Abstract
Autobiographical memories in our lives are critically dependent on temporal lobe structures. However, the contribution of CA1 neurons in the human hippocampus to the retrieval of episodic autobiographical memory remains elusive. In patients with a rare acute transient global amnesia, highly focal lesions confined to the CA1 field of the hippocampus can be detected on MRI. We studied the effect of these lesions on autobiographical memory using a detailed autobiographical interview including the remember/know procedure. In 14 of 16 patients, focal lesions in the CA1 sector of the hippocampal cornu ammonis were detected. Autobiographical memory was significantly affected over all time periods, including memory for remote periods. Impairment of episodic memory and autonoetic consciousness exhibited a strong temporal gradient extending 30 to 40 y into the past. These results highlight the distinct and critical role of human hippocampal CA1 neurons in autobiographical memory retrieval and for re-experiencing detailed episodic memories.
Autobiographical memory is one of the multifaceted forms of human memory that constitutes a major source for self-identity, self-continuity, and self-awareness of an individual's life. Episodic autobiographical recollection includes conscious awareness for specific episodic situations embedded in time and space, and the ability of mental reliving of episodes of a person's history along the subjective and mental time axis (1). Considering the phenomenological experience of remembering, episodic recollection involving re-experiencing requires autonoetic consciousness in contrast to semantic knowledge of memory, which is associated with noetic consciousness (2).
It is generally accepted that the hippocampus plays a critical role in episodic memory, including autobiographical memory, as neuroimaging data show hippocampal activation in autobiographical memory retrieval tasks, supporting that the hippocampus is an integral structure of the autobiographical network (3, 4). The involvement of the hippocampus culminates in the question of the temporal contribution of the hippocampal-neocortical interaction in the consolidation and recollection of remote episodic memory (5). One view, according to the standard model of consolidation, considers the hippocampus as a temporary relay structure that facilitates the transition of newly encoded hippocampus-dependant memory traces to its permanent storage and consolidation in neocortical structures, resulting in hippocampus-independent remote memories (6). This transitory role of the hippocampus is thought to be reflected in the temporal gradient of retrograde amnesia seen in patients with lesions to the medial temporal lobe (MTL). Evidence for this time-limited role of the hippocampus has come from lesion studies of amnestic patients and neuroimaging studies (7, 8). In contrast, the multiple trace theory (MTT) postulates a permanent contribution of the hippocampus in retrieving and recollection in terms of a binding module for episodic memories (9). This view proposes a creation and proliferation of a new memory trace whenever a memory gets reactivated, involving a joint ensemble of both hippocampal and neocortical neurons. Although hippocampal cornu ammonis (CA1) neurons in rodents play a pivotal role in the processing of hippocampus-dependent memory, the contribution of CA1 neurons in the human hippocampus to autobiographical memory and autonoetic consciousness remains elusive because of the rareness of a human model (10). A selective disruption of hippocampal circuits might allow deeper insights into the functional anatomy and processing of autobiographical information.
The transient global amnesia (TGA) is a unique amnestic syndrome that is characterized by a rapid onset of an antero- and retrograde amnesia (11). A TGA usually lasts 6 to 10 h but is formally limited up to 24 h. In TGA patients, highly focal lesions confined to the CA1 field of the hippocampal cornu ammonis can be detected in high-resolution MRI 24 to 72 h after the amnestic phase (12, 13). These MRI lesions can be considered the structural correlate of the amnestic deficit reflecting a transient diaschisis of CA1-dependent circuits in terms of functional disconnection of the hippocampus (11, 12, 14). Here, we studied the effect of selective, acute, and focal lesions of hippocampal CA1 neurons on episodic autobiographical memory and autonoetic consciousness in patients with an acute TGA. Some of the results have been published in abstract form (15).
Results
A total of 16 patients [11 women, 5 men, 68 ± 7 y (mean ± SD)] were included in the study and compared with 10 healthy controls (5 women, 5 men, mean 67.0 ± 7.1 y) (Table 1). The mean duration of attacks was 8.3 ± 1.9 h.
Table 1.
Clinical characteristics of the patients with TGA, including the distribution of MR-lesions
| Patient no. | Sex | Age (y) | Duration TGA (h) | Circumstances of onset/precipitating event | No. of CA1 lesions | Location of lesion |
| 1 | M | 64 | 10 | Shopping | 1 | R mid |
| 2 | M | 57 | 10 | Postcoital | 2 | L mid/ R mid |
| 3 | F | 64 | 8 | Emotional upset (argument), straining | 0 | — |
| 4 | F | 69 | 10 | Lifting heavy weights, straining | 1 | R mid |
| 5 | F | 74 | 9 | After vomiting | 1 | L ant |
| 6 | F | 65 | 6 | Emotional upset (seeing grandchild for the first time) | 2 | L mid/ R mid |
| 7 | F | 66 | 10 | Swimming in a lake | 1 | R mid |
| 8 | M | 66 | 9 | Postcoital | 1 | R post |
| 9 | F | 66 | 10 | After cycling | 2 | L ant/ L post |
| 10 | F | 72 | 9 | Gardening | 1 | L ant |
| 11 | M | 84 | 9 | Conversation | 1 | L ant |
| 12 | F | 56 | 8 | Office work | 0 | — |
| 13 | F | 65 | 9 | After febrile infection, emotional upset | 1 | L ant |
| 14 | F | 70 | 6 | After breakfast | 1 | R ant |
| 15 | M | 68 | 4 | After renovating | 1 | L mid |
| 16 | F | 78 | 6 | Pretravel arousal | 1 | R ant |
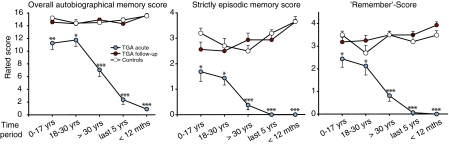
Overall Autobiographical Memory Score.
Acute patients exhibited a temporal gradient with a complete retrograde amnesia in the recent time periods. Recollection gradually improved to the first two episodes encompassing the first 30 y of life. Recollection in the first two episodes was significantly different from the level of the follow-up testing and the scores of the control group (Fig. 1).
Fig. 1.
Results on the (Left) overall autobiographical memory score, (Center) the strictly episodic score, and the (Right) remember-score over the time periods for patients in the acute episode (n = 16), during follow-up, and controls (n = 10). Mean ± SEM. Post hoc pair-wise comparisons (paired-samples t tests) between acute patients and follow-up per time period. *P < 0.05, **P < 0.01, ***P < 0.001.
TGA acute vs. follow-up.
An ANOVA yielded significant effects of TGA state (acute phase vs. follow up) [F(1,15) = 203.33, P < 0.0001], as well as an effect of time period [F(4,60) = 35.99, P < 0.0001] and an interaction effect of TGA state × time period [F(4,60) = 57.17, P < 0.0001]. Post hoc pair-wise comparisons showed differences in the acute to the follow-up every time.
TGA acute vs. controls.
We found significant effects of group: [F(1,24) = 109.07, P < 0.0001], time period [F(4,96) = 27.82 P < 0.0001] and an interaction effect of group × time period [F(4,96) = 34.077, P < 0.0001]. Post hoc comparisons between acute patients and controls were significantly different except for the time period 18 to 30 y (P = 0.06).
Follow-up vs. controls.
An ANOVA showed a nonsignificant effect of group but a significant effect of time period [F(4,96) = 4.14 P = 0.004]. The interaction effect group × time period was not significant. Post hoc comparisons between controls and follow-up were not significant.
Strictly Episodic Memory.
TGA acute vs. follow-up.
We found a significant effect of TGA state [F(1,24) = 327.25, P < 0.0001], an effect of time period [F(4,96) = 2.63, P < 0.043], and an interaction effect of TGA state × time period [F(4,96) = 17.40, P < 0.0001]. Post hoc comparisons showed that performances in the acute phase significantly differed from those in the follow-up in every time period (Fig. 1).
TGA acute vs. controls.
An ANOVA showed the same pattern: effects of group [F(1,24) = 150.53 P < 0.0001], and time period [F(2,96) = 6.75, P < 0.0001], as well as an interaction effect of group × time period [F(2,96) = 11.68, P < 0.0001]. Post hoc pair-wise comparisons showed that performances in the acute phase significantly differed to controls in every time period.
Follow-up vs. controls.
There were no differences among groups, a significant effect of time period [F(4,96) = 5.86, P < 0.0001] and no significant interaction effect group × time period. Post hoc comparisons between follow-up and controls were not significant.
Remember Responses Score.
TGA acute vs. follow-up.
In the remember responses score, significant effects of TGA state [F(1,24) = 237.15, P < 0.0001], time period [F(4,96) = 7.71 P < 0.0001], and an interaction effect of TGA state × time period [F(4,96) = 27.49, P < 0.0001] could be shown. Post hoc pair-wise comparisons showed that performances in the acute phase significantly differed from those in the follow-up in every time period (Fig. 1).
TGA acute vs. controls.
There were also significant effects of group [F(1,24) = 103.99, P < 0.0001], time period [F(2,96) = 8.71, P < 0.0001], and an interaction effect of group × time period [F(2,96) = 12.49, P < 0.0001]. Post hoc comparisons between acute patients and controls were significantly different, except for the time period 18 to 30 y old (P = 0.32).
Follow-up vs. controls.
An ANOVA yielded no significant effects of group, a significant effect of time period [F(4,96) = 2.97, P = 0.02], but no interaction effect group × time period. Post hoc comparisons were not significant.
TGA duration, lesion size, or laterality of lesions did not correlate with verbal and spatial learning in the acute phase. There was no correlation between the severity of the TGA and performance in the general cognitive domains. Because of pronounced floor effects there was no correlation between the extent of anterograde amnesia [Rey auditory verbal learning test (RAVLT) delayed recall] and the extent of retrograde amnesia (periods 3 and 4 in the autobiographical memory interview). Overall autobiographical memory and the strictly episodic memory scores were correlated (r = 0.733; P < 0.001). When subtracting the strictly episodic score from the overall autobiographical memory score, the remaining score shows a temporal gradient (P1 = 4.5; P2 = 6.0; P3 = 5.56; P4 = 2.38; P5 = 0.78, P < 0.001 ANOVA). Patients with a lesion located at the anterior hippocampus showed a more impaired autobiographical memory retrieval in recent time periods P3 to P5 than patients without lesions located anteriorly [P3 (≥ 30 y): 4.83 ± 3.84 vs. 8.40 ± 4.25; P4 (last 5 y): 1.50 ± 1.97 vs. 2.90 ± 3.38; P5 (last 12 mo): 0.57 ± 0.89 vs. 1.09 ± 0.91) (16). Acute patients were profoundly impaired in verbal and visoconstructive memory tests (Fig. 2). Patients did not differ from controls in naming and conceptual knowledge, general intellectual abilities, and visual attention in the follow-up testing (Table 2). All participants were right handed (laterality quotient patients: 90.5 ± 16.9; controls: 96.3 ± 7.6; mean ± SD; P > 0.05).
Fig. 2.
Verbal and visuo-spatial memory results of the RAVLT and the Rey-Osterrieth complex figure of TGA patients (acute and follow-up) and controls (mean ± SD). P values from independent-samples t tests. P values from paired-samples t tests; **P < 0.01.
Table 2.
General neuropsychometric results of patients with a TGA (follow-up) vs. controls (mean ± SD)
| TGA | Controls | t (df = 22) | P | |
| Age | 68 ± 7 | 67 ± 7 | 0.26 | 0.79 |
| TMT-A | 44.4 ± 9.5 | 40.4 ± 12.4 | 0.93 | 0.36 |
| TMT-B | 99.9 ± 23.7 | 102.0 ± 16.7 | −0.24 | 0.81 |
| MWT-B | 32.3 ± 2.3 | 33.2 ± 1.8 | −1.03 | 0.31 |
| RWT-S | 20.0 ± 4.2 | 21.6 ± 3.0 | −1.04 | 0.31 |
| RWT-P | 18.3 ± 5.9 | 16.8 ± 4.3 | 0.67 | 0.51 |
MWT-B, equivalent of the NART-Test; TMT, Trail-making test; RWT, Regensburg word fluency test.
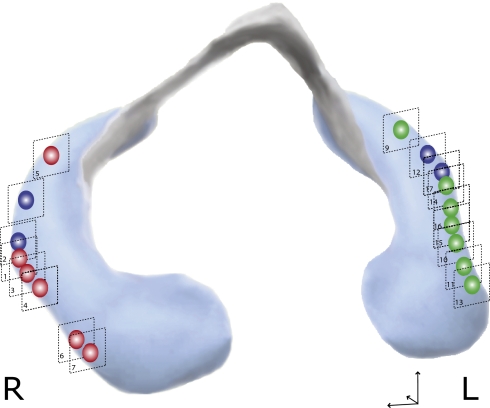
Fourteen of 16 patients had a total of 17 hippocampal lesions in a time window of 48 to 72 h after onset of symptoms (Fig. 3 and Table 1). The average size of CA1 lesions in its maximal extension in the coronal plane was 5.6 ± 2.3 mm2 (right side 5.0 ± 1.7 mm2; left side 6.5 ± 3.7 mm2; mean ± SD). A detailed analysis of the distribution of lesion showed that all lesions were selectively found in the area corresponding to the CA1 sector of the hippocampal cornu ammonis (Fig. 4). The number and laterality of lesions was not correlated with the number of vascular risk factors, the duration of the amnesia, age, and sex. A detailed whole-brain study did not show diffusion lesions outside the hippocampus (Fig. 5).
Fig. 3.
Three-dimensional model of the hippocampus showing the anterior-posterior distribution of hippocampal CA1 lesions. Lesions were located in the lateral hippocampus and were distributed along the anterior-posterior axis of the hippocampus. Green lesions indicate left lesions, red lesions show lesions in the right hippocampus, and blue shows two patients with bilateral lesions. Template modified after http://www9.biostr.washington.edu/da.html, used with permission, copyright 1997 University of Washington, USA.
Fig. 4.
(A) Anatomical template showing a representative coronary slice of the hippocampal cornu ammonis indicating sectors after Lorento de No. [Graphical template reproduced with permission from ref. 42 (Copyright 2005, Springer-Verlag).] (B) Synopsis of all DWI/TR2R lesions transferred to an anatomical template of the cornu ammonis. (C) MRI shows that lesions were confined to the CA1 area of the cornu ammonis.
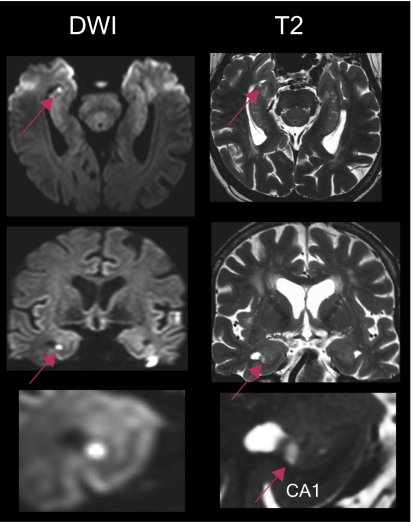
Fig. 5.
Whole-brain 3T MRI of one patient showing a focal lesion in the head of the right hippocampus. The lesion in the diffusion-weighted imaging correlates with the lesion in T2-weighted sequences (red arrow). The lesion as seen in the diffusion-weighted image can be clearly differentiated from the hyperintense susceptibility artifacts at the skull base. Imaging in the coronal plane show that the lesion is confined to the CA1 sector of the cornu ammonis (Bottom, red arrow).
Discussion
Our data show that autobiographical episodic memory in patients with TGA is affected over all time periods, including memory for remote periods. The memory loss is characterized by a pronounced temporal gradient. Because MRI lesions in these patients are confined to the Sommer sector, we suggest that human CA1 neurons are essential for the retrieval of remote episodic memory and are important for autonoetic consciousness.
Autobiographical memory is a theoretical concept referring to the remembering of personal events of an individual's life. The events consist of specific episodic situations embedded in time and space and include the ability of a vivid mental reliving. Autobiographical memory thus refers to autonoetic consciousness of a person's history along the subjective time axis and is bound to a sense of self-continuity, reliving, and emotional coloring (17).
The neuroanatomy of autobiographical memory has only recently been elucidated, describing a dynamic network that includes the MTL, posterior parietal midline region, visual cortex, and prefrontal cortex (18). The findings show a dynamic interaction in the hippocampal-neocortical connectivity upon retrieval of autobiographical memories (19, 20). The insight into the functional role of the hippocampus and the MTL in the processing of autobiographical memory has been greatly advanced by studying the amnestic deficit of patients with lesions to the hippocampus (10). In particular, the finding of a temporal gradient of retrograde amnesia has attracted much attention, leading to the assumption of a time-limited role of the hippocampus in the incremental storage of memories in extrahippocampal and neocortical structures in the process of systems consolidation. An alternative model suggests a permanent role of the hippocampus in the retrieval of autobiographical memory within a dynamically distributed hippocampal-neocortical network (9). The extension and the nature of the temporal gradient of the retrograde amnesia has been considered a correlate of the involvement of the hippocampus in the systems consolidation and retrieval of episodic memory (5). It thus has been suggested that the extent of retrograde amnesia is dependent on the degree of the damage to the hippocampus, extending from the last 2 y to more than several decades after extensive damage of the hippocampal formation (7). Memory deficits observed in hippocampal and MTL lesion studies, however, vary considerably across cases depending on the location and extent of lesions as well as methods of testing (6, 21). A considerable amount of studies failed to observe a temporally graded memory impairment (9).
Besides the hippocampus, recent neuroimaging results observed an activation of the MTL and neocortical regions in autobiographical memory retrieval irrespective the recentness or remoteness of memories (20, 22, 23). Frequently an activation of both hippocampi has been observed, whereas in some studies a laterality effect was detected. The right hippocampus exhibited a temporal gradient correlated with the remoteness of autobiographical memories, whereas the left hippocampus showed a constant activation, implying a permanent contribution to remembering autobiographical episodes along the time axis (24–26). On the other hand, a large body of studies did show a temporal gradient of hippocampal activation in autobiographical memory in terms of a disengagement of the hippocampus after a retention interval longer than 5 y, which was taken as evidence for a time-limited role of the hippocampus in systems consolidation (8, 27–29).
With regard to the retrograde amnesia in TGA, our observation is compatible with earlier reports in which a temporally graded deficit, including memory for autobiographical and public events in patients with a TGA, was observed (30, 31). This finding was initially taken as an impairment of MTL structures in TGA, thus anticipating later imaging findings. Some studies with TGA patients yielded no graded autobiographical memory loss in the sense of Ribot, possibly because of the variability of methods and testing, the temporal dynamics of the recovery from TGA, and hence the time point of testing (32). Notably, similar to our results, earlier studies in TGA also observed a temporal gradient with regard to semantic memory compatible with the possibility that hippocampal lesions also gradually affect semantic memory (30–34). Using PET, patients with Alzheimer's disease show a temporally graded autobiographical amnesia reflected in a gradual disengagement of the hippocampus in the recall of autobiographical memories for remote memories (29). In the early stages of Alzheimer's disease, a preferential degeneration of CA1 neurons has been shown in pathology studies, as well as in high-resolution volumetric imaging (35).
The finding of a pronounced temporal gradient of episodic autobiographical memory in TGA can be reconciled with the framework of the standard consolidation model in terms of a temporary involvement of the hippocampus in systems consolidation (10). The behavioral predictions of two models of systems memory consolidation—the standard consolidation model and the multiple trace theory—are often compared with regard to the temporal gradient of retrograde amnesia. Within the MTT model, it has been suggested that each retrieval and reactivation of an episodic memory leads to the formation of additional, hippocampus-dependent memory traces, so that memories retrieved more often, such as remote memories, will have more traces and thus exhibit a certain resilience against hippocampal damage. Thus, according to the MTT and standard model of consolidation, partial hippocampal damage, such as in TGA, may result in a temporally graded retrograde amnesia (5).
The lack of lateralization effects of CA1 lesions is possibly because of the fact that the TGA patients were older and an acknowledgment that hemispheral asymmetry is reduced during aging (36), leading to a bilateral hippocampal activation (19, 26). A differential antero-posterior activation of the hippocampus according to memory remoteness was shown inasmuch that activations belonging to recent memories are located in the anterior end of the hippocampus, whereas remote memories are distributed along the anterior-posterior axis (16). This finding is somewhat corroborated by our data, as patients with a lesion located at the anterior hippocampus showed a greater impairment in remote autobiographical memory.
Our results show that autonoetic consciousness is closely coupled to the recollection of episodic memory and suggest that CA1 neurons are critically involved in the conscious recollection of episodic memory. As in TGA, a similar temporal gradient of autonoetic consciousness is seen in patients with Alzheimer's disease (37), although other neuroimaging data suggest an invariant activation of the hippocampus during autonoetic consciousness (26).
Within the hippocampal memory system, CA1 neurons are critically involved the formation, consolidation, and retrieval of hippocampal-dependant memories. Recent high-resolution volumetry did show an association between CA1 volume and episodic memory retrieval (38). The impairment of autobiographical memory retrieval during the acute TGA thus mirrors a profound but transient impairment of CA1-dependent hippocampal function leading to an acute diaschisis and reduced connectivity within this network. This finding suggests that hippocampal CA1 neurons might have an important long-term role in the retrieval of remote memories. Indeed, neuroimaging findings suggest that lesions to the MTL impair the connectivity within the autobiographical network (39).
The functional impairment of CA1 lesions in the present study was based on the detection of hyperintense diffusion lesions that mirror an impaired cellular metabolism. With regard to the heterogenous literature regarding effects of hippocampal lesions on episodic memory, obviously factors such as extension of the lesion, covert lesions, and type of noxious impact may contribute to some of the controversies in the literature. These controversies might also refer to the observation concerning the magnitude of the amnestic deficit, inasmuch as acute focal CA1 lesions in TGA produce a much more profound impairment then do full bilateral or bilateral CA1 lesions. The difference of the impact of CA1 lesions in acute and chronic models is most likely a reflection of the plasticity in the hippocampal circuitry because functional reorganization and compensatory mechanisms are time-dependent. Patients in our study were tested a few hours after onset, whereas in most studies chronic patients have been tested. Diffusion-weighted imaging (DWI) is a very sensitive method for detecting impairments in cellular diffusion, making it unlikely that covert lesions went undetected. Using focal MR-spectroscopy (14), perfusion- and diffusion-weighted imaging (13, 40), and single-photon emission computed tomography (41), it could be shown that CA1 lesions in TGA are highly focal and not a peak reflection of global metabolic changes in the MTL.
In summary, we have provided evidence that autobiographical episodic memory in patients with a hippocampal CA1 lesion is significantly affected. This finding suggests that human CA1 neurons are essential for the retrieval of remote episodic memory and that they are important for autonoetic consciousness.
Methods
Study Cohort.
Patients presenting to our Neurological emergency unit and fulfilling established diagnostic criteria of a TGA were studied (11): (i) the presence of an anterograde amnesia, (ii) witnessed by an observer, (iii) no clouding of consciousness or loss of personal identity, (iv) cognitive impairment limited to amnesia, (v) no focal neurological or epileptic signs, (vi) no recent history of head trauma or seizures, and (vii) resolution of symptoms within 24 h. Patients were studied by one neurologist who remained on-call for this study 24/7. Age-matched healthy subjects were recruited as controls. Every participant gave informed consent to the study, which was approved by the Ethical Committee of the University of Kiel and which was conducted according to the Declaration of Helsinki. All patients had a standard neurological examination on admission and follow-up and underwent a structured interview to assess vascular and nonvascular risk factors and a history of cardiovascular and neurological diseases (Table 1).
General Neuropsychometric Evaluation.
Neuropsychological assessment was performed in the acute episode (< 4 h after onset) and at follow-up when patients were fully recovered from all TGA symptoms [controls 14 ± 1.2 d; TGA 15 ± 3.6 d, (mean ± SD)]. The test battery in the acute phase included the RAVLT and the Rey-Osterrieth/Taylor Complex Figure Test. During follow-up, memory was tested again using alternative versions of the RAVLT and the Complex Figure test. Naming and conceptual knowledge was tested using the Regensburg Word Fluency Test (RWT). Premorbid general intellectual ability was obtained using an equivalent of the National Adult Reading Test (NART). Attentional performance, cognitive speed, and flexibility was assessed using the Trail-Making Test A and B (TMT). Handedness was assessed by the Edinburgh Handedness Inventory. The level of education was graded according to the time needed for the achievement of the high-school diploma (13 y) or prediploma (10 y). In the patient and control group, six subjects featured 13 y of education.
Autobiographical Memory Assessment.
Episodic autobiographical memory.
For testing autobiographical memory an established questionnaire was used (25) assessing episodic memory over five specific time periods across the entire life span: (0–17 y old; 18–30 y old; ≥ 30 y old apart from the last 5 y; the last 5 y excluding the last 12 mo; and the last 12 mo). For each time period (except for the last 12 mo) subjects were asked to describe a detailed (i.e., information about the temporal and spatial context) specific event related to four topics: (i) a meeting or an event linked to a person, (ii) a job-related event, (iii) a trip or a journey, and (iv) a family event. For the fifth time period (the last 12 mo), subjects were asked to give a detailed description of seven fixed points of time: (i) last summer, (ii) Christmas or New Year's Day, (iii) last week, (iv) last weekend, (v) 2 d ago, (vi) yesterday, (vii) today. This approach allows a chronological reconstruction of retrograde amnesia extending to the most recent time period. As patients were assessed within 4 h after onset of the amnestic TGA interval, the protocol used here focused on the episodic memory domain of patients with an acute TGA when the retention span was limited to a few minutes. Because of the acuteness, we had to use a modified and simplified version of the protocol used by Piolino et al (25).
State of consciousness.
The episodic memory system is reflected in two different kinds of consciousness. The semantic system corresponds to a “noetic consciousness,” implicating the mere awareness of the internal and external world or simply “knowing” (17). The “autonoetic consciousness” of episodic memory incorporates the placement of one's own identity in the subjective time line, a sense of a vivid self-recollection connected with feelings, impressions, and perceptions that were distinctive for the remembered situation and the ability for a mental “time travel.” Noetic and autonoetic consciousness was assessed using the remember/know paradigm. To differentiate the episodicity of the subjects’ answers we distinguished between the following judgment categories: (i) Remember: if subjects were able to relive the accordant episode with surrounding context factors, they were requested to denote the answer as “remembered” (r); (ii) Know: An answer was annotated as “know” (k) when subjects just knew that they experienced an episode but were not able to re-experience the specific situation; (iii) Guess: If subjects just guessed (g) that the questioned episode could have been taken place but do not remember any contents or details they were asked to denote their answer as “guessed.”
Procedure.
Participants were asked to describe a detailed specific event related to four topics per time period. Instructions like “please describe a particular travel episode you experienced at the age of 0–17” were given. If the patient was not able to recollect a specific event spontaneously, cues (“a situation with your parents”) were given. A family relative was present during the acute and follow-up interview and verified reports. In the follow-up interview, patients recollected the same memory content as in the acute TGA state. We did not notice confabulations or false memories. The subjects were then asked to select one of the categories (i.e., remember, know, or guess) related to the memorized and reported event. We explained these categories carefully and in detail after each recollection and asked the subjects to decide between these categories.
Scoring.
Three different total scores per time period were constituted (25, 37):
Overall autobiographical memory score.
This score includes episodic as well as semantic types of recall. The described events were scored on a four-point episodic scale and calculated as the sum of given points per time period (37). In this score, a maximum of four points per time point could be gained and maximally four points × four topics could be reached per time period. In this episodic scale, the specificity of the content, the temporal and spatial location and the richness of details per time period were stated: (i) four points were given if the recollection was situated in time and space and comprehended many contextual details; (ii) three points were given if the recollection was situated in time and space but did not comprehend any details; (iii) two points were given if a repeated or general recollection situated in time and space was provided; (iv) one point was given if a repeated or general recollection was not situated in time and space; (v) zero points were given if there was no recollection or general information about the topic.
Strictly episodic memory score.
For differentiating overall autobiographical memories from “true episodic” information, we used the strictly episodic memory score, which takes into account all four-point answers solely. In this score, only the number of the four-point answers per time period was scored (maximally four points per time period).
Remember responses score.
As we focused on the study of episodic memory in TGA we restricted our analysis to the remember responses, similar to the approach by Piolino et al. (37). The remember responses score is calculated from the sum of “remembered”-denoted answers, devoid of “know” and “guess” answers.
Neuroradiological Study.
Standard whole-brain MRIs of patients were performed 24 to 72 h after onset of TGA symptoms when the detectability of hippocampal lesions is highest (13). High-resolution MRIs were performed on a 3T unit (Philips Intera Achieva). The following sequences were acquired: Dual TSE (proton density- and T2-weighted), DWI-Echo Planar Imaging (SE-EPI) and T2-weighted, transverse oblique plane parallel to the hippocampus and coronal perpendicular to the hippocampus with subsequent maps of the apparent diffusion coefficient (ADC), slice thickness 2 mm. All MRIs were studied with respect to structural abnormalities in the whole brain, including temporal and frontal lobe structures. Only those lesions were considered a CA1 hippocampal lesion in a consensus agreement if they were detectable in both DWI- and T2-weighted images (Fig. 3). For this agreement, hyperintense DWI and T2 lesions (and hypointense lesions on ADC maps) had to be in identical locations within the different sectors of the cornu ammonis in the coronal plane and the rostral-occipital position (Fig. 3).
Statistical Analysis.
Statistical analysis was conducted using SPSS 17.0. Parametric tests (t tests) for independent samples were used for metric measures. Repeated-measures ANOVAs including two within-subjects factors; (i) TGA state and (ii) time periods were computed in patients in the acute TGA phase versus same subjects in the follow-up. Further repeated-measures ANOVAs including one within-subjects factor (time periods) and a between-subjects factor (patients in the acute phase, patients in the follow-up, and controls) were computed. The Levene statistic was computed to test for violations of variance homogeneity across groups and conditions. Frequency tables for the dichotomous or categorical variables were analyzed by means of the Pearson Chi-Square test. Correlations between the magnitude of TGA symptoms and the behavioral measures were quantified by means of the Pearson coefficient. Post hoc pair-wise comparisons were carried out to discover differences between groups and time periods.
Acknowledgments
This study has been supported by Deutsche Forschungsgemeinschaft SFB 654 and TP A14 (to T.B.) and by the Faculty of Medicine, University of Kiel, Germany.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Conway MA, Pleydell-Pearce CW. The construction of autobiographical memories in the self-memory system. Psychol Rev. 2000;107:261–288. doi: 10.1037/0033-295x.107.2.261. [DOI] [PubMed] [Google Scholar]
- 2.Tulving E. Episodic memory: From mind to brain. Annu Rev Psychol. 2002;53:1–25. doi: 10.1146/annurev.psych.53.100901.135114. [DOI] [PubMed] [Google Scholar]
- 3.Eldridge LL, Knowlton BJ, Furmanski CS, Bookheimer SY, Engel SA. Remembering episodes: A selective role for the hippocampus during retrieval. Nat Neurosci. 2000;3:1149–1152. doi: 10.1038/80671. [DOI] [PubMed] [Google Scholar]
- 4.Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. Annu Rev Neurosci. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frankland PW, Bontempi B. The organization of recent and remote memories. Nat Rev Neurosci. 2005;6(2):119–130. doi: 10.1038/nrn1607. [DOI] [PubMed] [Google Scholar]
- 6.Squire LR, Clark RE, Knowlton BJ. Retrograde amnesia. Hippocampus. 2001;11(1):50–55. doi: 10.1002/1098-1063(2001)11:1<50::AID-HIPO1019>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 7.Rempel-Clower NL, Zola SM, Squire LR, Amaral DG. Three cases of enduring memory impairment after bilateral damage limited to the hippocampal formation. J Neurosci. 1996;16:5233–5255. doi: 10.1523/JNEUROSCI.16-16-05233.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haist F, Bowden Gore J, Mao H. Consolidation of human memory over decades revealed by functional magnetic resonance imaging. Nat Neurosci. 2001;4:1139–1145. doi: 10.1038/nn739. [DOI] [PubMed] [Google Scholar]
- 9.Winocur G, Moscovitch M, Bontempi B. Memory formation and long-term retention in humans and animals: convergence towards a transformation account of hippocampal-neocortical interactions. Neuropsychologia. 2010;48:2339–2356. doi: 10.1016/j.neuropsychologia.2010.04.016. [DOI] [PubMed] [Google Scholar]
- 10.Squire LR, Bayley PJ. The neuroscience of remote memory. Curr Opin Neurobiol. 2007;17(2):185–196. doi: 10.1016/j.conb.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bartsch T, et al. Focal lesions of human hippocampal CA1 neurons in transient global amnesia impair place memory. Science. 2010;328:1412–1415. doi: 10.1126/science.1188160. [DOI] [PubMed] [Google Scholar]
- 12.Bartsch T, et al. Selective affection of hippocampal CA-1 neurons in patients with transient global amnesia without long-term sequelae. Brain. 2006;129:2874–2884. doi: 10.1093/brain/awl248. [DOI] [PubMed] [Google Scholar]
- 13.Bartsch T, Alfke K, Deuschl G, Jansen O. Evolution of hippocampal CA-1 diffusion lesions in transient global amnesia. Ann Neurol. 2007;62:475–480. doi: 10.1002/ana.21189. [DOI] [PubMed] [Google Scholar]
- 14.Bartsch T, et al. Focal MR spectroscopy of hippocampal CA-1 lesions in transient global amnesia. Neurology. 2008;70:1030–1035. doi: 10.1212/01.wnl.0000306633.06027.33. [DOI] [PubMed] [Google Scholar]
- 15.Bartsch T, et al. Human hippocampal CA1 neurones are critically involved in autobiographical memory and autonoetic consciousness. 2010 Poster (600.15) presented at Neuroscience 2010, San Diego, CA. [Google Scholar]
- 16.Gilboa A, Winocur G, Grady CL, Hevenor SJ, Moscovitch M. Remembering our past: Functional neuroanatomy of recollection of recent and very remote personal events. Cereb Cortex. 2004;14:1214–1225. doi: 10.1093/cercor/bhh082. [DOI] [PubMed] [Google Scholar]
- 17.Tulving E. Memory and consciousness. Can Psychol. 1985;26(1):1–12. [Google Scholar]
- 18.Cabeza R, St Jacques P. Functional neuroimaging of autobiographical memory. Trends Cogn Sci. 2007;11:219–227. doi: 10.1016/j.tics.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 19.Maguire EA, Frith CD. Aging affects the engagement of the hippocampus during autobiographical memory retrieval. Brain. 2003;126:1511–1523. doi: 10.1093/brain/awg157. [DOI] [PubMed] [Google Scholar]
- 20.Viard A, et al. Patterns of hippocampal-neocortical interactions in the retrieval of episodic autobiographical memories across the entire life-span of aged adults. Hippocampus. 2010;20(1):153–165. doi: 10.1002/hipo.20601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spiers HJ, Maguire EA, Burgess N. Hippocampal amnesia. Neurocase. 2001;7:357–382. doi: 10.1076/neur.7.5.357.16245. [DOI] [PubMed] [Google Scholar]
- 22.Ryan L, et al. Hippocampal complex and retrieval of recent and very remote autobiographical memories: Evidence from functional magnetic resonance imaging in neurologically intact people. Hippocampus. 2001;11:707–714. doi: 10.1002/hipo.1086. [DOI] [PubMed] [Google Scholar]
- 23.Piolino P, et al. Reliving lifelong episodic autobiographical memories via the hippocampus: A correlative resting PET study in healthy middle-aged subjects. Hippocampus. 2008;18:445–459. doi: 10.1002/hipo.20406. [DOI] [PubMed] [Google Scholar]
- 24.Maguire EA, Frith CD. Lateral asymmetry in the hippocampal response to the remoteness of autobiographical memories. J Neurosci. 2003;23:5302–5307. doi: 10.1523/JNEUROSCI.23-12-05302.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Piolino P, et al. Autobiographical memory, autonoetic consciousness, and self-perspective in aging. Psychol Aging. 2006;21:510–525. doi: 10.1037/0882-7974.21.3.510. [DOI] [PubMed] [Google Scholar]
- 26.Viard A, et al. Hippocampal activation for autobiographical memories over the entire lifetime in healthy aged subjects: An fMRI study. Cereb Cortex. 2007;17:2453–2467. doi: 10.1093/cercor/bhl153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Niki K, Luo J. An fMRI study on the time-limited role of the medial temporal lobe in long-term topographical autobiographic memory. J Cogn Neurosci. 2002;14:500–507. doi: 10.1162/089892902317362010. [DOI] [PubMed] [Google Scholar]
- 28.Piefke M, Weiss PH, Zilles K, Markowitsch HJ, Fink GR. Differential remoteness and emotional tone modulate the neural correlates of autobiographical memory. Brain. 2003;126:650–668. doi: 10.1093/brain/awg064. [DOI] [PubMed] [Google Scholar]
- 29.Eustache F, et al. ‘In the course of time’: A PET study of the cerebral substrates of autobiographical amnesia in Alzheimer's disease. Brain. 2004;127:1549–1560. doi: 10.1093/brain/awh166. [DOI] [PubMed] [Google Scholar]
- 30.Hodges JR, Ward CD. Observations during transient global amnesia. A behavioural and neuropsychological study of five cases. Brain. 1989;112:595–620. doi: 10.1093/brain/112.3.595. [DOI] [PubMed] [Google Scholar]
- 31.Kritchevsky M, Squire LR. Transient global amnesia: Evidence for extensive, temporally graded retrograde amnesia. Neurology. 1989;39:213–218. doi: 10.1212/wnl.39.2.213. [DOI] [PubMed] [Google Scholar]
- 32.Guillery-Girard B, et al. The dynamic time course of memory recovery in transient global amnesia. J Neurol Neurosurg Psychiatry. 2004;75:1532–1540. doi: 10.1136/jnnp.2003.024968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Evans J, Wilson B, Wraight EP, Hodges JR. Neuropsychological and SPECT scan findings during and after transient global amnesia: evidence for the differential impairment of remote episodic memory. J Neurol Neurosurg Psychiatry. 1993;56:1227–1230. doi: 10.1136/jnnp.56.11.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kritchevsky M, Zouzounis J, Squire LR. Transient global amnesia and functional retrograde amnesia: Contrasting examples of episodic memory loss. Philos Trans R Soc Lond B Biol Sci. 1997;352:1747–1754. doi: 10.1098/rstb.1997.0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kerchner GA, et al. Hippocampal CA1 apical neuropil atrophy in mild Alzheimer disease visualized with 7-T MRI. Neurology. 2010;75:1381–1387. doi: 10.1212/WNL.0b013e3181f736a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dolcos F, Rice HJ, Cabeza R. Hemispheric asymmetry and aging: Right hemisphere decline or asymmetry reduction. Neurosci Biobehav Rev. 2002;26:819–825. doi: 10.1016/s0149-7634(02)00068-4. [DOI] [PubMed] [Google Scholar]
- 37.Piolino P, et al. Autobiographical memory and autonoetic consciousness: Triple dissociation in neurodegenerative diseases. Brain. 2003;126:2203–2219. doi: 10.1093/brain/awg222. [DOI] [PubMed] [Google Scholar]
- 38.Mueller SG, Chao LL, Berman B, Weiner MW. Evidence for functional specialization of hippocampal subfields detected by MR subfield volumetry on high resolution images at 4 T. Neuroimage. 2011;56:851–857. doi: 10.1016/j.neuroimage.2011.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Addis DR, Moscovitch M, McAndrews MP. Consequences of hippocampal damage across the autobiographical memory network in left temporal lobe epilepsy. Brain. 2007;130:2327–2342. doi: 10.1093/brain/awm166. [DOI] [PubMed] [Google Scholar]
- 40.Toledo M, et al. Lack of evidence for arterial ischemia in transient global amnesia. Stroke. 2008;39:476–479. doi: 10.1161/STROKEAHA.107.498303. [DOI] [PubMed] [Google Scholar]
- 41.Yang Y, et al. Cerebellar hypoperfusion during transient global amnesia: An MRI and oculographic study. J Clin Neurol. 2009;5(2):74–80. doi: 10.3988/jcn.2009.5.2.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Duvernoy HM. The Human Hippocampus. 3rd Ed. Berlin, Heidelberg: Springer Verlag; 2005. [Google Scholar]