Abstract
Ultralow velocity zones are the largest seismic anomalies in the mantle, with 10–30% seismic velocity reduction observed in thin layers less than 20–40 km thick, just above the Earth’s core-mantle boundary (CMB). The presence of silicate melts, possibly a remnant of a deep magma ocean in the early Earth, have been proposed to explain ultralow velocity zones. It is, however, still an open question as to whether such silicate melts are gravitationally stable at the pressure conditions above the CMB. Fe enrichment is usually invoked to explain why melts would remain at the CMB, but this has not been substantiated experimentally. Here we report in situ high-pressure acoustic velocity measurements that suggest a new transformation to a denser structure of MgSiO3 glass at pressures close to those of the CMB. The result suggests that MgSiO3 melt is likely to become denser than crystalline MgSiO3 above the CMB. The presence of negatively buoyant and gravitationally stable silicate melts at the bottom of the mantle, would provide a mechanism for observed ultralow seismic velocities above the CMB without enrichment of Fe in the melt. An ultradense melt phase and its geochemical inventory would be isolated from overlying convective flow over geologic time.
Keywords: dynamics, early Earth evolution, high-pressure experiment, sound velocity measurement, pressure-induced polymorphism
The buoyancy relations between silicate melts and crystals in a deep terrestrial magma ocean are the primary constraints on the possible chemical stratification of Earth’s interior (1, 2). The nature of silicate melts under high-pressure conditions is therefore critically important for elucidating the formation and differentiation of the Earth through massive primordial melting of the proto-Earth. Extensive melting of the proto-Earth and the formation of a deep magma ocean, induced by massive planetesimal collisions and possibly encompassing an entire planet, facilitated metal-silicate (core-mantle) segregation, with the subsequent fractional crystallization of silicate melt leading to chemical and gravitational equilibrium (3–6). Because of the high compressibility of melts, a density crossover between crystals and coexisting magmas is expected in the course of fractional crystallization in a deep magma ocean, which would enhance chemical differentiation and could result in a stratified structure of the Earth’s interior (7, 8). The possible presence of dense, gravitationally stable magmas deep within the Earth at pressures above 100 gigapascals (GPa) has thus been proposed as a consequence of partial melting and a remnant of a deep magma ocean (9, 10), which might explain the observation of anomalously ultralow seismic velocities (ULVZ) above the core-mantle boundary (CMB) (11). However, the behavior of silicate melts under such extreme pressures is poorly understood, and it is still an open question as to whether a density crossover between silicate melt and coexisting mantle phases occurs in the D′′ region above the CMB (approximately 2,900 km depth). Density measurements on silicate melts at relatively low pressures below 15 GPa suggested that gravitationally stable melt compositions in the deep interior are restricted to iron-rich compositions such as basaltic melts (7, 8). The high densities of magnesium-silicate perovskite, (MgFe)O ferropericlase, and Ca-perovskite—the main phases in the lower mantle—make it difficult for melts to be negatively buoyant under lowermost mantle conditions. Although measurements on silicate melts at high pressures are beyond current experimental capabilities, MgSiO3 glass can be used as an analogue for the most abundant component of the silicate melts in a deep magma ocean (12). Experimental investigations of the high-pressure structure of the MgSiO3 glass up to a pressure of 39 GPa strongly suggest that changes in the Si-O and Mg-O coordination number are a critical to the densification mechanism of MgSiO3 glass (13, 14). However, little is known about further densification above approximately 40 GPa due to experimental challenges and the lack of suitable in situ structural probes. In comparison with spectroscopy on crystals, the significant signal weakening and broadening in studying the structure of glasses/melts has so far prevented experiments under extreme high-pressure conditions. Acoustic wave velocity measurements are one of the most promising approaches for detecting structural changes of glasses and melts, inasmuch as the sound velocity directly reflects the density and elasticity, regardless of whether a sample is crystalline or amorphous. Our newly developed in situ high-pressure Brillouin scattering spectroscopic system has recently proven to be highly suitable for measuring acoustic velocities under ultrahigh pressure conditions of approximately 200 GPa (15, 16), corresponding to a depth of approximately 3,500 km in the Earth and thereby permitting the simulation of pressures in a deep magma ocean. Here we report in situ Brillouin scattering results for MgSiO3 glass at pressures up to 203 GPa, revealing a systematic change in the velocity-pressure trajectory above 130 GPa. We infer this to be a unique transition to a denser structure that is likely associated with the onset of a change in coordination number to higher than sixfold.
Results and Discussion
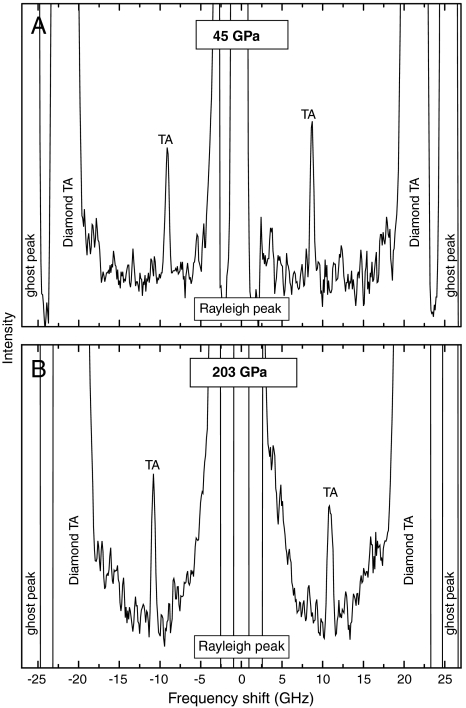
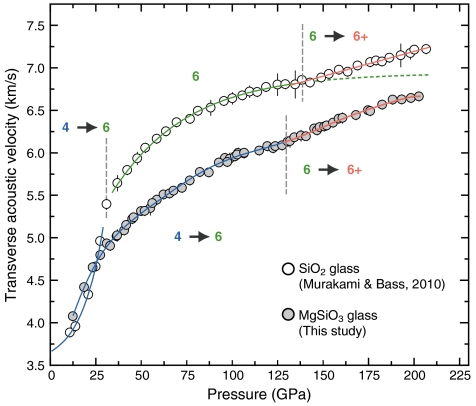
Very sharp Brillouin peaks from the transverse acoustic modes of MgSiO3 glass were obtained over the entire pressure range up to 203 GP, as shown in Fig. 1. We did not observe peaks from longitudinal acoustic waves at pressures higher than 40 GPa. The longitudinal modes were masked by those of the diamond transverse acoustic modes. The full width at half maximum of the transverse acoustic peaks at lower pressure (Fig. 1A) and higher pressure (Fig. 1B) were identical within the uncertainties [0.44(3) and 0.46(3) GHz, respectively], and no significant peak broadening was observed with pressure (Fig. S1 and Table S1). This indicates that the hydrostaticity in the sample chamber did not change drastically with increasing pressure. Such high quality Brillouin scattering signals under extreme pressure conditions resulted in velocity uncertainties of 0.6% on average throughout the pressure range we explored (Table S1). Fig. 2 shows the results for the transverse acoustic wave velocities for MgSiO3 glass as a function of pressure together with those of SiO2 glass that we recently reported (16).
Fig. 1.
High-pressure Brillouin spectra at 45 GPa (A) and 203 GPa (B). TA: transverse acoustic mode of MgSiO3 glass.
Fig. 2.
Transverse acoustic wave velocities of MgSiO3 glass (gray circles) as a function of pressure up to 203 GPa. The white circles indicate the results on SiO2 glass by Murakami and Bass (16). The velocity trajectories for the distinct pressure regimes are shown as blue, green, and orange lines with increasing pressure. Numbers indicate the potential Si-O coordination number within each pressure regime. Approximate pressure boundary for each Si-O coordinated structure are shown as the vertical dot lines. Error bars are ± 1σ from mean.
All data were fit by polynomial functions to evaluate the change in trend of the transverse acoustic wave velocity of MgSiO3 glass as a function of pressure, which gives a remarkably good fit with an adjusted R-square of 0.9986 as shown in Fig. 2. The pressure at which the change in trend of the velocity profile occurs was then determined by evaluating the second derivative of the fitted function with respect to the pressure and determining the point at which d2VT/dP2 is a maximum. This point was found to be at 133 GPa. The transverse acoustic wave velocity of MgSiO3 glass increased continuously from 12 to 133 GPa in a convex-upward trend. The pressure derivatives of transverse acoustic velocity (dVT/dP) from 12 GPa to 133 GPa decrease gradually by one order of magnitude, from 58.5 to 7.4 (m/ sec )/GPa. By contrast, we found a significantly steeper velocity trajectory upon subsequent pressure increase from 133 to 203 GPa. This velocity profile is fit well by a logarithmic function with dVT/dP of 10.2 (m/ sec )/GPa at 133 GPa, showing that the velocity gradient increases by approximately 43% compared to the lower pressure trend below 133 GPa (5.3 (m/ sec )/GPa).
In comparison with our recent high-pressure acoustic velocity results on SiO2 glass (16), we found a remarkably consistent trend with an anomalous velocity gradient increase above 130 GPa (Fig. 2). Our results for SiO2 glass along with those of previous studies (17–24) indicate that the velocity-pressure trends in SiO2 glass can be interpreted as a gradual transition from four- to sixfold coordination of Si below 40 GPa, predominantly sixfold coordination from 40 to 140 GPa, and a transition from sixfold to a higher coordination state above 140 GPa (16) (Fig. 2). This notable similarity in the velocity-pressure curves for MgSiO3 and SiO2 glasses above 130 GPa is a strong indicator that the structural transformation of MgSiO3 glass corresponds to a change in the Si-O coordination number from sixfold to a higher coordination state. The absence of a sharp exponential velocity increase at low pressures, as observed in SiO2 glass, suggests that the gradual coordination change from four- to sixfold occur over a wider pressure range for MgSiO3 glass (25). Given that the velocity profile of MgSiO3 glass from 40 to 140 GPa always exhibits a steeper trend than that of SiO2 glass, which is dominated by sixfold coordination (Fig. 2), it is reasonable to expect that the gradual change in Si-O coordination number from four- to sixfold in MgSiO3 glass is continuous over a broad pressure range up to approximately 130 GPa. This is possibly due to the presence of network-modifying Mg2+ cations acting to impede the Si-O coordination change from 4 → 6, at least at lower pressures (13, 14). Such a gradual coordination change over a broad pressure range is consistent with recent computational simulations on MgSiO3 melt using first-principles molecular dynamics (25).
Although further experimentation on the effect of temperature and chemistry is needed to obtain insight on the behavior of more realistic magma ocean compositions, the identification of a unique densification mechanism for MgSiO3 glass above 130 GPa, closely matching the pressure at the CMB region (approximately 135 GPa), could have a major affect on the density contrast between melts and coexisting solids phases in a deep terrestrial magma ocean. Previous experiments on amorphous MgSiO3 at pressures below 10 GPa indicate that higher temperature should promote relaxation of the glass, thus making the structural transition to an ultradensified melt at somewhat lower pressure (13) and above the CMB. A recent computational study examined the pressure-density relationship between of MgSiO3 melt with predominately six-coordinated Si, and MgSiO3 perovskite, the principal phase in the lower mantle. These simulations show that the densities of melt and MgSiO3 perovskite converge as the pressure of the CMB is approached, but that the amorphous phase remains positively buoyant (25). The present finding of a unique denser structure of MgSiO3 glass, possibly associated with a Si-O coordination number change to higher than six, is highly suggestive of a density crossover between MgSiO3 melt and coexisting mantle minerals in the deep terrestrial magma ocean that is likely to be achieved near the CMB region. Combining the previous analysis of shock wave Hugoniot results for MgSiO3 (12), MgSiO3 melt that is a few percent denser than crystalline MgSiO3 can be inferred under CMB conditions. Virtually all previous efforts to explain the presence of melt in D′′ have invoked a strong partitioning of Fe and possibly Ca into the melt phase, but there is no experimental or theoretical justification for such partitioning at CMB pressure-temperature conditions. Other studies have suggested Fe enrichment of the melt via interaction of with the molten Fe alloy of the outer core. However, experiments have shown that oxides of the lowermost mantle would in fact be depleted in Fe through interaction with molten Fe-rich alloys (26, 27). The ultrahigh density melt state implied by the present study is attained through changes in the atomic structure of the amorphous phase, without the addition of heavy elements such as Fe and Ca to the melt, thereby strengthening models with dense melt at the bottom of Earth’s mantle (8–10). With this buoyancy relationship, one can infer possible dynamical behavior of the dense melt in the course of crystallization of a magma ocean in the early Earth, although further investigations are required for more quantitative discussions. Ultradense melts, with whatever inventory of incompatible elements they might concentrate, may be dynamically (and chemically) isolated from convection in overlying solids and melts over geologic time. Partially molten regions may currently persist in the narrow pressure gap between the top of the outer core and the neutral buoyancy line for melt, providing a possible explanation for the distinctively thin structure of ULVZs, which are typically less than 40 km (11).
Because extensive melting of the protoplanets is believed to be almost inevitable during the formation of the terrestrial planets (3–6) it is perhaps likely that gravitationally stable dense magmas in a deep magma ocean are a fairly common phenomenon for the larger terrestrial planets. The presence of such ultradense magma should significantly enhance chemical differentiation, especially in deeper parts of the planets, and would therefore have important implications for the chemical evolution of not only the Earth but also giant extrasolar terrestrial planets, such as recently discovered super-Earth (28).
Methods
The MgSiO3 glass was prepared from the synthetic stoichiometric MgSiO3 gel that has been used for the previous high-pressure experiments on the stability of the MgSiO3 postperovskite phase (29). The gel powder was placed in a platinum crucible, melted in air in a furnace at 1650 °C and quenched by immersing the base of the crucible in water. The composition and homogeneity of the final glass was confirmed by electron probe microanalysis and polarized microscope observation.
In situ high-pressure Brillouin scattering measurements of acoustic wave velocities were performed at room temperature with a symmetric diamond anvil cell, an argon laser (λ = 514.5 nm) as a light source, and Sandercock-type six-pass tandem Fabry–Perot interferometer to analyze the scattered light. The incident laser beam was focused to a spot size of approximately 20 μm. In all measurements, we used a symmetric scattering geometry with a 50° external scattering angle. A prepressed plate of MgSiO3 glass powder was loaded into a 50 μm hole drilled in the rhenium gasket, without a pressure-transmitting medium. The sample was compressed with 150 μm culet beveled diamond anvils. Pressure was determined using the Raman T2g mode of the diamond anvil (30) by measuring several points around the central area of the sample where the probe laser for Brillouin scattering measurements was irradiated. Representative Raman spectra and their differential spectra dI/dν from the different points are shown in Fig. S1. The high-frequency edge of the Raman band was defined as a minimum of the dI/dν, which is determined by curve fitting using a Gaussian function. As shown in Fig. S1 and Table S1, we obtained very sharp peaks for the dI/dν function at each pressure point, even at Mbar pressures, yielding measured pressures that were typically consistent within 1 GPa. Brillouin spectra were collected on the compressed MgSiO3 glass at 59 pressures in two separate runs from 12 to 203 GPa, in pressure increments of 2–6 GPa. The collecting time for a single Brillouin measurement was from 30 min to 12 h. At each pressure, the raw Brillouin spectra of Stokes and anti-Stokes peaks were fitted with a Gaussian function to determine peak locations. Additional experimental details are provided elsewhere (15).
Supplementary Material
Acknowledgments.
We thank Kohei Hatano for his valuable comments on data analysis, and Ed Stolper and anonymous reviewers for constructive suggestions. This study was supported by the Grant-in-Aid for Challenging Exploratory Research (21654075) by the Ministry of Education, Culture, Sports, Science and Technology, Japan (M.M.), and by the US National Science Foundation through Grants EAR-0738871 and the Consortium for Materials Properties Research in Earth Sciences (COMPRES) EAR 10-43050 (J.D.B.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1109748108/-/DCSupplemental.
References
- 1.Stolper EM, Walker D, Harger BH, Hays JF. Melt segregation from partially molten source regions: the importance of melt density and source region size. J Geophys Res. 1981;86:6261–6271. [Google Scholar]
- 2.Ohtani E. The primordial terrestrial magma ocean and its implication for stratification of the mantle. Phys Earth Planet In. 1985;38:70–80. [Google Scholar]
- 3.Stevenson DJ. In: Origin of the Earth. Newsom HE, Jones JH, editors. Oxford: Oxford University Press; 1990. pp. 231–249. [Google Scholar]
- 4.Cameron AG, Benz WB. The origin of the moon and the single impact. Icarus. 1991;92:204–216. doi: 10.1016/0019-1035(89)90129-2. [DOI] [PubMed] [Google Scholar]
- 5.Tonks WB, Melosh HJ. Magma ocean formation due to giant impact. J Geophys Res. 1993;98:5319–5333. [Google Scholar]
- 6.Canup RM. Dynamics of lunar formation. Annu Rev Astron Astr. 2004;42:441–475. [Google Scholar]
- 7.Agee CB, Walker D. Static compression and olivine flotation in ultrabasic silicate liquid. J Geophys Res. 1989;98:3437–3449. [Google Scholar]
- 8.Ohtani E, Maeda M. Density of basaltic melt at high presure and stability of the melt at the base of thelower mantle. Earth Planet Sc Lett. 2001;193:69–75. [Google Scholar]
- 9.Labrosse S, Hernlund JW, Coltice NA. Crystallizing dense magma ocean at the base of the earth's mantle. Nature. 2007;450:866–869. doi: 10.1038/nature06355. [DOI] [PubMed] [Google Scholar]
- 10.Williams Q, Garnero EJ. Seismic evidence for partial melting at the base of earth’s mantle. Science. 1996;273:1528–1530. [Google Scholar]
- 11.Garnero EJ, Revenaugh J, Williams Q, Lay T, Kellogg LH. In: The Core-Mantle Boundary Region. Gurnis M, Wysession ME, Knittle E, Buffett BA, editors. Washington, DC: American Geophysical Union; 1998. [Google Scholar]
- 12.Akins JA, Luo S-N, Asimow PD, Ahrens TJ. Shock-induced melting of MgSiO3 perovskite and implications for melts in earth’s lowermost mantle. Geophys Res Lett. 2004;31:L14612. [Google Scholar]
- 13.Gaudio SJ, Sen S, Lesher CE. Pressure-induced structural changes and densification of vitreous MgSiO3. Geochim Cosmochim Acta. 2008;72:1222–1230. [Google Scholar]
- 14.Lee SK, et al. X-ray raman scattering study of MgSiO3 glass at high pressure: implication for triclustered MgSiO3 melt in earth’s mantle. Proc Natl Acad Sci USA. 2008;105:7925–7929. doi: 10.1073/pnas.0802667105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murakami M, et al. Sound velocity of MgSiO3 post-perovskite phase: a constraints on the D′′ discontinuity. Earth Planet Sc Lett. 2007;259:18–23. [Google Scholar]
- 16.Murakami M, Bass JD. Spectroscopic evidence for ultrahigh-pressure polymorphism in SiO2 glass. Phys Rev Lett. 2010;104:025504. doi: 10.1103/PhysRevLett.104.025504. [DOI] [PubMed] [Google Scholar]
- 17.Hemley RJ, Mao H, Bell PM, Mysen B. Raman spectroscopy of SiO2 glass at high-pressure. Phys Rev Lett. 1986;57:747–750. doi: 10.1103/PhysRevLett.57.747. [DOI] [PubMed] [Google Scholar]
- 18.Williams Q, Jeanloz R. Soectroscopic evidence for pressure-induced coordination changes in slicate-glass and melts. Science. 1988;239:902–904. doi: 10.1126/science.239.4842.902. [DOI] [PubMed] [Google Scholar]
- 19.Meade C, Hemley RJ, Maeda M. High-pressure X-ray diffraction of SiO2 glass. Phys Rev Lett. 1992;69:1387–1390. doi: 10.1103/PhysRevLett.69.1387. [DOI] [PubMed] [Google Scholar]
- 20.Zha C-S, Hemley RJ, Mao H, Duffy TS, Meade C. Acoustic velocities and refractive index of SiO2 glass to 57.5 GPa by Brillouin scattering. Phys Rev B. 1994;50:13105–13112. doi: 10.1103/physrevb.50.13105. [DOI] [PubMed] [Google Scholar]
- 21.El’kin F, Brazhkin V, Khvostantsev L, Tsiok O, Lyapin A. In situ study of the mechanism of formation of pressure-densified SiO2 glasses. JETP Lett+ 2002;75:342–347. [Google Scholar]
- 22.Loerting T, Brazhkin V, Morishita T. Multiple amorphous-amorphous transitions. Adv Chem Phys. 2009;143:29–82. [Google Scholar]
- 23.Lin J-F, et al. Electronic bonding transition in compressed SiO2 glass. Phys Rev B. 2007;75:012201. [Google Scholar]
- 24.Sato T, Funamori N. Sixfold-coordinated amorphous polymorph of SiO2 under high pressure. Phys Rev Lett. 2008;101:255502. doi: 10.1103/PhysRevLett.101.255502. [DOI] [PubMed] [Google Scholar]
- 25.Stixrude L, Karki BB. Structure and freezing of MgSiO3 liquid in earth’s lower mentle. Science. 2005;310:297–299. doi: 10.1126/science.1116952. [DOI] [PubMed] [Google Scholar]
- 26.Ozawa H, et al. Chemical equilibrium between ferropericlase and molten iron to 134 GPa and implications for iron content at the bottom of the mantle. Geophys Res Lett. 2008;35:L05308. [Google Scholar]
- 27.Ozawa H, et al. Experimental study of reaction between perovskite and molten iron to 146 GPa and implications for chemically distinct buoyant layer at the top of the core. Phys Chem Miner. 2009;36:355–363. [Google Scholar]
- 28.Rivera EJ, et al. A ∼7.5 earth-mass planet orbiting the nearby star, GJ876. Astrophys J. 2005;634:625–640. [Google Scholar]
- 29.Murakami M, Hirose K, Kawamura K, Sata N, Ohishi Y. Post-perovskite phase transition in MgSiO3. Science. 2004;304:855–858. doi: 10.1126/science.1095932. [DOI] [PubMed] [Google Scholar]
- 30.Akahama Y, Kawamura H. High-pressure Raman spectroscopy of diamond anvils to 250 GPa. J Appl Phys. 2004;96:3748–3751. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.