Abstract
Successful priming of adaptive immune responses is crucially dependent on innate activation signals that convert resting antigen-presenting cells (APCs) into immunogenic ones. APCs expressing the relevant innate pattern recognition receptors can be directly activated by pathogen-associated molecular patterns (PAMPs) to become competent to prime T-cell responses. Alternatively, it has been suggested that APCs could be activated indirectly by proinflammatory mediators synthesized by PAMP-exposed cells. However, data obtained with CD4+ T cells suggest that inflammatory signals often cannot substitute for direct pattern recognition in APC activation for the priming of T helper responses. To test whether the same is true for CD8+ T cells, we studied cytotoxic T lymphocyte development in vitro and in mixed chimeric mice in which coexisting APCs can either present a preprocessed model antigen or directly recognize a given PAMP, but not both. We show that indirectly activated APCs promote antigen-specific proliferation of naïve CD8+ T cells but fail to support their survival and cytotoxic T lymphocyte differentiation. Furthermore, CD8+ T cells primed by indirectly activated APCs are unable to reject tumors. Thus, inflammation cannot substitute for direct recognition of single PAMPs in CD8+ T-cell priming. These findings have important practical implications for vaccine design, indicating that adjuvants must be judiciously chosen to trigger the relevant pattern recognition receptors in APCs.
Keywords: vaccine, dendritic cells, adjuvanticity
The induction of adaptive immune responses is controlled by innate pattern recognition receptors (PRRs) sensing pathogen-associated molecular patterns (PAMPs) (1). Antigen-presenting cells (APCs) couple innate recognition to adaptive immunity (2). The effectiveness of vaccines therefore relies on the generation of adequate innate signals to activate APCs. In the case of non-live vaccines, adjuvants containing PAMPs or synthetic analogs provide the innate impetus for APC activation. APCs expressing the relevant PRRs can be directly activated by PAMPs, or instead, indirectly by proinflammatory factors produced by a range of hematopoietic and non-hematopoietic cells upon exposure to the same PAMPs. This observation has led to the notion that inflammation can substitute for direct microbial recognition by the priming APC for the induction of adaptive immunity (3–5). In this vein, little attention is being paid to the mode of APC activation when adjuvants for vaccines and maturation stimuli for dendritic cells (DCs) immunotherapy are chosen. However, there is increasing evidence that indirectly activated APCs differ qualitatively from directly activated ones (6–8). Most strikingly, indirectly activated DCs fail to produce “signal 3” molecules needed by naïve T cells along with cognate peptide/MHC and costimulation for their effector differentiation (9). Priming of naïve CD4+ T cells by indirectly activated APCs results in the clonal expansion of T-cell populations lacking helper function (6, 8), despite the availability of signal 3 from non-presenting DCs in the vicinity. However, it is not clear whether indirectly activated DCs also lack the ability to induce cytotoxic T lymphocyte (CTL) responses, because the priming requirements for the differentiation of effector T cells from naïve CD4+ vs. CD8+ T cells are known to differ substantially (10). Here, we explored the ability of indirectly activated APCs to assist the expansion and differentiation of CD8+ T-cell populations with effector functions in vitro and in vivo. We found that indirectly activated APCs have the capacity to promote the proliferation of naïve CD8+ T cells but that this does not lead to sustained clonal expansion and does not suffice to generate effector T cells endowed with CTL properties. These findings have major implications for vaccine design and reinforce the notion that inflammation can amplify but not substitute for PRR signaling in coupling innate to adaptive immunity.
Results
Indirectly Activated APCs Fail to Promote CTL Differentiation and Survival in Vitro.
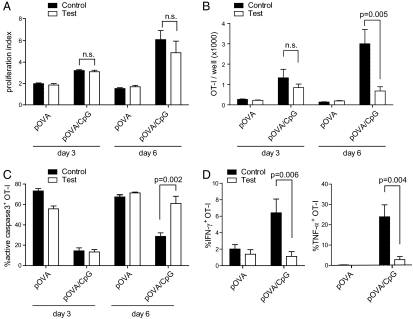
To assess the capacity of indirectly activated APCs to prime effector CD8+ T-cell responses, we started with an in vitro model. Highly purified naïve OT-I CD8+ T cells [bearing a T-cell receptor specific for ovalbumin residues 257–264 (pOVA) presented by H-2Kb] were carboxyfluorescein diacetate succinimidyl ester (CFSE)-labeled and cocultured with spleen APCs from tlr9+/+ H2Kb−/− + tlr9−/− H2Kb+/+ (test cultures) or tlr9+/+ H2Kb−/− + tlr9+/+ H2Kb+/+ mice (control cultures). Upon addition of a TLR9 agonist (CpG-containing DNA oligonucleotide), DCs in analogous cultures undergo all typical signs of maturation, including up-regulating MHC class II molecules, CD40, CD80, and CD86, but do not produce cytokines such as IL-6, IL-12/23p40, and IFN-I (6). Therefore, when pOVA is provided, the test cultures act as a model in which one can study the priming of naïve CD8+ T cells by APCs that are activated exclusively in an indirect fashion (i.e., by inflammatory mediators produced by nonpresenting cells stimulated through TLR9).
A congenic marker (CD45.1) allowed for specific gating of OT-I cells for analysis of CFSE dilution profiles at day 3 of culture and revealed that CpG substantially enhanced proliferation. This was true even when the presenting APCs were unable to directly sense the TLR9 ligand, because the OT-I cells underwent a comparable number of divisions in control and test cultures (Fig. 1A). A similar pattern was observed after 6 d of culture, when OT-I cells in CpG-supplemented control or test cultures had continued to proliferate strongly, whereas they divided only poorly in the absence of CpG. Thus, direct APC activation is dispensable to promote the proliferation of naïve CD8+ T cells, at least in a system in which antigen processing is bypassed by provision of preprocessed peptide.
Fig. 1.
Indirectly activated APCs promote proliferation but not survival or effector differentiation of CD8+ T cells in vitro. (A) Proliferative index (average number of division per divided cell) of OT-I cells primed in vitro under control or test conditions at the indicated time points. (B) Quantification of the total number of viable OT-I cells recovered from control and test cultures. (C) Percentage of OT-I cells staining positive for active caspase 3. (D) Assessment of antigen-induced effector cytokine production at day 6 of culture. The percentage of IFN-γ+ (Left) or TNF-α+ (Right) OT-I cells from control or test cultures is shown. Error bars indicate the SD within technical triplicates. N.s., not significant. Data are representative of three independent experiments (A–D).
Despite similar proliferation in both control and test cultures, differences were observed when numbers of viable OT-I cells were determined. At day 3, the total number of OT-I cells in pOVA/CpG-treated control and test cultures did not differ significantly (Fig. 1B), despite a five- to sevenfold increased yield compared with cultures without CpG. However, by day 6, the number of OT-I cells in pOVA/CpG-stimulated control cultures had increased in control but not in test cultures. Likewise, generational analysis of dividing T cells revealed decreased frequencies of highly divided cells in test cultures (Fig. S1). Given that this difference could not be attributed solely to differences in cell division (Fig. 1A), we hypothesized that the T cells in test cultures undergo apoptosis more readily, especially at later culture times. Consistent with this notion, nearly two-thirds of the OT-I cells in test cultures containing pOVA/CpG were apoptotic by day 6 and virtually indistinguishable from cells stimulated with pOVA only (Fig. 1C). In contrast, OT-I cells primed in control cultures maintained high viability. Together, these results suggest that direct APC activation is required to rescue newly primed CD8+ T cells from activation-induced cell death even if it is largely dispensable for the induction of T-cell proliferation.
Next, we sought to investigate the functionality of the expanded CD8+ T cells. Cultures without CpG failed to generate OT-I cells capable of producing either IFN-γ or TNF-α (Fig. 1D). As expected, the addition of CpG to control cultures provided the necessary signals to allow OT-I differentiation into cells producing IFN-γ and TNF-α. In contrast, a markedly reduced fraction of OT-I cells in pOVA/CpG-stimulated test cultures produced the two effector cytokines. A similar pattern emerged with respect to the lytic machinery: OT-I cells recovered from test cultures contained significantly less granzyme B and considerably fewer cells degranulated upon encounter with target cells (Fig. S2). This is reminiscent of the phenotype of CD8+ T cells given antigen and costimulation in vitro but no “signal 3” cytokines (11). Analogous test cultures in which only non-presenting cells were able to respond to TLR7 agonists yielded comparable results: naïve OT-I cells failed to differentiate and expanded poorly, whereas the respective control cultures yielded large numbers of effector cells (Fig. S3). In conclusion, these experiments reveal a need for direct pattern recognition by the presenting cell for both the survival and effector differentiation of newly activated CD8+ T cells in vitro.
Impaired Expansion and Effector Differentiation of CD8+ T Cells Primed by Indirectly Activated APCs in Vivo.
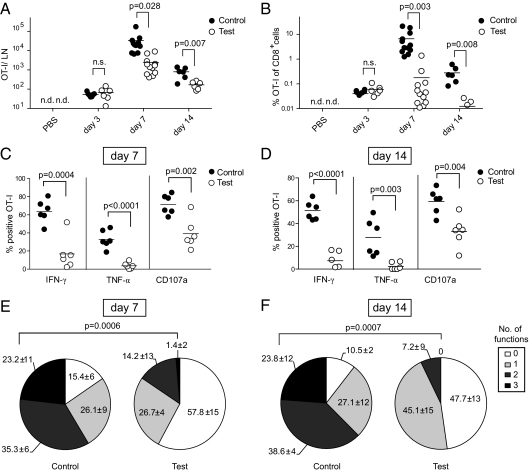
To validate these findings in vivo, we developed a mixed bone marrow chimeric mouse model in which half the APCs cannot directly recognize CpG but can present pOVA to OT-I cells, whereas the other half of APCs can respond to CpG directly but cannot present pOVA. In these chimeric mice (tlr9+/+ H2Kb−/− + tlr9−/− H2Kb+/+ → wt-B6; hereafter “test chimeras”), pOVA/Kb-specific T cells are primed exclusively by indirectly activated APCs (tlr9−/− H2Kb+/+) but are nevertheless exposed to the inflammatory environment created by non-presenting cells that sense CpG directly (tlr9+/+ H2Kb−/−). Mixed chimeras in which all cells can respond to CpG were used as controls (tlr9+/+ H2Kb−/− + tlr9+/+ H2Kb+/+ → wt-B6; henceforth “control chimeras”). Chimeras were infused with a low number of naïve OT-I cells and subsequently immunized with pOVA/CpG in the footpads. In unimmunized mice, the number OT-I cells in the draining lymph node was generally below the detection limit. Three days after immunization, the numbers and frequencies of OT-I cells were similar in both groups but were reduced by more than one order of magnitude in test chimeras at the peak of the response (day 7) and day 14 (Fig. 2 A and B). The defective expansion of OT-I cells could signify that CpG had no adjuvant effect in test chimeras. However, this was not the case because the number of OT-I cells in test chimeras immunized with pOVA/CpG was significantly increased at day 7 compared with mice that received pOVA only (Fig. S4). Similarly, our earlier study demonstrated that indirectly activated DCs are superior to non-activated DCs at stimulating CD4+ T-cell expansion in vivo (6). We conclude that, in vivo as in vitro, indirectly activated APCs cannot sustain CD8+ T-cell expansion.
Fig. 2.
CD8+ T cells primed by indirectly activated APCs fail to expand and differentiate in vivo. (A and B) Impaired expansion of CD8+ T-cell populations primed by indirectly activated DCs. The absolute number (A) and frequency (B) of OT-I cells in the popliteal lymph nodes from control and test chimeras were analyzed at the indicated time points. Pooled data from four independent experiments are shown (n = 6–12). N.d., not detectable. N.s., not significant. (C–F) Cytokine secretion and degranulation upon restimulation of OT-I cells primed 7 (C and E) or 14 d (D and F) earlier. (C and D) Percentage of OT-I cells staining positive for IFN-γ, TNF-α, or CD107a in control and test chimeras. (E and F) Functionality profiles of OT-I cells from control or test chimeras are indicated as percentage of none-, mono, bi-, or trifunctional OT-I cells (IFN-γ, TNF-α, surface CD107a). Pooled data from two independent experiments, each with three animals per group, are shown.
The functionality of the OT-I T cells was determined next. Seven days after immunization, two-thirds of OT-I cells in test chimeras produced IFN-γ upon restimulation, and approximately half as many stained positive for TNF-α (Fig. 2C). In contrast, the frequency of cytokine-positive OT-I cells in test chimeras was strongly reduced. To gauge the lytic ability on a per-cell basis, we measured the capacity of OT-I cells to degranulate in response to target cell encounter. The majority of OT-I cells primed in control chimeras degranulated upon recognition of target cells, but only half as many did so in test chimeras (Fig. 2C). Similarly, OT-I granzyme B levels in test chimeras were significantly reduced compared with controls and were close to the level found in naïve CD8+ T cells (Fig. S5A). This decrease in degranulation and granzyme B content was reflected in a markedly reduced CTL activity (Fig. S5B). The functional defect of OT-I cells in test chimeras was not due to slower kinetics in the acquisition of effector function, given that we observed a virtually identical phenotype regarding IFN-γ, TNF-α, and degranulation on day 14 after immunization (Fig. 2D).
The ability to perform single effector functions is a relatively poor indicator of the functionality of T cells (12). We therefore examined whether priming in control or test chimeras endowed OT-I cells with one or more effector functions, namely the ability to make IFN-γ and TNF-α and to degranulate. At day 7 after immunization, the frequency of bifunctional OT-I cells in test chimeras was less than half that of controls and diminished further at day 14 (Fig. 2 E and F). The discrepancy was even more pronounced with respect to cells expressing all three effector functions. Only a remnant population was found in test animals at day 7 and had vanished completely by day 14, whereas a quarter was persistently trifunctional in controls.
Taken together, these data demonstrate that inflammation can assist the proliferative response of CD8+ T cells recognizing antigen on indirectly activated APCs, but this does not lead to sustained clonal expansion or generate CTL effectors. Thus, at least with respect to the PAMPs studied here, inflammation cannot substitute for direct pattern recognition in the priming of functional CD8+ T-cell responses.
CD8+ T Cells Primed by Indirectly Activated APCs Do Not Protect from Tumors.
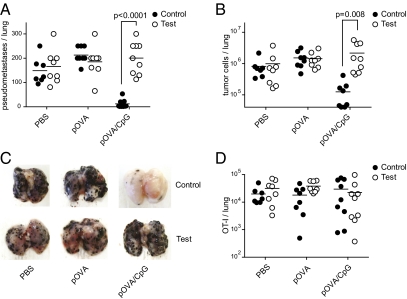
The most stringent measure of the quality of a T-cell response is its protective capacity. Therefore, immunized test and control chimeras were challenged by i.v. injection of B16 melanoma cells expressing ovalbumin (B16-OVA) and analyzed 18 d later. The total number of lung pseudometastases and tumor cells in PBS- or pOVA-immunized animals was comparable in both groups (Fig. 3 A–C). Inclusion of CpG into the pOVA inoculum dramatically changed the outcome. Control chimeras immunized with pOVA/CpG were virtually free of pseudometastases and tumor cells (Fig. 3 A–C). In sharp contrast, test chimeras vaccinated with pOVA/CpG were unable to control the outgrowth of B16-OVA tumor cells in the lungs and in fact had the highest tumor burden of all experimental groups.
Fig. 3.
CD8+ T cells primed by indirectly activated APCs are not protective. Chimeric mice were immunized as in Fig. 2 and challenged with B16-OVA tumors 2 wk later. Eighteen days after tumor challenge the lungs were analyzed. (A) Pseudometastases burden per lung, (B) number of tumor cells per lung, (D) and absolute number of OT-I cells per lung are shown. (C) Representative lungs of control and test chimeric mice immunized as indicated are shown. Pooled data from two of three independent experiments are shown (n = 7–10, A–D).
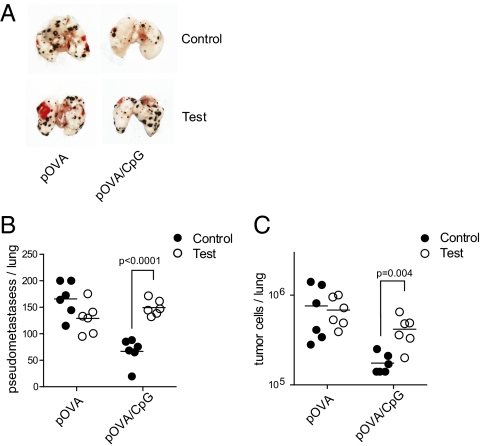
The clonal expansion of OT-I cells in test chimeras was reduced by at least one order of magnitude compared with controls at the day of tumor challenge (Fig. 2 A and B). This raises the question whether the failure of test chimeras to limit tumor growth is due to insufficient antigen-specific CD8+ T cells or due to their reduced functionality on a per-cell basis. Arguing for the latter, the absolute number of OT-I cells in the lungs of pOVA/CpG-primed control and test chimeras was comparable 18 d after tumor challenge (Fig. 3D). Nevertheless, we formally tested the functionality on a per-cell basis by transferring equal numbers of OT-I cells that were primed in control or test cultures into wild-type mice that received B16-OVA cells shortly before. OT-I cells primed in control conditions were fully competent in rejecting tumors in the adoptive hosts (Fig. 4 A–C). In contrast, T cells primed under test conditions were unable to efficiently clear the tumors, indicating a functional defect on a per-cell basis (Fig. 4 A–C). Taken together, these data demonstrate that APCs activated exclusively by the PAMP-induced inflammatory milieu are unable to mount protective antitumor CD8+ T-cell responses.
Fig. 4.
Impaired per-cell functionality of CD8+ T cells primed by indirectly activated APCs. (A–C) OT-I cells were primed under test and control conditions as in Fig. 1. After 4 d, cultures were harvested, and equal numbers of OT-I cells were adoptively transferred into naïve wild-type mice that received B16-OVA tumor cells 3 h before. Eighteen days later, the tumor burden in their lungs was analyzed. (A) Representative lungs of recipients of OT-I cells from control or test cultures. (B and C) Quantification of the number of pseudometastases (B) and tumor cells (C) per lung. Data are representative of two independent experiments (n = 6, A–C).
Discussion
The data presented here indicate that APCs activated in trans by inflammatory mediators alone can present preprocessed antigens and promote proliferation of responding naïve CD8+ T cells. Such APCs show all of the typical signs of “maturation,” including high costimulatory potential. However, they cannot rescue CD8+ T cells from activation-induced cell death or instruct differentiation into CTL. These results have several implications for our understanding of immunobiology. First, they do not fit typical “two signal” models of T-cell activation and therefore reinforce the notion that T-cell priming by APCs requires an additional “signal 3” that is the key determinant of immunogenicity (9). In this vein, other studies demonstrate that phenotypically mature DCs do not necessarily induce immunity (13–15). Second, our findings suggest that at least one part of “signal 3” must be delivered by the presenting APC and cannot be provided in trans by nonpresenting cells in the microenvironment. Finally, our data reinforce previous proposals that APCs can only become competent to provide “signal 3” when they receive a direct pathogen signal rather than just being exposed to an inflammatory milieu. Similarly, TNF receptor or type I IFN receptor signaling is necessary but not sufficient to render DCs immunogenic (7, 14, 16, 17). It is important to emphasize that our data do not exclude a role for the inflammatory milieu in promoting adaptive immunity. The autocrine and paracrine action of cytokines such as IFN-α/β and TNF-α is clearly very important for the induction of costimulation on APCs (18–20), and the effectiveness of certain adjuvants in T-cell priming depends on their ability to trigger not only APCs but also other cell types, including ones of non-hematopoietic origin (16, 17). Thus, inflammation is clearly necessary but not sufficient to couple innate recognition to adaptive immunity. It will be important to determine whether the effects seen here with two distinct adjuvants, CpG and R848, are generally applicable to other innate stimuli. In particular, because the IL-1R signals via MyD88, one might envisage that stimuli that induce production of high levels of IL-1α or IL-1β could lead to a type of bystander APC activation that mimics direct activation induced by a TLR agonist. Nevertheless, our data strongly argue that caution should be taken when choosing simple adjuvants for CTL priming to ensure that they activate the appropriate APCs.
Our experimental approach relied in part on the use of radiation chimeras, in which some APCs, such as Langerhans cells, remain of host origin. We believe there are two main reasons for the absence of a “masking” effect of such cells in our model. First, radioresistant APCs do not efficiently prime T cells in vivo (21). Second, the deliberate use of soluble antigen and PAMP allows draining and circumvents the need for active transport from the peripheral injection site to the local lymph node by migrating DCs (22). In addition, immigrant Langerhans cells are outnumbered by resident DCs in the draining lymph node. In our model, naïve T cells will therefore encounter antigen primarily presented on lymph node-resident, donor-derived DCs. We therefore believe that the inability of Langerhans cells to rescue the priming of a robust CD8+ effector T-cell population in test chimeras can be attributed to a combination of the reasons discussed above. However, we cannot completely exclude a minor contribution of Langerhans cells to the expansion of OT-I populations. Nevertheless, if such a contribution exists and if it does lead to the differentiation of effectors, that contribution to the overall response is small, as evident from the data presented here.
Several important issues remain open in this study. Of particular relevance is the question of help from CD4+ T cells. The CTL response studied here is help-independent. We have used preprocessed antigen lacking helper epitopes rather than intact protein to allow for the discrimination between effects on CD8+ T cells and CD4+ T cells. Nevertheless, it would be interesting to investigate whether fully competent T helper cells (i.e., CD4+ T cells that were primed by directly activated DCs) could rescue the defective CTL response primed by indirectly activated DCs. Circumstantial evidence from experiments on the expansion of HSV-specific CD8+ T cells indicate that a partial defect remains in a help-competent environment (23), and it would be interesting to determine whether this extends to CTL function. Another issue concerns the T-cell responses in mucosal and other peripheral surfaces. TLR4 on radioresistant airway cells is necessary and sufficient for priming DC-dependent Th2 responses to LPS-containing inhaled house dust mite allergen (4). It is therefore conceivable that the requirement for cognate pattern recognition in T-cell priming is not absolute in certain peripheral tissues or under chronic inflammatory conditions. Alternatively, the necessity for the recognition of PAMPs by the priming cell could be restricted to certain types of responses, such as Th1 and CTL priming, and does not apply to Th2-type immunity.
Independent from possible exceptions, our findings have important practical implications for vaccination and immunotherapy. They suggest that the efficacy of vaccines depends on the ability of adjuvants to activate APCs through engagement of their PRRs. This in turn requires careful matching of the adjuvant choice to the repertoire of PRRs expressed by APCs. Thus, the absence of TLR9 on human conventional DCs could in part explain the disappointing outcome of some clinical trials of CpG to treat cancer (24, 25). Differential expression of PRRs among APC subsets further complicates the selection of suitable adjuvants. An attractive cellular target for vaccination strategies are the CD8α+ DCs and their equivalents in tissues, owing to their superior ability to present exogenous antigen on MHC class I and capacity to provide robust “signal 3” (26). In contrast to DCs belonging to other subsets, the CD8α+ DCs lack the expression of TLR7 (27, 28). This suggests that TLR7 agonists are unlikely to be potent adjuvants for vaccines requiring cross-presentation of antigen, whereas adjuvants engaging TLR3 and other PRRs expressed by CD8α+ DCs are predicted to hold greater potential. Tumor-specific CD8+ T cells expand to high frequencies, for example in melanoma patients, but generally lack CTL activity and thus fail to control the tumors (29, 30). Choosing appropriate adjuvants for tumor vaccines could realize the full tumoricidal potential of specific CTL precursors and thus result in a more favorable clinical outcome. The recent identification of the human equivalent of murine CD8α+ DCs, advances in technologies to target antigen specifically to DC subsets, and an increasing understanding of the mechanisms and rules of adjuvanticity should allow for the clinical potential of DCs to be tapped and for the rational design of new and better vaccines aimed at eliciting CTL responses against tumors and infected cells (31, 32).
Materials and Methods
Mice.
Wild-type C57BL/6 (WT-B6) mice were purchased from Janvier Elevage (Le Genest St. Isle, France). WT-B6, OT-I, and P14 mice on a CD45.1 congenic background, tlr9−/−-B6, tlr7−/−-B6, myd88−/−-B6, H2Kb−/−-B6, and H2Db−/−-B6 mice were bred and housed at the Eidgenössische Technische Hochschule Zürich (Switzerland) or RCC Ltd. (Füllinsdorf, Switzerland) under specific pathogen-free conditions. All mice were used at 6–12 wk of age and were sex- and age-matched within experiments. For the generation of mixed chimeric mice, WT-B6 recipient mice were γ-irradiated with 950 Rad and subsequently reconstituted with equal amounts of T- and NK cell-depleted donor bone marrow cells from H2Kb−/−-B6 + tlr9+/+-B6 mice (control chimeras) or H2Kb−/−-B6 + tlr9−/−-B6 mice (test chimeras) (2–4 × 106 cells in total). Where indicated, irradiated WT-B6 or tlr7−/−-B6 hosts were reconstituted with H2Db−/−-B6 + tlr7+/+-B6 (control chimeras) or H2Db−/−-B6 + tlr7−/−-B6 (test chimeras) bone marrow, respectively. Six to eight weeks after reconstitution, mice were tested for chimerism on the basis of the expression of H-2Kb on peripheral blood leukocytes. Chimeras were only used for experiments if the genotype ratio of blood leukocytes was close to 1:1. Animal husbandry and procedures were carried out in accordance with institutional policies and have been reviewed and permitted by the cantonal veterinary office.
Reagents and Media.
CpG oligonucleotide 1668 (TCCATGACGTTCCTGATGCT) was synthesized by Microsynth. R848 was purchased from Enzo Life Sciences (Lausen, Switzerland). The ovalbumin-derived SIINFEKL peptide (residues 257–264, pOVA) and the lymphocytic choriomeningitis virus glycoprotein-derived KAVYNFATM peptide (residues 33–41, gp33) were synthesized by EMC Microcollections. For all in vivo experiments, reagents were formulated in sterile PBS. Cells were cultured in complete medium (RPMI 1640 with 10% heat-inactivated FCS, 100 units/mL of penicillin, 100 mg/mL of streptomycin, 2 mM glutamine, and 50 μM 2-mercaptoethanol).
Adoptive Transfers and Immunizations.
Single cell suspensions from spleens and lymph nodes of OT-I mice were obtained by enzymatic digestion with Liberase/DNase (Roche Diagnostics), and enriched for CD8+ cells using magnetic-activated cell sorting (MACS) technology (Miltenyi Biotech). The cells were stained according to the FACS staining procedure as described below, and naïve OT-I cells (CD8+ CD45.1+ CD44low CD11c− CD11b− CD4− Gr1− CD49b−) were purified using a BD FACSAria cell sorter (BD Biosciences). Resulting OT-I preparations were >98% pure by the above criteria and viable. Purified OT-I cells (5 × 103) were transferred i.v. into recipient mice 1 d before immunization. Mice were immunized s.c. in the hind footpad with 1 μg pOVA in combination with or without 3 μg CpG in 30 μL PBS. Where indicated, mice were infused with 1.4 × 106 MACSorted CD8+ T cells from the spleen and lymph nodes of P14 mice (bearing a T-cell receptor specific for gp33 presented by H-2Db) and subsequently immunized s.c. with 25 μg gp33 in combination with 6.25 μg R848 per flank.
Tumor Challenge.
The melanoma cell line B16-OVA, expressing an OVA-GFP fusion protein, has been previously described (33). In experiments in which the OT-I cells were primed in vivo, 2.5 × 105 B16-OVA cells were injected i.v. To measure CTL functionality on a per-cell basis, mice were injected with 1 × 105 B16-OVA cells, 6 h before adoptive transfer of 5 × 104 in vitro-primed OT-I cells. Eighteen days after tumor challenge mice were anesthetized (by i.p. injection of 2 mg ketamine, 0.4 mg xylacine, and 60 μg acepromacine formulated in PBS) and perfused with PBS to remove circulating blood cells from the lungs. Pseudometastases in the lung were counted manually by visual inspection. The total numbers of lung resident OT-I cells and tumor cells were measured by flow cytometry. Tumor cells were identified according to their GFP expression. A defined number of CaliBRITE3 beads (BD Biosciences) were added to each sample before acquisition to extrapolate the absolute number of OT-I and B16-OVA cells per organ.
In Vitro T-Cell Stimulation Assay.
For in vitro experiments, OT-I cells were labeled with 1 μM CFSE (Molecular Probes) for 6 min at 37 °C. OT-I cells (2 × 103) were cocultured with a total of 2 × 105 of 1:1 mixtures of splenocytes from tlr9−/− + H2Kb−/− or WT + H2Kb−/− in flat-bottom 96-well plates (test and control cultures, respectively). Cells were then stimulated with pOVA (final concentration 10−9 to 10−11 M), with or without CpG (final concentration 0.5 μg/mL) or R848 (final concentration 3 μM) and incubated at 37 °C for the indicated time periods. For the detection of cytokine production and degranulation, T cells were restimulated for 6 h at 37 °C in complete medium supplemented with 1 μM pOVA in the presence of 2 μM monensin A, 10 μg/mL brefeldin A, and 0.5 μg/mL anti-CD107a-APC.
In Vivo CTL Assay.
In vivo CTL assays were performed as previously described (33). In brief, freshly isolated splenocytes from WT-B6 (CD45.1) mice were incubated with 1 μM, 0.05 μM, or 1 μM gp33 for 1 h at 37 °C in complete media, washed, and labeled with 2.5 μM, 0.5 μM, or 0.1 μM CFSE, respectively, for 6 min at 37 °C in PBS. After extensive washing, the three splenocyte fractions were pooled in equal ratio and injected i.v. (5 × 106 per mouse). The next day mice were killed, and the CD45.1+ population in the spleen was analyzed for the presence of CFSE+ cells by flow cytometry. Specific killing was calculated as a ratio of pulsed to unpulsed target cells (100 × [1 − (% CFSE peptide/% CFSE no peptide)]).
Flow Cytometry.
All stainings were carried out in ice-cold FACS buffer (PBS, 2 mM EDTA, 1% heat-inactivated FCS, 0.1% sodium azide) at 30 min in the dark, except when cells were labeled for cytometric sorting, for which ice-cold sterile PBS containing 2 mM EDTA and 1% heat-inactivated FCS was used. The following antibodies (indicating target-fluorochrome) were purchased from Biolegend: CD45.1-PE, CD45.1-APC, CD45.2-Pacific Blue, CD8α-PerCP, CD4-FITC, CD44-PE, IFN-γ-PE-Cy7, TNF-α-APC, CD107a-FITC, and CD107a-APC. Anti-active caspase 3-FITC, CD11c-FITC, CD11b-FITC, Gr-1-FITC, CD49b-FITC, and mouse IgG1-PE isotype control was purchased from BD Biosciences. Anti-granzyme B-PE was purchased from Caltag Laboratories. Staining for active caspase 3 was performed according to standard protocols using paraformaldehyde fixation and BD Perm/wash buffer (BD Biosciences). Staining for granzyme B was performed according to standard protocols using paraformaldehyde fixation and permeabilization buffer (eBioscience). For the detection of cytokine production and degranulation by T cells, single cell suspensions from popliteal lymph nodes excised from experimental animals were restimulated for 6 h at 37 °C in complete medium supplemented with 1 μM pOVA, in the presence of 2 μM monensin A, 10 μg/mL brefeldin A, and 0.5 μg/mL anti-CD107a–FITC. After surface marker staining as described above, cells were fixed and permeabilized for 10 min with BD lysing solution (BD Biosciences) + 0.1% Tween, washed, and then stained intracellularly for IFN-γ and TNF-α in FACS buffer. Data were acquired on a LSRII flow cytometer and analyzed (including determination of the proliferative index and generational analysis) using FlowJo software (Treestar). Background responses of unstimulated cells were subtracted.
Statistical Analyses.
Statistical analyses were performed using a two-tailed unpaired Student t test using GraphPad Prism.
Supplementary Material
Acknowledgments
We thank members of the A.O. group for discussion and technical support, members of the C.R.e.S. group for advice, Heather Hinton (Roche) for critical reading of the manuscript and discussion, and the Rodent Center HCI (Eidgenössische Technische Hochschule Zurich) for animal husbandry. This work was funded by the Swiss Life Jubiläumsstiftung and UBS AG on behalf of a client. R.S. is supported by the Holcim Foundation for the Advancement of Scientific Research, C.R.e.S. is supported by Cancer Research UK and a prize from Fondation Bettencourt-Schueller, and A.O. is supported by the Swiss National Science Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1108945108/-/DCSupplemental.
References
- 1.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5:987–995. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 2.Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 3.Gallucci S, Lolkema M, Matzinger P. Natural adjuvants: endogenous activators of dendritic cells. Nat Med. 1999;5:1249–1255. doi: 10.1038/15200. [DOI] [PubMed] [Google Scholar]
- 4.Hammad H, et al. House dust mite allergen induces asthma via Toll-like receptor 4 triggering of airway structural cells. Nat Med. 2009;15:410–416. doi: 10.1038/nm.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Santini SM, et al. Type I interferon as a powerful adjuvant for monocyte-derived dendritic cell development and activity in vitro and in Hu-PBL-SCID mice. J Exp Med. 2000;191:1777–1788. doi: 10.1084/jem.191.10.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spörri R, Reis e Sousa C. Inflammatory mediators are insufficient for full dendritic cell activation and promote expansion of CD4+ T cell populations lacking helper function. Nat Immunol. 2005;6:163–170. doi: 10.1038/ni1162. [DOI] [PubMed] [Google Scholar]
- 7.Nolte MA, Leibundgut-Landmann S, Joffre O, Reis e Sousa C. Dendritic cell quiescence during systemic inflammation driven by LPS stimulation of radioresistant cells in vivo. J Exp Med. 2007;204:1487–1501. doi: 10.1084/jem.20070325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hou B, Reizis B, DeFranco AL. Toll-like receptors activate innate and adaptive immunity by using dendritic cell-intrinsic and -extrinsic mechanisms. Immunity. 2008;29:272–282. doi: 10.1016/j.immuni.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaliński P, Hilkens CM, Wierenga EA, Kapsenberg ML. T-cell priming by type-1 and type-2 polarized dendritic cells: The concept of a third signal. Immunol Today. 1999;20:561–567. doi: 10.1016/s0167-5699(99)01547-9. [DOI] [PubMed] [Google Scholar]
- 10.Curtsinger JM, et al. Inflammatory cytokines provide a third signal for activation of naive CD4+ and CD8+ T cells. J Immunol. 1999;162:3256–3262. [PubMed] [Google Scholar]
- 11.Curtsinger JM, Lins DC, Johnson CM, Mescher MF. Signal 3 tolerant CD8 T cells degranulate in response to antigen but lack granzyme B to mediate cytolysis. J Immunol. 2005;175:4392–4399. doi: 10.4049/jimmunol.175.7.4392. [DOI] [PubMed] [Google Scholar]
- 12.Seder RA, Darrah PA, Roederer M. T-cell quality in memory and protection: Implications for vaccine design. Nat Rev Immunol. 2008;8:247–258. doi: 10.1038/nri2274. [DOI] [PubMed] [Google Scholar]
- 13.Albert ML, Jegathesan M, Darnell RB. Dendritic cell maturation is required for the cross-tolerization of CD8+ T cells. Nat Immunol. 2001;2:1010–1017. doi: 10.1038/ni722. [DOI] [PubMed] [Google Scholar]
- 14.Menges M, et al. Repetitive injections of dendritic cells matured with tumor necrosis factor alpha induce antigen-specific protection of mice from autoimmunity. J Exp Med. 2002;195:15–21. doi: 10.1084/jem.20011341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang A, et al. Disruption of E-cadherin-mediated adhesion induces a functionally distinct pathway of dendritic cell maturation. Immunity. 2007;27:610–624. doi: 10.1016/j.immuni.2007.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Longhi MP, et al. Dendritic cells require a systemic type I interferon response to mature and induce CD4+ Th1 immunity with poly IC as adjuvant. J Exp Med. 2009;206:1589–1602. doi: 10.1084/jem.20090247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sato A, Iwasaki A. Induction of antiviral immunity requires Toll-like receptor signaling in both stromal and dendritic cell compartments. Proc Natl Acad Sci USA. 2004;101:16274–16279. doi: 10.1073/pnas.0406268101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Honda K, et al. Selective contribution of IFN-alpha/beta signaling to the maturation of dendritic cells induced by double-stranded RNA or viral infection. Proc Natl Acad Sci USA. 2003;100:10872–10877. doi: 10.1073/pnas.1934678100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoshino K, Kaisho T, Iwabe T, Takeuchi O, Akira S. Differential involvement of IFN-beta in Toll-like receptor-stimulated dendritic cell activation. Int Immunol. 2002;14:1225–1231. doi: 10.1093/intimm/dxf089. [DOI] [PubMed] [Google Scholar]
- 20.Sundquist M, Wick MJ. TNF-alpha-dependent and -independent maturation of dendritic cells and recruited CD11c(int)CD11b+ Cells during oral Salmonella infection. J Immunol. 2005;175:3287–3298. doi: 10.4049/jimmunol.175.5.3287. [DOI] [PubMed] [Google Scholar]
- 21.Allenspach EJ, Lemos MP, Porrett PM, Turka LA, Laufer TM. Migratory and lymphoid-resident dendritic cells cooperate to efficiently prime naive CD4 T cells. Immunity. 2008;29:795–806. doi: 10.1016/j.immuni.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Itano AA, et al. Distinct dendritic cell populations sequentially present antigen to CD4 T cells and stimulate different aspects of cell-mediated immunity. Immunity. 2003;19:47–57. doi: 10.1016/s1074-7613(03)00175-4. [DOI] [PubMed] [Google Scholar]
- 23.Davey GM, et al. Cutting edge: priming of CD8 T cell immunity to herpes simplex virus type 1 requires cognate TLR3 expression in vivo. J Immunol. 2010;184:2243–2246. doi: 10.4049/jimmunol.0903013. [DOI] [PubMed] [Google Scholar]
- 24.Kadowaki N, et al. Subsets of human dendritic cell precursors express different toll-like receptors and respond to different microbial antigens. J Exp Med. 2001;194:863–869. doi: 10.1084/jem.194.6.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmidt C. Clinical setbacks for toll-like receptor 9 agonists in cancer. Nat Biotechnol. 2007;25:825–826. doi: 10.1038/nbt0807-825. [DOI] [PubMed] [Google Scholar]
- 26.den Haan JM, Lehar SM, Bevan MJ. CD8(+) but not CD8(-) dendritic cells cross-prime cytotoxic T cells in vivo. J Exp Med. 2000;192:1685–1696. doi: 10.1084/jem.192.12.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Doxsee CL, et al. The immune response modifier and Toll-like receptor 7 agonist S-27609 selectively induces IL-12 and TNF-alpha production in CD11c+CD11b+CD8- dendritic cells. J Immunol. 2003;171:1156–1163. doi: 10.4049/jimmunol.171.3.1156. [DOI] [PubMed] [Google Scholar]
- 28.Edwards AD, et al. Toll-like receptor expression in murine DC subsets: Lack of TLR7 expression by CD8 alpha+ DC correlates with unresponsiveness to imidazoquinolines. Eur J Immunol. 2003;33:827–833. doi: 10.1002/eji.200323797. [DOI] [PubMed] [Google Scholar]
- 29.Lee PP, et al. Characterization of circulating T cells specific for tumor-associated antigens in melanoma patients. Nat Med. 1999;5:677–685. doi: 10.1038/9525. [DOI] [PubMed] [Google Scholar]
- 30.Pittet MJ, et al. High frequencies of naive Melan-A/MART-1-specific CD8(+) T cells in a large proportion of human histocompatibility leukocyte antigen (HLA)-A2 individuals. J Exp Med. 1999;190:705–715. doi: 10.1084/jem.190.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tacken PJ, de Vries IJ, Torensma R, Figdor CG. Dendritic-cell immunotherapy: From ex vivo loading to in vivo targeting. Nat Rev Immunol. 2007;7:790–802. doi: 10.1038/nri2173. [DOI] [PubMed] [Google Scholar]
- 32.Villadangos JA, Shortman K. Found in translation: the human equivalent of mouse CD8+ dendritic cells. J Exp Med. 2010;207:1131–1134. doi: 10.1084/jem.20100985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sancho D, et al. Tumor therapy in mice via antigen targeting to a novel, DC-restricted C-type lectin. J Clin Invest. 2008;118:2098–2110. doi: 10.1172/JCI34584. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.