtubule Stability in Rab5-Silenced Cells.
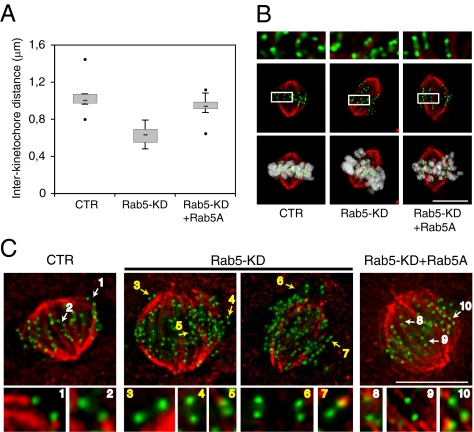
Coordinated chromosome movements largely rely on kinetochore microtubule dynamics, which in turn, are responsible for the generation of tension across sister kinetochores (25). We evaluated whether tension at kinetochores was defective in late prometaphase/metaphase-silenced cells by measuring the interkinetochore distance of kinetochore pairs. We found that interkinetochore spacing was reduced in Rab5-depleted cells compared with control cells (average = 0.63 ± 0.21 μm compared with 1.2 ± 0.27 μm in control cells) (Fig. 3 A and B). Reexpression of Rab5A in the Rab5-silenced cells recovered interkinetochore distance to control levels (average = 0.94 ± 0.26 μm) (Fig. 3 A and B). We also investigated the stability of kinetochore microtubules in the Rab5-silenced cells. Cells were briefly chilled at 4 °C to induce depolymerization of microtubules not stably attached to kinetochores. We noticed that stable kinetochore microtubules formed in the cells depleted of Rab5. Interestingly, although in control cells, kinetochores were invariably attached to microtubules, a subset of unattached kinetochores was observed in all of the Rab5-silenced cells analyzed (Fig. 3C). Reexpression of Rab5A in the Rab5-silenced cells rescued the stability of kinetochore microtubules (Fig. 3C). Overall, these data show that, in cells depleted of Rab5, strong pulling forces across kinetochores are defective and the stability of kinetochore microtubules is reduced.
Fig. 3.
Reduced tension at kinetochores and decreased stability of kinetochore microtubules in Rab5-silenced cells. U2OS cells stably expressing the inducible Rab5A silencing-resistant plasmid were silenced with control siRNA oligos (CTR) or oligos for Rab5 and mock-treated (Rab5-KD) or treated with doxycycline to reexpress Rab5A (Rab5-KD + Rab5A). (A) Box plot of Interkinetochore distance (in micrometers) measured in prometaphase–metaphase cells. Horizontal lines are the median; >60 kinetochore pairs in which both sister kinetochores were on the same focal plane were analyzed in each condition. P value = 5.1 × 10−05. (B) Representative images of cells quantified in A silenced as indicated on the bottom and stained with anti–α-tubulin (red) anticentromere, ACA (green) antibodies and DAPI (gray). (C) Images are deconvolution microscopic Z projections of cells stained with anti–α-tubulin (red) and ACA (green) antibodies. Attachment of microtubules to kinetochores was assessed as in ref. 17. The images and enlargements correspond to the merge of selected focal planes. Two representative examples of Rab5-silenced cells are shown. White arrows show biorented and attached kinetochores, and yellow arrows show defective ones. All of the kinetochores visualized in control cells displayed attached microtubules bundles; conversely, 4–10 unattached kinetochores per cell were detected in Rab5-silenced cells.
Rab5 Is Required to Localize CENP-F to Kinetochores.
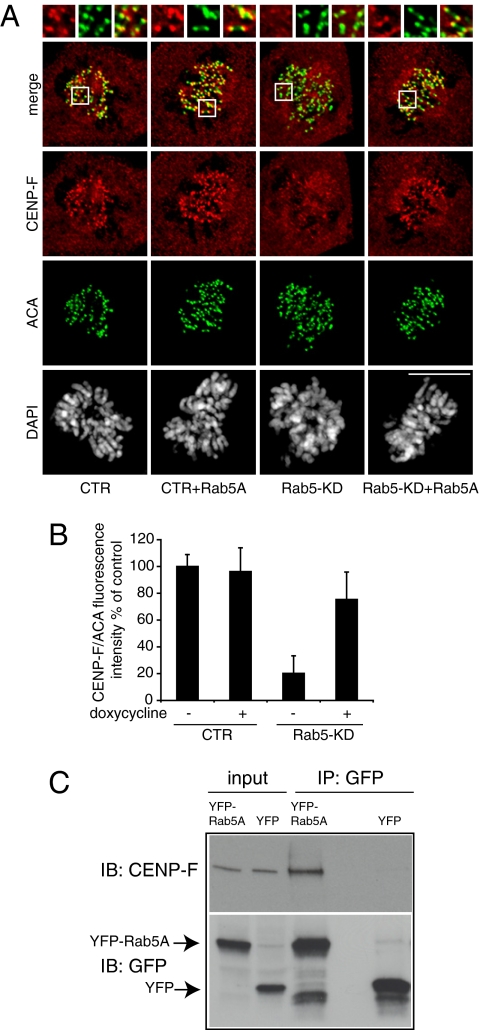
Unstable kinetochore microtubule interactions may be caused by defects in outer kinetochore assembly, particularly of those components involved in spindle microtubule capture or stabilization such as the Ndc80 complex (26) or centromere-associated proteins CENP-F (17) and CENP-E (27). Therefore, we analyzed the kinetochore localization of these proteins. The amount of CENP-F at kinetochores was severely reduced in Rab5-silenced cells compared with control cells (Fig. 4 A and B). Reexpression of Rab5A in the Rab5-silenced cells recovered CENP-F localization to kinetochores (Fig. 4 A and B). Conversely, the recruitment of HEC1, a subunit of the Ndc80 complex, whose silencing prevents the formation of stable kinetochore fibers (26), was not affected on depletion of Rab5 (Fig. S3). Of note, although HEC1 is essential for the stability of kinetochore microtubules, depletion of CENP-F does not prevent their formation, but it affects stable capture, resulting in defects (17, 18) similar to the defects observed in the Rab5-silenced cells (Fig. 3C).
Fig. 4.
Rab5 is required to efficiently localize CENP-F to kinetochores. (A) Confocal Z projections of images from cells silenced and treated as reported on the bottom and stained with anti–CENP-F (red), ACA (green) antibodies, and DAPI (gray). Enlargements of kinetochores are boxed in the merged image and shown along the top. CENP-F localization to kinetochores is reduced in Rab5-silenced cells compared with control cells and recovers, in the Rab5-silenced cells, on reexpression of Rab5A. (B) Quantification of pixel intensity (percent of control). Values represent the mean ± SEM of 30 well-resolved kinetochores in three different cells. Only cells in middle to late prometaphase were measured. P value for Rab5-KD vs. control is 0.0002. (C) CENP-F coimmunoprecipitates with Rab5. Total cellular lysates (3 mg) from mitotic U2OS cells stably expressing YFP-Rab5A or YFP alone were immunoprecipitated with anti-GFP antibody. Input = 60 μg. Immunoblot (IB) was as shown on the left. YFP-Rab5A and YFP bands are indicated by arrows.
We noticed that, in early prophase, Rab5 and CENP-F concentrated around the nuclear envelope (Fig. S4A) and that a partial overlap between Rab5 and CENP-F stainings could be detected adjacent to some kinetochores (Fig. S4A). To further investigate this finding, we took advantage of the in situ proximity ligation assay (is PLA). The high sensitivity of this technique allowed us to score positive signals in the cells probed with both anti-Rab5A and anti–CENP-F antibodies but not in cells probed with purified IgG and CENP-F antibody, which were invariably close to kinetochores (Fig. S4B). Inspired by these results, we investigated whether Rab5 could interact with CENP-F in mitosis. To this end, mitotic lysates of U2OS cell stably expressing either YFP-Rab5A or YFP were immunoprecipitated with anti-GFP antibody. A fraction of CENP-F coimmunoprecipitated with YFP-Rab5A but not with YFP alone (Fig. 4C), indicating that a pool of CENP-F is in complex with Rab5.
At late G2 phase, CENP-F localizes to the nuclear envelope, where it participates in centrosome tethering to the nuclear surface (15). We investigated whether Rab5 might be required to target CENP-F to the nuclear envelope and if it could be required for centrosomes anchoring. We readily detected, as reported in ref. 15, centrosome detachment from the nuclear envelope in the CENP-F–silenced cells but not in the Rab5-depleted cells (Fig. S5). Consistently, we noticed that, in contrast to what was observed at kinetochores (Fig. 4 A and B), Rab5 silencing did not prevent CENP-F localization to the nuclear envelope (Fig. S6A). These results show that Rab5 is not part of the pathway that, at late G2, recruits CENP-F to anchor centrosomes to the nuclear envelope.
We observed that the fluorescence intensity of CENP-F and lamin B1, a component of the nuclear lamina, was increased at the nuclear envelope of Rab5-silenced prophase cells compared with control cells (Fig. S6 A–C). This increase was not observed in the Rab5-silenced cells expressing Rab5A (Fig. S6 B and C). Because in C. elegans, silencing of Rab5 retards nuclear envelope disassembly, resulting in increased levels of B-type lamin at nuclear envelope (10), we investigated whether Rab5 might participate to nuclear envelope disassembly in mammalian cells. To this end, we analyzed the duration of prophase (from the point at which chromosome condensation becomes evident until prometaphase, when the disassembly of the nuclear envelope is complete) in silenced U2OS cells expressing H2B-GFP, and we found that this transition was slightly delayed in cells depleted of Rab5 (median = 35 min and n = 80 compared with median = 25 min and n = 84 in control cells) (Fig. S6 D and E). Together, our data suggest that Rab5, by participating in nuclear envelope disassembly, regulates the kinetics of release of CENP-F and lamin B1 from the nuclear envelope.
Of note, reduced localization of CENP-F to kinetochores in Rab5-silenced cells is unlikely to be an exclusive result of its delayed release from the nuclear envelope, because Rab5-silencing retards nuclear envelope disassembly but does not prevent it. Furthermore, CENP-F does not need to associate first with the nuclear envelope to subsequently target the kinetochore (14, 15). Hence, based on the observed Rab5–CENP-F interaction, we propose that Rab5, by entering in a complex with CENP-F, controls its accumulation at kinetochores after the completion of nuclear envelope disassembly.
Simultaneous Depletion of Rab5 and CENP-F Does Not Enhance the Mitotic Defects Caused by Silencing of Either Rab5 or CENP-F.
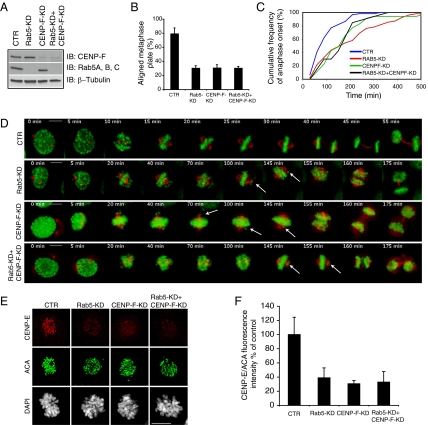
Functional ablation of CENP-F impairs chromosome congression and results in mitotic delay and aberrant anaphase chromosome segregation (16–18, 20) reminiscent of the defects that we found in the Rab5-silenced cells. Based on this parallel, the observed interaction of Rab5 with CENP-F and Rab5’s requirement for CENP-F localization to kinetochores, we knocked down CENP-F in the Rab5-silenced cells to test whether the aberrant mitotic phenotypes would be exacerbated or not. Rab5 and/or CENP-F were depleted to >80% compared with control U2OS cells by RNAi-mediated silencing (Fig. 5A). First, we scored the percentage of metaphase-arrested silenced cells with uncongressed chromosomes, and we found that it was comparable in the cells depleted of either or both Rab5 and CENP-F (Fig. 5B). Second, we investigated chromosome congression and the duration of mitosis in the silenced cells by time-lapse videomicroscopy (Fig. 5 C and D and Movie S7). Depletion of either or both Rab5 and CENP-F yielded similar defects in chromosome alignment and segregation (Fig. 5D and Movie S7). Of note, the mitotic delay, measured in cells in which both proteins were depleted, was comparable with the delay caused by single silencing of either Rab5 or CENP-F (Fig. 5C) (median in CENP-F–KD or Rab5-KD = 160 min, median in Rab5-KD + CENP-F–KD = 165 min). Third, because ablation of CENP-F affects the localization of CENP-E to kinetochores (16, 17, 28), we measured the latter parameter in midlate prometaphase cells depleted of Rab5 or CENP-F, and we found that it was decreased to similar extent regardless of whether Rab5 or CENP-F alone or both proteins were depleted (Fig. 5 E and F). These results indicate that Rab5 and CENP-F operate in the same pathway to regulate chromosome congression.
Fig. 5.
Depletion of Rab5 and/or CENP-F causes comparable chromosome alignment defects and mitotic delay. (A) Total cellular lysates (50 μg) from cells silenced as reported along the top were IB as indicated. (B) The bar graph represents the quantification of metaphase-arrested cells with aligned chromosomes silenced as in A. Mean values ± SD (n = 3, 100 cells/condition per experiment) are shown. P values ≤ 0.0057. (C) Cumulative frequency plot showing the time spent in mitosis of cells silenced as reported in the legend (>30 cells were scored in each condition; P values ≤ 0.0005). (D) Still images from Movie S7 of cells silenced as reported on the left. Time is in minutes; t = 0 is defined as late prophase. Arrows point at misaligned chromosomes. (E) Confocal Z projections of middle to late prometaphase cells silenced as reported along the top and stained with anti–CENP-E (red), ACA (green) antibodies, and DAPI (gray). (F) Quantification of pixel intensity (percent of control) showing that CENP-E localization to kinetochores is reduced to a similar extent in cells depleted of either or both Rab5 and CENP-F. Values represent the mean ± SEM of 30 well-resolved kinetochores in three different cells. P ≤ 0.009.
Discussion
In this study, we show a function of Rab5 in chromosome congression. Because Rab5 is required for chromosome alignment in Drosophila melanogaster (29) as well, this function seems to be conserved through evolution.
Our data indicate that Rab5 interacts with CENP-F in mitosis and regulates its accumulation at kinetochores. Interestingly, another Rab family member, Rab6A′, has been found to control the localization of p150Glued, one of the components that transiently associates with kinetochores (30).
Reduction in CENP-F activity affects chromosome alignment, impairs the establishment of stable kinetochore microtubule interactions, prolongs mitosis, reduces the kinetochore localization of the mitotic kinesin CENP-E, and results in chromosome missegregation at anaphase (16–20), reminiscent of the defects that we observed, to similar extent, in the Rab5-silenced cells. Despite that additional proteins are likely involved in the molecular circuitry underlying Rab5 function, the lack of additive effects in cells simultaneously depleted of Rab5 and CENP-F argues that CENP-F is an important target of Rab5 in the pathway to chromosome congression.
We show that, as reported in other model organisms (10, 29), Rab5 also modulates the kinetics of nuclear envelope disassembly in mammalian cells and participates in the release of components of the nuclear envelope required for spindle stability, such as lamin B1 and CENP-F. Given that lamin B1 promotes the formation of a fibro-membranous spindle matrix that tethers spindle assembly factors and also requires nuclear distribution protein nudE-like (NudEL), a CENP-F–interacting partner (15, 31–33), our data suggest the possibility that Rab5 might also be involved in some of the functions exerted by the spindle matrix.
In addition, considering our data and the work of Bolhy et al. (15), we propose that two pathways likely control CENP-F localization. The first pathway depends on Nup133 that, before nuclear envelope breakdown, recruits CENP-F and the NudE/EL–dynein-dynactin complex to the nuclear envelope for centrosome tethering. The second pathway requires Rab5, and it is responsible for localizing CENP-F at kinetochores from prophase to the anaphase onset to aid chromosome congression.
In conclusion, our findings point at Rab5 as an important regulator in the CENP-F–mediated mechanism that ensures the correct execution of mitotic programs, thereby guarding against chromosomal instability, one of the hallmarks of cancer.
Materials and Methods
U2OS cells were grown in DMEM supplemented with 10% FBS (Gibco). Derivatives of U2OS conditionally expressing the silencing-resistant version of Rab5A or empty vector were generated by transfecting the corresponding plasmids with lipofectamine (Invitrogen) according to the manufacturer's instructions. Stable transfectants were selected with puromycin (2 μg/mL; Sigma) and grown in DMEM supplemented with 10% Tet system-approved FBS (Clontech).
In the RNAi experiments, siRNA oligo duplexes from Dharmacon were transfected with Oligofectamine (according to manufacturer's instructions) and harvested 72–96 h after transfection. siRNA for Rab5 was previously described (7). siRNA for Rab21 was performed with Rab21-specific siGENOME SMARTpool. siRNA for CENP-F was as described in ref. 28. Double Rab5 and CENP-F silencing was performed transfecting siRNA oligos for Rab5. After 24 h, these cells were transfected with CENP-F oligos and processed 48 h later.
Expression of the Rab5A silencing-resistant construct in the inducible cell lines was achieved by adding doxycycline (2.5 μg/mL; Sigma-Aldrich) 24 h before harvesting cells. Controls were performed with siCONTROL Non-Targeting siRNA (Dharmacon).
For aligned metaphase plate measurements, cells were treated with 10 μM 26S proteasome inhibitor MG132 (Sigma-Aldrich) for 2 h before fixation.
Descriptions of expression vectors and antibodies, immunoprecipitation experiments, videomicroscopy, immunofluorescence, image quantifications, and in situ proximity ligation assays are in SI Materials and Methods.
Supplementary Material
Acknowledgments
We gratefully acknowledge Andrea Musacchio for insightful discussion and critical reading of the manuscript. We thank Luisa Capalbo and David Glover for sharing results. We also thank Guido Serini and Dario Parazzoli for excellent technological support. This work was supported by grants from the Associazione Italiana per la Ricerca sul Cancro START UP program, the European Community (FP6), the Association for International Cancer Research (AICR), and Fondazione Piemontese per la Ricerca sul Cancro Intramural Grant 2010 (to L.L.). G.S. was the recipient of a fellowship from AICR. This work was also supported by the Danish National Research Foundation, the Danish Cancer Society, the Danish Research Council, and European Commission Projects Infla-Care, DDResponse, and CZ.1.05/2.1.00/01.0300 (to S.J. and J.B.). Additional support for this work was from the Regione Piemonte, the Technological Platforms for Biotechnology (DRUIDI), the Converging Technologies (PHOENICS), the Industrial Research 2009 (BANP), the Cassa di Risparmio di Torino Foundation, and Italian Ministry of Health Oncological Research Program 2006 (to F.B. and L.L.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1103516108/-/DCSupplemental.
References
- 1.Bucci C, et al. The small GTPase rab5 functions as a regulatory factor in the early endocytic pathway. Cell. 1992;70:715–728. doi: 10.1016/0092-8674(92)90306-w. [DOI] [PubMed] [Google Scholar]
- 2.Stenmark H, et al. Inhibition of rab5 GTPase activity stimulates membrane fusion in endocytosis. EMBO J. 1994;13:1287–1296. doi: 10.1002/j.1460-2075.1994.tb06381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lanzetti L, et al. The Eps8 protein coordinates EGF receptor signalling through Rac and trafficking through Rab5. Nature. 2000;408:374–377. doi: 10.1038/35042605. [DOI] [PubMed] [Google Scholar]
- 4.Lanzetti L, Palamidessi A, Areces L, Scita G, Di Fiore PP. Rab5 is a signalling GTPase involved in actin remodelling by receptor tyrosine kinases. Nature. 2004;429:309–314. doi: 10.1038/nature02542. [DOI] [PubMed] [Google Scholar]
- 5.Palamidessi A, et al. Endocytic trafficking of Rac is required for the spatial restriction of signaling in cell migration. Cell. 2008;134:135–147. doi: 10.1016/j.cell.2008.05.034. [DOI] [PubMed] [Google Scholar]
- 6.Bucci C, et al. Co-operative regulation of endocytosis by three Rab5 isoforms. FEBS Lett. 1995;366:65–71. doi: 10.1016/0014-5793(95)00477-q. [DOI] [PubMed] [Google Scholar]
- 7.Huang F, Khvorova A, Marshall W, Sorkin A. Analysis of clathrin-mediated endocytosis of epidermal growth factor receptor by RNA interference. J Biol Chem. 2004;279:16657–16661. doi: 10.1074/jbc.C400046200. [DOI] [PubMed] [Google Scholar]
- 8.Zerial M, McBride H. Rab proteins as membrane organizers. Nat Rev Mol Cell Biol. 2001;2:107–117. doi: 10.1038/35052055. [DOI] [PubMed] [Google Scholar]
- 9.Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol. 2009;10:513–525. doi: 10.1038/nrm2728. [DOI] [PubMed] [Google Scholar]
- 10.Audhya A, Desai A, Oegema K. A role for Rab5 in structuring the endoplasmic reticulum. J Cell Biol. 2007;178:43–56. doi: 10.1083/jcb.200701139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lanzetti L, Di Fiore PP. Endocytosis and cancer: An ‘insider’ network with dangerous liaisons. Traffic. 2008;9:2011–2021. doi: 10.1111/j.1600-0854.2008.00816.x. [DOI] [PubMed] [Google Scholar]
- 12.Sauer G, et al. Proteome analysis of the human mitotic spindle. Mol Cell Proteomics. 2005;4:35–43. doi: 10.1074/mcp.M400158-MCP200. [DOI] [PubMed] [Google Scholar]
- 13.Liao H, Winkfein RJ, Mack G, Rattner JB, Yen TJ. CENP-F is a protein of the nuclear matrix that assembles onto kinetochores at late G2 and is rapidly degraded after mitosis. J Cell Biol. 1995;130:507–518. doi: 10.1083/jcb.130.3.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hussein D, Taylor SS. Farnesylation of Cenp-F is required for G2/M progression and degradation after mitosis. J Cell Sci. 2002;115:3403–3414. doi: 10.1242/jcs.115.17.3403. [DOI] [PubMed] [Google Scholar]
- 15.Bolhy S, et al. A Nup133-dependent NPC-anchored network tethers centrosomes to the nuclear envelope in prophase. J Cell Biol. 2011;192:855–871. doi: 10.1083/jcb.201007118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang Z, et al. Silencing mitosin induces misaligned chromosomes, premature chromosome decondensation before anaphase onset, and mitotic cell death. Mol Cell Biol. 2005;25:4062–4074. doi: 10.1128/MCB.25.10.4062-4074.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bomont P, Maddox P, Shah JV, Desai AB, Cleveland DW. Unstable microtubule capture at kinetochores depleted of the centromere-associated protein CENP-F. EMBO J. 2005;24:3927–3939. doi: 10.1038/sj.emboj.7600848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holt SV, et al. Silencing Cenp-F weakens centromeric cohesion, prevents chromosome alignment and activates the spindle checkpoint. J Cell Sci. 2005;118:4889–4900. doi: 10.1242/jcs.02614. [DOI] [PubMed] [Google Scholar]
- 19.Feng J, Huang H, Yen TJ. CENP-F is a novel microtubule-binding protein that is essential for kinetochore attachments and affects the duration of the mitotic checkpoint delay. Chromosoma. 2006;115:320–329. doi: 10.1007/s00412-006-0049-5. [DOI] [PubMed] [Google Scholar]
- 20.Laoukili J, et al. FoxM1 is required for execution of the mitotic programme and chromosome stability. Nat Cell Biol. 2005;7:126–136. doi: 10.1038/ncb1217. [DOI] [PubMed] [Google Scholar]
- 21.Chavrier P, Parton RG, Hauri HP, Simons K, Zerial M. Localization of low molecular weight GTP binding proteins to exocytic and endocytic compartments. Cell. 1990;62:317–329. doi: 10.1016/0092-8674(90)90369-p. [DOI] [PubMed] [Google Scholar]
- 22.Lénárt P, et al. The small-molecule inhibitor BI 2536 reveals novel insights into mitotic roles of polo-like kinase 1. Curr Biol. 2007;17:304–315. doi: 10.1016/j.cub.2006.12.046. [DOI] [PubMed] [Google Scholar]
- 23.Simpson JC, et al. A role for the small GTPase Rab21 in the early endocytic pathway. J Cell Sci. 2004;117:6297–6311. doi: 10.1242/jcs.01560. [DOI] [PubMed] [Google Scholar]
- 24.Pellinen T, et al. Small GTPase Rab21 regulates cell adhesion and controls endosomal traffic of beta1-integrins. J Cell Biol. 2006;173:767–780. doi: 10.1083/jcb.200509019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nezi L, Musacchio A. Sister chromatid tension and the spindle assembly checkpoint. Curr Opin Cell Biol. 2009;21:785–795. doi: 10.1016/j.ceb.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 26.DeLuca JG, et al. Hec1 and nuf2 are core components of the kinetochore outer plate essential for organizing microtubule attachment sites. Mol Biol Cell. 2005;16:519–531. doi: 10.1091/mbc.E04-09-0852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yao X, Abrieu A, Zheng Y, Sullivan KF, Cleveland DW. CENP-E forms a link between attachment of spindle microtubules to kinetochores and the mitotic checkpoint. Nat Cell Biol. 2000;2:484–491. doi: 10.1038/35019518. [DOI] [PubMed] [Google Scholar]
- 28.Johnson VL, Scott MI, Holt SV, Hussein D, Taylor SS. Bub1 is required for kinetochore localization of BubR1, Cenp-E, Cenp-F and Mad2, and chromosome congression. J Cell Sci. 2004;117:1577–1589. doi: 10.1242/jcs.01006. [DOI] [PubMed] [Google Scholar]
- 29.Capalbo L, D'Avino PP, Archambault V, Glover DM. Rab5 GTPase controls chromosome alignment through Lamin disassembly and relocation of the NuMA-like protein Mud to the poles during mitosis. Proc Natl Acad Sci USA. 2011;108:17343–17348. doi: 10.1073/pnas.1103720108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miserey-Lenkei S, et al. A role for the Rab6A’ GTPase in the inactivation of the Mad2-spindle checkpoint. EMBO J. 2006;25:278–289. doi: 10.1038/sj.emboj.7600929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vergnolle MA, Taylor SS. Cenp-F links kinetochores to Ndel1/Nde1/Lis1/dynein microtubule motor complexes. Curr Biol. 2007;17:1173–1179. doi: 10.1016/j.cub.2007.05.077. [DOI] [PubMed] [Google Scholar]
- 32.Tsai MY, et al. A mitotic lamin B matrix induced by RanGTP required for spindle assembly. Science. 2006;311:1887–1893. doi: 10.1126/science.1122771. [DOI] [PubMed] [Google Scholar]
- 33.Ma L, et al. Requirement for Nudel and dynein for assembly of the lamin B spindle matrix. Nat Cell Biol. 2009;11:247–256. doi: 10.1038/ncb1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.