Abstract
Phases of the iron–oxygen binary system are significant to most scientific disciplines, directly affecting planetary evolution, life, and technology. Iron oxides have unique electronic properties and strongly interact with the environment, particularly through redox reactions. The iron–oxygen phase diagram therefore has been among the most thoroughly investigated, yet it still holds striking findings. Here, we report the discovery of an iron oxide with formula Fe4O5, synthesized at high pressure and temperature. The previously undescribed phase, stable from 5 to at least 30 GPa, is recoverable to ambient conditions. First-principles calculations confirm that the iron oxide here described is energetically more stable than FeO + Fe3O4 at pressure greater than 10 GPa. The calculated lattice constants, equation of states, and atomic coordinates are in excellent agreement with experimental data, confirming the synthesis of Fe4O5. Given the conditions of stability and its composition, Fe4O5 is a plausible accessory mineral of the Earth’s upper mantle. The phase has strong ferrimagnetic character comparable to magnetite. The ability to synthesize the material at accessible conditions and recover it at ambient conditions, along with its physical properties, suggests a potential interest in Fe4O5 for technological applications.
Keywords: solid Earth, mineral physics, extreme conditions, density functional theory
Iron oxides have a widespread occurrence, being composed of the two most abundant elements on Earth. They are the most common strong magnetic phases in nature and have vast technological uses including semiconductors, pigments, catalysts, and iron extraction (1). Iron oxides are also important biominerals and are used in biomedical applications (2). At ambient pressure, six crystalline forms of iron oxides exist, including wüstite FeO, magnetite Fe3O4, and four phases of Fe2O3 (1, 3–7 and references therein). The basic atomic arrangements of these phases are simple; however, they show extensive nonstoichiometry and complex defect structures (8, 9) that complicate the understanding and modeling of their properties. The possibility to host extensive amounts of defects explains their solid-state redox activity. For thousands of years iron oxides have attracted scientific curiosity and challenged the modeling of their physical and chemical properties, so it is compelling to report the discovery of a phase that is not simply a polymorph of known iron oxides, but a distinct compound with formula Fe4O5.
Synthesis and Structure of Fe4O5
Fe4O5 was first synthesized in the diamond anvil cell from the breakdown products of siderite (FeCO3) at about 10 GPa and 1,800 K. A spherical crystal of about 8 μm in diameter grew in less than a minute upon heating (Fig. 1). Diffraction patterns were collected at high pressure and ambient temperature using highly focused synchrotron X-rays. The single crystal diffraction pattern (Fig. S1) was indexed with an orthorhombic C-centered cell with a = 2.8430(7) Å, b = 9.700(8) Å, c = 12.29(6) Å. Structural solution and refinement converged to a structure isomorphic to CaFe3O5 (10), with space group Cmcm; therefore, we propose the crystal to be a distinct oxide of stoichiometry Fe4O5 (Table 1). Refined and ab initio calculated atomic coordinates at 10 GPa are in excellent agreement (Table 2); occupancies of all sites are full within uncertainty (about 5%), supporting the inferred stoichiometry. The atomic arrangement of Fe4O5 can be described as a stacking along the c axis of layers of edge-sharing FeO6 octahedra and layers of face-sharing trigonal prisms, hosting Ca in the prototype phase (Fig. 2). There is close similarity between the structures of Fe4O5 and h-Fe3O4, the high-pressure polymorph of magnetite (11, 12). In the latter, edge-sharing octahedral layers are made of equivalent sites, whereas in the former two nonequivalent octahedral sites are arranged in a thicker layer. The similarity in the stacking perpendicular to the octahedral layers accounts for the similarity of two of the cell parameters of the two oxides; the thicker octahedral layer along the c axis in Fe4O5 accounts for a c-cell parameter that is about 3 Å longer than the analogous direction in h-Fe3O4.
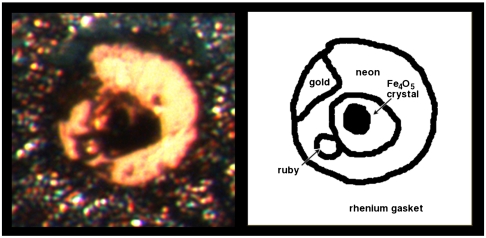
Fig. 1.
The single crystal of Fe4O5 synthesized in the diamond anvil cell at high pressure after laser heating. The sample chamber, about 60 μm in diameter, viewed through a diamond anvil at 10 GPa and its sketch show the rounded opaque crystal of Fe4O5 grown in the heated area. The sample is well separated from the gasket, ruby, and the anvils by the inert neon medium.
Table 1.
Summary of instrumental parameters and refinement statistical parameters for the single crystal structural analysis
| Beamline | 13 IDD, GSECARS, APS, ANL |
| Wavelength, Å | 0.3344 |
| Pressure, GPa | 10 |
| Temperature, K | 298 |
| Composition | Fe4O5 |
| Symmetry | orthorhombic, Cmcm |
| Lattice parameters a, b, c, Å | 2.8430 (7), 9.700 (8), 12.29 (6) |
| Volume, Å3 | 339 (2) |
| Z | 4 |
| Rint | 0.032 |
| Refl. range | -2 ≤ h ≤ 2, -7 ≤ k ≤ 7, -2 ≤ l ≤ 4 |
| θ range, ° | 3.5 ≤ θ ≤ 9.1 |
| N. reflections | 34 |
| N. independent | 20 |
| N. I > 2σ(I) | 20 |
| Refinement | F2 |
| R | 0.050 |
| wR2 | 0.134 |
Table 2.
Atomic fractional coordinates of Fe4O5
| Atom | Wyckoff position | Method | x | y | z |
| Fe1 | 4a | 0 | 0 | 0 | |
| Fe2 | 8f | SXD | 0 | 0.2616(4) | 0.1131(11) |
| DFT | 0 | 0.2623 | 0.1161 | ||
| XRPD | 0 | 0.2657 (4) | 0.1160 (3) | ||
| Fe3 | 4c | SXD | 0 | 0.5048(6) | 1/4 |
| DFT | 0 | 0.4995 | 1/4 | ||
| XRPD | 0 | 0.5038 (5) | 1/4 | ||
| O1 | 4c | SXD | 0 | 0.158(3) | 1/4 |
| DFT | 0 | 0.1567 | 1/4 | ||
| XRPD | 0 | 0.174 (2) | 1/4 | ||
| O2 | 8f | SXD | 0 | 0.365(3) | 0.55(1) |
| DFT | 0 | 0.3681 | 0.5375 | ||
| XRPD | 0 | 0.3566 (12) | 0.5389(10) | ||
| O3 | 8f | SXD | 0 | 0.085 (3) | 0.643 (9) |
| DFT | 0 | 0.0838 | 0.6422 | ||
| XRPD | 0 | 0.090 (1) | 0.6372 (9) |
SXD, X-ray structural refinement of single-crystal (10 GPa); XRPD, powder diffraction data (10.5 GPa); DFT, ab initio calculation (10 GPa).
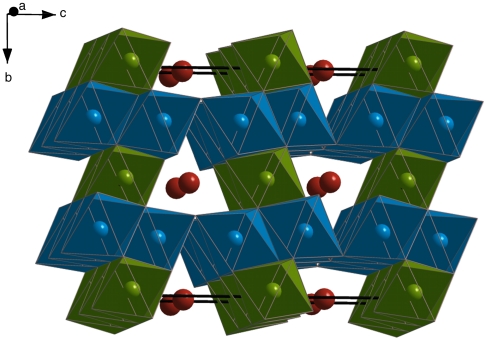
Fig. 2.
Structure of Fe4O5. Green and blue octahedra (sites Fe1 and Fe2, respectively) illustrate nonequivalent edge-sharing FeO6 groups forming layers perpendicular to the c axis. Layers are alternated by channels where iron, represented as red spheres (Site Fe3), is arranged in larger triangular prisms sharing faces along the direction of the a axis.
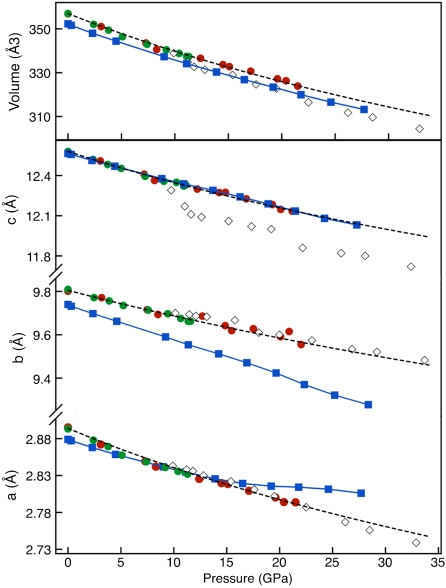
After synthesis and without further heating, the compressibility of the single crystal was measured up to 30 GPa (Fig. 3). The single crystal c lattice parameter shows a much greater decrease compared to powder diffraction data and first-principles calculations that are described later in this paper. We attribute this behavior to nonhydrostatic conditions developed as soon as pressure was increased after the synthesis. The single crystal grew at 10 GPa after the load was released from about 40 GPa. The sample chamber became very thin in the new compression cycle, because the gasket was deformed during the first compression and the crystal likely bridged the anvils. The single crystal c axis, nearly parallel to the load axis, shows therefore an apparent greater compressibility than in the powder samples.
Fig. 3.
Compressibility of Fe4O5. Pressure dependence of lattice parameters and volume of the unit cell. Data are from single crystal (open diamonds), two separate powder diffraction experiments (red and green circles), and from first-principles calculations (blue squares). Dashed black trends, interpolated from powder diffraction data, are reported as a visual aid. Standard deviations are smaller than the symbol sizes.
In order to explore the stability range of Fe4O5 in a pure Fe-O system, several syntheses were performed starting from mixtures of iron and Fe3O4 in appropriate proportions. Fe4O5 was readily synthesized at 10 and at 20 GPa, upon heating at 1,500–2,200 K. In most cases the high-pressure phase shows a rather coarse grain size (Fig. S2). Two samples were decompressed without further heating to ambient pressure. Remarkably, we found that Fe4O5 is a retrievable phase. One of the samples was heated during decompression; at about 5 GPa we observed its breakdown to magnetite and wüstite, showing that the observed phase has indeed intermediate composition between the two cubic oxides. The sample homogeneity and chemical gradients were monitored by collecting diffraction patterns in 2D grids across the sample area before and after most heating cycles. The analysis of individual patterns shows variable mixtures of fine unreacted material and more coarse high-pressure phases, not systematic chemical gradients. Because of the challenges in recovering and preparing the samples synthesized at high pressure as well as in measuring the Fe/O ratio, we could not independently determine the composition of the proposed phase. The bulk Fe/O ratio was, however, monitored from phase abundances determined by Rietveld refinement of the averaged patterns. Throughout the experiments the calculated Fe/O ratio shows random variations between 0.81 and 0.77, suggesting that no appreciable redox reaction occurred. Wide oscillation patterns were collected where Fe4O5 appeared to be the dominant phase and to have a finer size. The pattern of a fine grain powder collected at 10.5 GPa and that of a slightly textured sample collected at ambient pressure were selected for powder diffraction structural analysis (Fig. S3). Atomic fractional coordinates were refined for the pattern at 10.5 GPa; they were instead fixed to the values found for the single crystal in the analysis of the ambient conditions pattern. The Rietveld analysis converged with very satisfactory statistical parameters (Table S1) in both cases. Calculated atomic coordinates at 10.5 GPa are in good agreement with those from the refinement of the single crystal diffraction data and from first-principles calculations (Table 2).
Energetics and Stability of Fe4O5
To the best of our knowledge, Fe4O5 has not been previously described. Therefore, complementary first-principles calculations were carried out to examine the viability of Fe4O5 in terms of its energetics and phase stability under pressure. The enthalpies and volume-compression data of FeO, h-Fe3O4 [the high-pressure polymorph of magnetite (11, 12)] and Fe4O5 were calculated using an effective Hubbard parameter of Ueff = 2 eV for Fe-d electrons (see Materials and Methods, Fig. S4, and Table S2). The calculated lattice constants for Fe4O5 at 0 K and 0 GPa are a = 2.88 Å, b = 9.75 Å, c = 12.58 Å, in excellent agreement with the experimental values (Table S2). The calculated bulk moduli of FeO and h-Fe3O4 are 186.3 GPa and 184.4 GPa, respectively, in close agreement with previous studies (13, 14). The calculated bulk modulus of Fe4O5 is 185.7 GPa, in excellent agreement with the predicted value using Vegard’s law (ca. 0.5% deviation).
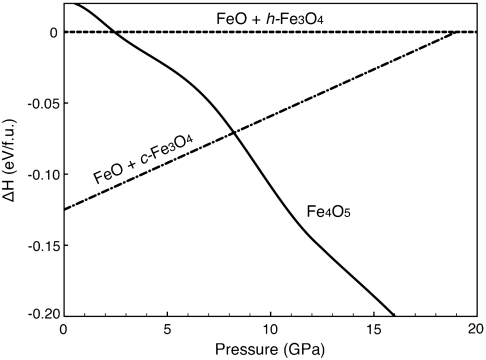
The enthalpy of Fe4O5 is computed and compared with the sum of the enthalpies of its possible breakdown products (FeO, cubic and orthorhombic Fe3O4, Fig. 4), revealing that Fe4O5 is about 0.096 eV less stable than h-Fe3O4 + FeO and about 0.783 eV less stable than c-Fe3O4 + FeO at 0 GPa. However, as pressure increases, Fe4O5 becomes energetically more favorable than the breakdown products (Fig. 4), providing complementary evidence that the synthesis of Fe4O5 is energetically plausible at high pressures. At 11 GPa, the average lengths of the Fe-O bonds are 1.944 Å (4a) and 2.076 Å (8f) in the octahedra and 2.089 Å (4c) in the trigonal prism. There is a significant charge transfer of about 1.6 e from Fe atoms to O atoms. Density functional calculations show that the proposed iron oxide is strongly ferrimagnetic (SI Text). The calculated lattice constants are a = 2.84 Å, b = 9.55 Å, and c = 12.34 Å at 11 GPa, close to experimental values. The structural changes of Fe4O5 under pressure can be closely followed by comparing the volume-compression data theoretically obtained from the first-principles calculations to the experimental data. In order to simulate the compression under hydrostatic conditions, all atoms and lattice constants were allowed to relax at each pressure point. The agreement between theory and experiment is remarkably good (cf. Fig. 3 and Table 2), providing a clear confirmation of Fe4O5. Nevertheless, further confirmation of its composition by direct methods will be pursued.
Fig. 4.
Enthalpies of Fe4O5 and of the plausible breakdown oxides FeO and h-Fe3O4. To examine the relative stability of Fe4O5 with respect to its possible breakdown products (FeO and Fe3O4 in the ambient and high-pressure structures), the enthalpy differences, ΔHc = H(Fe4O5) - [H(FeO) + H(c-Fe3O4)] and ΔHh = H(Fe4O5) - [H(FeO) + H(h-Fe3O4)], were calculated as a function of pressure. The solid, dashed, and dot-dashed lines represent the enthalpies of Fe4O5, ΔHh (the reference), and ΔHc, respectively. ΔHc is extracted from the literature (15). The Fe4O5 structure is favored over the sum of breakdown products at 10 GPa.
Physics, Geophysics, Geochemistry, and Material Science Implications.
The stoichiometry of the phase here described falls within the most puzzling region of the Fe-O phase diagram, between wüstite and magnetite (7, 16–19). With increasing temperature, at ambient pressure, the Fe-O phase diagram at 44 at. % Fe shows coexistence of iron and magnetite up to about 900 K, wüstite and magnetite up to 1,700 K, magnetite and liquid up to 1,900 K, and liquid at higher temperature. Coexisting wüstite and magnetite exhibit extensive degrees of nonstoichiometry and complex defect structures, depending on the synthesis temperature and cooling path. The two cubic phases share the same oxygen array and can grow coherently (8, 20, 21), providing a nearly continuous range of structures with random defect distributions, clustered defects, and coherently grown distinct phases. The high-pressure behavior of the binary phase diagram is relatively unexplored. With the exception of the Fe-rich portion (22–24), relevant to planetary cores, only the polymorphism of known oxides is well investigated. Wüstite is stable to very high pressure and temperature (25), whereas magnetite undergoes a phase transition at about 10 GPa (26) into a nonrecoverable high-pressure phase having strong similarities with the phase presented in this work. Our experiment suggests that Fe4O5 is stable from about 5 GPa to at least 30 GPa. This phase, with lower symmetry, layered structure, and all six-coordinated iron, is favored over the largely nonstoichiometric framework structures hosting four- and six-coordinated iron, showing that pressure changes the character of atomic ordering of iron oxides in the most complex, intriguing region of the binary phase diagram.
Density functional theory (DFT) calculations show that Fe4O5 is ferrimagnetic. Although the high-pressure polymorph of magnetite is known to be a semiconductor (26, 27), a detailed account of the electronic structure of Fe4O5 is beyond the scope of this study and will be the subject of a forthcoming investigation along with experimental efforts. As in the isostructural calcium ferrite (28), electron delocalization is expected among the edge-sharing iron sites with short Fe-Fe distances (Fig. S5). Furthermore, in Fe4O5 strong interactions should be expected among the face-sharing Fe3 sites. Given its distinctive physical properties, the relatively low pressure of synthesis and its recoverability, Fe4O5 is a potentially interesting material for technological applications. The isomorphism with CaFe3O5 suggests that the doping with large cations is likely to decrease the synthesis pressure while concurrently affecting physical properties.
The stability of oxides in the deep Earth is a complex function of pressure, temperature, oxygen fugacity, bulk composition, and partitioning equilibria among coexisting minerals. It is therefore difficult to predict the occurrence of Fe4O5 in the deep interior without extensive investigations of systems with realistic composition. Nonetheless, it is important to note that Fe4O5, stable at the P-T conditions of the Earth’s upper and lower mantles, is more likely to occur than Fe3O4 from the viewpoint of oxygen fugacity given its more reduced composition. As geophysical research advances, the description of the Earth’s interior becomes increasingly complex, including structural, mineralogical, and chemical lateral heterogeneity. Oxygen fugacity and bulk chemical fluctuations might likely determine variations in the stabilities of accessory oxide minerals. The occurrence of mixtures of wüstite and ferrite inclusions in diamonds (29) provides direct evidence of the existence of compositions close to Fe4O5 in the Earth’s mantle. It follows that Fe4O5 is a plausible accessory phase of the Earth’s interior at least in locally Fe-rich portions at highly zoned shallow depths. Being isostructural with CaFe3O5, Fe4O5 and h-Fe3O4 would likely host significant substitutions of calcium and other large cations, not common in spinels and wüstite, representing a possible different scenario for the element partitioning of iron oxides at depth. It should be noted that the geophysical and geochemical importance of Fe4O5 goes beyond its occurrence or abundance, revising the iron–oxygen phase diagram, which is a fundamental reference for the thermodynamics and oxygen fugacity of the deep Earth.
As all oxides of elements of multiple closely accessible valence states, we expect Fe4O5 to show defect structures depending on pressure, temperature, and oxygen fugacity. In Fe4O5, in addition to vacancies and interstitials that are common in the cubic oxides, stacking disorder might occur. The Fe4O5 and h-Fe3O4 structures have similar trigonal-prism units that are intercalated by octahedral layers of different thickness, and therefore stacking disorder involving Fe4O5 and h-Fe3O4 octahedral layers is plausible. As the structures of iron oxides are common to many binary systems and complex solid solutions, we anticipate that a rich set of isostructural compounds and solid solutions with tunable properties may be synthesized.
Materials and Methods
The first experiment, where the single crystal of Fe4O5 was obtained, was performed using a crystal of siderite (FeCO3) from Ivigtut, Greenland, as starting material. Several further syntheses were performed from mixtures of pure commercial magnetite and iron fine powders. Samples were loaded in diamond anvil cells along with the pressure gauges, e.g., gold (30) and a ruby sphere (31), and with Ne as a pressure medium. Ruby and gold were placed at the edge of the sample chamber; they were neither in contact with the sample nor were they heated. Experiments were conducted at the stations 13IDD, of GeoSoilEnviroCARS, Center for Advanced Radiation Sources, and 16IDB of High Pressure Collaborative Access Team, at the Advanced Photon Source, Argonne National Laboratory. Double-sided heating was performed with fiber lasers (32, 33). The structure was probed in situ with X-ray beams of 37 and 30 keV focused to 5 × 5 μm. Single crystal and powder diffraction patterns were collected with MAR165CCD detectors. Diffraction images were collected while the samples were rotated around the vertical axis by 30° to 70° according to the limitations imposed by the angular opening of the diamond anvil cell. GSE_ADA, RSV (34), Shelxl97 (35), FIT2D (36), General Structure Analysis System (GSAS) (37), and Endeavour (38) were used for data processing.
First-principles total energy calculations were performed using spin-polarized DFT as implemented in the Vienna ab initio simulation package (39). Structural and magnetic properties of Fe, FeO, h-Fe3O4, and Fe4O5 were calculated using the generalized gradient approximation (GGA) with the parameterization of Perdew and Wang (PW91) (40). The plane-wave cutoff energy for the electronic wave functions was set to 500 eV, ensuring the convergence of the total energy of the system to within 2 × 10-6 eV/atom. A periodic unit cell containing four formula units was used in the calculations for FeO (B1), h-Fe3O4 (Cmcm), and Fe4O5 (Cmcm). The Brillouin zone was sampled using the Monkhorst–Pack special k-point scheme (41) with a 3 × 3 × 3 mesh for structural optimization and total energy calculations. In general, the PW91 functional tends to underestimate the lattice constants of iron and iron oxides; the GGA+U method was introduced in the total energy calculations (42). The resulting lattice constants of Fe and magnetite are close to the measured values when Ueff = 2 (SI Text).
Supplementary Material
Acknowledgments.
We thankfully acknowledge the Department of Mineral Sciences, Smithsonian Institution for providing sample NMNH 17893-2 for research. The authors thank the anonymous reviewers for their valuable comments and suggestions. The University of Nevada, Las Vegas (UNLV) High Pressure Science and Engineering Center is supported by Department of Energy-National Nuclear Security Administration (NNSA) Cooperative Agreement DE-FC52-06NA262740. This work was performed at GeoSoilEnviroCARS (Sector 13), and at High Pressure Collaborative Access Team (HPCAT) (Sector 16), Advanced Photon Source (APS), Argonne National Laboratory (ANL). GeoSoilEnviroCARS is supported by the National Science Foundation—Earth Sciences (EAR-0622171) and Department of Energy (DOE)–Geosciences (DE-FG02-94ER14466). HPCAT is supported by Carnegie Institution of Washington, Carnegie/DOE Alliance Center, UNLV, and Lawrence Livermore National Laboratory through funding from DOE-NNSA, DOE-Basic Energy Services (BES), and National Science Foundation. APS is supported by DOE-BES, under Contract DE-AC02-06CH11357. E.K. and P.F.W. acknowledge funding through Subcontract 135947 with Los Alamos National Lab within the Department of Energy’s Fuel Cycle Research and Development program.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1107573108/-/DCSupplemental.
References
- 1.Cornell R, Schwertmann U. The Iron Oxides: Structure, Properties, Reactions, Occurrences, and Uses. Weinheim, Germany: Wiley-VCH; 2003. [Google Scholar]
- 2.Gupta A, Gupta M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials. 2005;26:3995–4021. doi: 10.1016/j.biomaterials.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 3.Bragg W. The structure of magnetite and the spinels. Nature. 1915;95:561–561. [Google Scholar]
- 4.Goldschmidt VM. Geochemische Verteilungsgesetze der Elemente. Skrifter Norske Videnskaps-Akad, Oslo I Mat Naturv Klasse. 1926:112–117. [Google Scholar]
- 5.Thewlis J. The structure of ferromagnetic ferric oxide. Philos Mag. 1931;12:1089–1106. [Google Scholar]
- 6.Hagg G. The crystal structure of magnetic ferrioxide, gamma Fe2O3. Z Phys Chem B. 1935;E29:95–103. [Google Scholar]
- 7.Darken L, Gurry R. The system iron oxygen. I. The wüstite field and related equilibria. J Am Chem Soc. 1945;67:1398–1412. [Google Scholar]
- 8.Andersson B, Sletnes JO. Decomposition and ordering in Fe1-xO. Acta Crystallogr A. 1977;33:268–276. [Google Scholar]
- 9.Schweika W, Hoser A, Martin M, Carlsson AE. Defect structure of ferrous oxide Fe1-xO. Phys Rev B Condens Matter. 1995;51:15771–15788. doi: 10.1103/physrevb.51.15771. [DOI] [PubMed] [Google Scholar]
- 10.Evrard O, Jeannot F, Tannieres N, Aubry J. Determination of orthorhombic cell of CaFe3O5 by isotypy with Ca3Y2Fe19O30. Rev Chimi Miner. 1976;13:492–496. [Google Scholar]
- 11.Fei Y, Frost DJ, Mao HK, Prewitt CT, Hausermann D. In situ structure determination of the high-pressure phase of Fe3O4. Amer Mineral. 1999;84:203–206. [Google Scholar]
- 12.Haavik C, Stolen S, Fjellvag H, Hanfland M, Hausermann D. Equation of state of magnetite and its high-pressure modification: Thermodynamics of the Fe-O system at high pressure. Amer Mineral. 2000;85:514–523. [Google Scholar]
- 13.Alfredsson M, et al. Structural and magnetic phase transitions in simple oxides using hybrid functionals. Mol Simul. 2005;31:367–377. [Google Scholar]
- 14.Klotz S, et al. Magnetism and the Verwey transition in Fe3O4 under pressure. Phys Rev B Condens Matter. 2008;77:012411. [Google Scholar]
- 15.Lazor P, Shebanova O, Annersten H. High-pressure study of stability of magnetite by thermodynamic analysis and synchrotron x-ray diffraction. J Geophys Res. 2004;109:B05201. [Google Scholar]
- 16.Darken L, Gurry R. The system iron oxygen. II. Equilibrium and thermodynamics of liquid oxide and other phases. J Am Chem Soc. 1946;68:798–816. [Google Scholar]
- 17.Wriedt H. The Fe-O (iron-oxygen) system. J Phase Equilib. 1991;12:170–200. [Google Scholar]
- 18.Sundman B. An assessment of the Fe-O system. J Phase Equilib. 1991;12:127–140. [Google Scholar]
- 19.Fabrichnaya O, Sundman B. The assessment of thermodynamic parameters in the Fe-O and Fe-Si-O systems. Geochim Cosmochim Acta. 1997;61:4539–4555. [Google Scholar]
- 20.Wang YG, Ping D, Guo J. High-resolution transmission-electron-microscopy observation of the ultra-fine structure of natural magnetite. J Appl Crystallogr. 1994;27:96–102. [Google Scholar]
- 21.Uchida H, et al. Investigation of synthetic Mg1.3V1.7O4 spinel with MgO inclusions: Case study of a spinel with an apparently occupied interstitial site. Amer Mineral. 2007;92:1031–1037. [Google Scholar]
- 22.McCammon CA, Liu C. The effects of pressure and temperature on nonstoichiometric wüstite, FexO: The iron-rich phase-boundary. Phys Chem Miner. 1984;10:106–113. [Google Scholar]
- 23.Ringwood AE, Hibberson W. The system Fe-FeO revisited. Phys Chem Miner. 1990;17:313–319. [Google Scholar]
- 24.Seagle CT, Heinz DL, Campbell AJ, Prakapenka VB, Wanless ST. Melting and thermal expansion in the Fe-FeO system at high pressure. Earth Planet Sci Lett. 2008;265:655–665. [Google Scholar]
- 25.Ozawa H, Hirose K, Tateno S, Sata N, Ohishi Y. Phase transition boundary between B1 and B8 structures of FeO up to 210 GPa. Phys Earth Planet Int. 2010;179:157–163. [Google Scholar]
- 26.Schollenbruch K, Woodland AB, Frost DJ, Langenhorst F. Detecting the spinel-post-spinel transition in Fe3O4 by in situ electrical resistivity measurements. High Press Res. 2009;29:520–524. [Google Scholar]
- 27.Xu W, Machavariani G, Rozenberg G, Pasternak M. Mössbauer and resistivity studies of the magnetic and electronic properties of the high-pressure phase of Fe3O4. Phys Rev B Condens Matter. 2004;70:174106. [Google Scholar]
- 28.Gerardin R, Millon RE, Brice J, Evrard O, Lecaer OG. Electronic transfer of intervalence between unequal sites—The study of CaFe3O5 using Mössbauer spectrometry. J Phys Chem Solids. 1985;46:1163–1171. [Google Scholar]
- 29.Wirth R, Dobrzhinetskaya LF, Harte B, Green HW. Tubular Mg-ferrite in Mg-wüstite inclusions in diamond of superdeep origin: Control of Fe-valence by crystallographic structure. EOS Transactions AGU. 2009;90:14–18. Fall Meeting Suppl, Abstract V54B-07. http://www.agu.org/meetings/fm09/waisfm09.html. [Google Scholar]
- 30.Fei Y, et al. Toward an internally consistent pressure scale. Proc Natl Acad Sci USA. 2007;104:9182–9186. doi: 10.1073/pnas.0609013104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mao HK, Xu J, Bell P. Calibration of the ruby pressure gauge to 800-kbar under quasi-hydrostatic conditions. J Geophys Res. 1986;91:4673–4676. [Google Scholar]
- 32.Meng Y, Shen G, Mao HK. Double-sided laser heating system at HPCAT for in situ x-ray diffraction at high pressures and high temperatures. J Phys Condens Matter. 2006;18:S1097–S1103. doi: 10.1088/0953-8984/18/25/S17. [DOI] [PubMed] [Google Scholar]
- 33.Prakapenka VB, et al. Advanced flat top laser heating system for high pressure research at GSECARS: Application to the melting behavior of germanium. High Press Res. 2008;28:225–235. [Google Scholar]
- 34.Dera P, et al. High-pressure polymorphism of Fe2P and its implications for meteorites and Earth’s core. Geophys Res Lett. 2008;35(L10301) [Google Scholar]
- 35.Sheldrick GM. A short history of SHELX. Acta Crystallogr A. 2008;64:112–122. doi: 10.1107/S0108767307043930. [DOI] [PubMed] [Google Scholar]
- 36.Hammersley A, Svensson S, Hanfland M, Fitch A, Hausermann D. Two-dimensional detector software: From real detector to idealised image or two-theta scan. High Press Res. 1996;14:235–248. [Google Scholar]
- 37.Larson A, Von Dreele R. General structure analysis system (GSAS) LANL Report LAUR 86-748. 2000.
- 38.Crystal Impact. Endeavour 1.7. Bonn, Germany: Crystal Impact GbR; 2009. [Google Scholar]
- 39.Kresse G, Furthmuller J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys Rev B Condens Matter. 1996;54:11169–11186. doi: 10.1103/physrevb.54.11169. [DOI] [PubMed] [Google Scholar]
- 40.Perdew J, Wang Y. Accurate and simple analytic representation of the electron-gas correlation- energy. Phys Rev B Condens Matter. 1992;45:13244–13249. doi: 10.1103/physrevb.45.13244. [DOI] [PubMed] [Google Scholar]
- 41.Monkhorst H, Pack J. Special points for Brillouin-zone integrations. Phys Rev B Condens Matter. 1976;13:5188–5192. [Google Scholar]
- 42.Dudarev S, Botton G, Savrasov S, Humphreys C, Sutton A. Electron-energy-loss spectra and the structural stability of nickel oxide: An LSDA+U study. Phys Rev B Condens Matter. 1998;57:1505–1509. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.