Abstract
The axoneme forms the essential and conserved core of cilia and flagella. We have used cryo-electron tomography of Chlamydomonas and sea urchin flagella to answer long-standing questions and to provide information about the structure of axonemal doublet microtubules (DMTs). Solving an ongoing controversy, we show that B-tubules of DMTs contain exactly 10 protofilaments (PFs) and that the inner junction (IJ) and outer junction between the A- and B-tubules are fundamentally different. The outer junction, crucial for the initiation of doublet formation, appears to be formed by close interactions between the tubulin subunits of three PFs with unusual tubulin interfaces; other investigators have reported that this junction is weakened by mutations affecting posttranslational modifications of tubulin. The IJ consists of an axially periodic ladder-like structure connecting tubulin PFs of the A- and B-tubules. The recently discovered microtubule inner proteins (MIPs) on the inside of the A- and B-tubules are more complex than previously thought. They are composed of alternating small and large subunits with periodicities of 16 and/or 48 nm. MIP3 forms arches connecting B-tubule PFs, contrary to an earlier report that MIP3 forms the IJ. Finally, the “beak” structures within the B-tubules of Chlamydomonas DMT1, DMT5, and DMT6 are clearly composed of a longitudinal band of proteins repeating with a periodicity of 16 nm. These findings, discussed in relation to genetic and biochemical data, provide a critical foundation for future work on the molecular assembly and stability of the axoneme, as well as its function in motility and sensory transduction.
Keywords: microtubule stability, cilia, axoneme, ciliopathies, cytoskeleton
Cilia and flagella play important roles in cellular motility, signal transduction, embryonic development, and human disease (1–3). The most conserved features of these organelles are the nine outer doublet microtubules (DMTs) that assemble as extensions of the triplet microtubules (MTs) of centriolar basal bodies. Biochemical and structural evidence indicates that the molecular architecture of DMTs has been conserved since their evolution in eukaryotic cells ∼850 Mya (4).The DMT is formed by structural proteins (e.g., tubulin, tektin) that provide for the attachment of effector molecules (e.g., dyneins, regulatory signaling proteins) in a highly specific and repetitive manner. Posttranslational modifications of the DMTs are also critical for the mechanochemical function of dynein arms (5, 6). A better understanding of the assembly and function of cilia and flagella, as well as their role in disease, therefore requires a greater appreciation of the fundamental structure of the DMT.
Although the polymerization of singlet MTs in vitro is routine, DMTs or triplet MTs have thus far not been assembled in vitro. This is probably attributable to the complex proteome of the DMTs (7). Also not understood are the assembly mechanisms of DMTs and how they establish the different periodicities of attachment sites for associated complexes, such as the dynein arms or radial spokes. In the past 50 y, axonemes have been studied in great detail using EM, including several recent cryo-electron tomography (ET) studies (8–14). However, few axonemal structures have attracted more controversy about their architecture than the DMTs. First, whereas the A-tubules are composed of 13 protofilaments (PFs) (15) similar to native cytoplasmic MTs, the number of PFs in the B-tubules, 10 or 11, has been the subject of ongoing debate (16). Second, the nature of the junctions that the B-tubule makes with the A-tubule is unknown. The inner junction (IJ) and outer junction are sterically distinct, because different faces of the tubulin subunits participate in forming the two junctions. In addition, several genetic mutations appear to affect the inner vs. outer junction differentially, resulting in unusual phenotypes and disease in vertebrates (17–19). Finally, although structurally periodic microtubule inner proteins (MIPs*) that associate with the inner surfaces of the DMT walls were independently reported in 2006 (8, 10), these studies differed significantly with respect to the descriptions of these structures.
In this paper, we use cryo-ET and subtomogram averaging of intact flagella and axonemes from Chlamydomonas and sea urchin sperm to answer several questions and controversies unequivocally, as well as to provide information about the structure of DMTs. We discuss these findings in relation to what is known about the functional analyses of DMT components and their assembly. These results will be necessary to guide and interpret future functional analyses.
Results
Improved Resolution of the DMT Structure.
Several cryotomograms were reconstructed from rapidly frozen intact axonemes from WT and mutant Chlamydomonas and from intact sea urchin sperm flagella or isolated DMTs. These 3D reconstructions were used for subtomographic averaging of the 96-nm axoneme repeat unit (Fig. 1, Fig. S1, and Table 1). The significant improvement in resolution compared with our previous publications (e.g., compare Fig. S2 A–F with A′–F′) was mainly achieved in three ways: (i) by a local alignment approach concentrated on the DMTs, which reduced the influence of associated structures that might show structural variability, and thus introduce alignment errors; (ii) by advancements in the subtomogram averaging software PEET (http://bio3d.colorado.edu/PEET/) (10); and (iii) by increasing the number of averaged repeat units. Depending on the number of particles averaged and data quality, the resolution, based on the 0.5 Fourier shell correlation criterion, ranged from 3.3 to 3.9 nm for Chlamydomonas and sea urchin sperm flagella (Fig. 1C and Table 1). The increased signal-to-noise ratio and resolution in the averages generated clear images that revealed unprecedented details about the DMT structure (Figs. 1–4 and Movie S1). Nominally, the resolution of our 96-nm repeats is not better than the 3.0 nm reported for subtomographic averages of 16-nm repeating units of isolated DMTs from sea urchin sperm flagella (8). However, in contrast to isolated DMTs, the cylindrical organization of intact axonemes is ideal for averaging repeats with many different orientations in relation to the tilt axis, and thus for compensating for the missing wedge of data typical for single-axis ET (Fig. S3). Therefore, the resolution of our final averages was isotropic in all directions without missing wedge distortions (Fig. 1 A, B, and D–F, and Fig. S3).
Fig. 1.
Cryo-ET provides an overview of the 3D structure of DMTs. Tomographic slices (A and B) and isosurface renderings (D–F) of averaged axonemal repeats from Chlamydomonas pseudo-WT (pWT; Table 1) show cross-sectional (A and D), longitudinal (B), and oblique (E and F) views of the DMT. The red lines in A indicate the cutting plane of the slice shown in B. In the surface renderings, only the DMT core is shown, whereas all peripheral structures [e.g., inner or outer dynein arm (IDA or ODA, respectively)] were removed but their positions are indicated in A (surface rendering overview with associated structures is shown in Fig. S1). PF numbers [according to Linck and Stephens (16)] are colored pink in the A-tubule (At) and dark blue in the B-tubule (Bt). In B, prominent left-handed helical lines with an 8-nm axial periodicity are apparent, probably corresponding to the helical lattice of tubulin subunits (28, 49). The IJ and trimeric outer junction (OJ) have distinct structures. Colored arrowheads point to MIP1 (light blue), MIP2 (red), MIP3 (yellow), and MIP4 (orange). DMT cross-sections are viewed from a proximal orientation (flagellar base) toward a distal (flagellar tip) orientation, and in the longitudinal view, the left side is proximal. The DMT orientations, labels, and colors shown here are used consistently in all subsequent figures unless otherwise noted and are valid for all panels. (C) Resolution of the DMT averages used in this study ranged from 3.3 to 3.9 nm (0.5 criterion of the Fourier shell correlation method). More details are provided in Table 1. (Scale bar: 10 nm.)
Table 1.
Strains used in this study and averaging details*
| Name | Strain(s) | Ref. | Structure visualized | Averaged repeats | Resolution, nm | Shown in figures |
| Chlamydomonas | ||||||
| pWT | pf2-4::PF2-GFP† | 14 | DMTs (DMT1–9) | 720 | 3.4 | 1 A, B, and D–F; 2; 3 A–F; 4 C–J; S1; S2 A′–F′; S3 F and G |
| DMTs without beak-MIP (DMT2, DMT3, DMT4, and DMT7–9) | 590 | 3.4 | 4 A and B | |||
| DMTs with beak-MIP (DMT1, DMT5, and DMT6) | 210 | 3.9 | 4 K–N | |||
| drc Mutants‡ | pf3 (CC-1026) | 14, 22 | Inner AB-junction without hole (DMT1–9) | 1,980 | 3.3 | 3 G and H |
| ida6 (CC-3090) | 23 | |||||
| 4D6 (CaM-IP2 amiRNAi) | 24 | |||||
| S. purpuratus (sea urchin) | ||||||
| Flagella | DMTs (DMT1–9) | 470 | 3.6 | S2 A′′–F′′, G, H, and J | ||
| Isolated DMT | Inner AB-junction (DMT1–9) | 118 | 3.8 | S2I | ||
*Some Chlamydomonas data were refined from data originally published by Nicastro et al. (10) and Heuser et al. (14) and were combined with previously undescribed data to improve resolution.
†A pseudo-WT (pWT) strain was obtained by transformation of the pf2 mutant with the WT PF2-GFP gene, which rescues the structural defects in the pf2 mutant (14).
‡Axonemal repeats from three different mutants with defects in the assembly of the N-DRC base plate were averaged together.
Fig. 4.
MIP1–4 and beak-MIP in Chlamydomonas axonemes show complex organization. Axonemal repeats from either all nine pseudo-WT DMTs (A–J) or only from DMT1, DMT5, and DMT6, which have the beak-MIP (K–N), were averaged. The MIP structures are shown in tomographic slices (A, C, E, G, I, K, and M) and isosurface renderings (B, D, F, H, J, L, and N) in cross-sectional (A, B, K, and L) and longitudinal views (C–J, M, and N). The blue, red, yellow, and orange lines in B and the green line in L indicate the cutting plane of the slices shown in C, E, G, I, and M, respectively. PFs of the A- and B-tubules (At and Bt) were numbered in pink and dark blue, respectively. In the isosurface renderings, the electron densities for MIP1–4 and the beak-MIP are color-coded (including the arrows and arrowheads pointing toward the MIPs): MIP1a and MIP1b (light and dark blue, respectively), MIP2a and MIP2b (light and dark red, respectively), MIP3a and MIP3b (yellow and olive, respectively), MIP4 (orange), and beak-MIP (green; also black and green brackets in L and M, respectively). The black arrows in E and H point to the hole in the IJ-density. (Scale bar: 20 nm).
B-Tubule Has 10 PFs.
Although there is general agreement that the A-tubules of most species are formed by 13 PFs (15), the number of PFs within the B-tubule has been controversial, being either 10 or 11 PFs (16). Our data clearly show a B-tubule with 10 PFs, of which at least PFs B2 to B10† appear to be identical (Fig. 1 A and D). Most lateral PF interactions in the DMT resemble those typically found in cytoplasmic 13 PF MTs [i.e., on the outside of the DMT, the PFs are separated by deep grooves and appear aligned parallel with the MT axis without supertwist (Fig. 1E)]. Inter-PF contacts are established at the bottom of the grooves (Fig. 1 D–F), whereas the inner MT surface appears to be relatively smooth at this resolution (Fig. 1F). The maximum thickness of the MT wall measured at the center of a PF is ∼4 nm. In cross-section, the A-tubule is slightly oval-shaped, with the short axis running perpendicular to the DMT “partition” and the five PFs of the A-tubule bounded by the two junctions with the B-tubule (PFs A10–A13 and A1) (Fig. 1 A and D).
Distinctly Different Outer and Inner A- and B-Tubule Junctions.
The outer A- and B-tubule junction (AB-junction) is built by three PFs (A10, A11, and B1) that interact closely in a trimeric fashion (Fig. 3 A and C and Fig. S1). The distance between PFs A11 and B1 appears to be larger than that between PFs A10 and B1, indicating a larger interface and/or the presence of accessory proteins between A10 and B1. In the center between the three PFs runs a channel devoid of significant electron density (Figs. 2 and 3 A–C). The cross-section of most PFs, including A10, A11, and B2–B10, appears triangular- to oval-shaped, with a vertex oriented outward (most PFs in Figs. 2A and 3C), which generates the grooved appearance of the outer MT surface, as expected from what is known about typical 13 PF MT lattices. However, the outer surface of the trimeric outer junction seems remarkably flat in the region of PF B1, and tomographic cross-sections do not show the typical orientation of an oval density for B1 (Figs. 1A, 2, and 3C). X-ray structures of tubulin placed into the B1 density in the conventional orientation clearly reach into the channel that lacks obvious electron density in our averages, making this orientation unlikely (Fig. 3C).
Fig. 3.
Outer and inner AB-junctions are fundamentally different. Isosurface renderings (A, C, D, F, and H) and tomographic slices (B, E, and G) of averaged axonemal repeats from Chlamydomonas pseudo-WT (A–F) and drc mutants (details are provided in Table 1) that lack the hole in the IJ (G and H) are displayed in cross-sectional (A, C, and D) and longitudinal (B and E–H) views. Pink and dark blue numbers identify the PFs of the A- and B-tubules (At and Bt), respectively. Arrowheads point to MIP1 (light blue), MIP2 (red), MIP3 (yellow), and MIP4 (orange). Red lines in A and D indicate the orientation of the longitudinal slices in B, E, and G, respectively. Details of the outer junction (OJ; cyan outline) in an overview (A) and in a close-up (C) of the area indicated by the dashed box in A. PFs A10, A11, and B1 surround a channel (cyan arrow in B and C) that lacks significant EM density. The crystal structures of tubulin dimers were placed in the EM density and filtered to a resolution of ∼2 nm (pink and blue densities); note that the displayed orientation of tubulin-PF B1 (density highlighted in red) is not consistent with the observed empty channel. Details of the IJ (purple-colored density) that connects PFs A1 and B10 in an overview (D) and in a side-by-side comparison between DMTs with a WT phenotype (E and F) and drc mutants that lack major components of the nexin–dynein regulatory complex (N-DRC) (G and H). Longitudinal sections through the IJ-density reveal ladder rung-like densities with 8-nm periodicity (indicated by 3 white lines in E–H), instead of a continuous filamentous structure like the PFs (e.g., compare IJ with PFs A11–A13 in B). In DMTs with a WT phenotype (E and F), the IJ-rung closest to the N-DRC base plate is missing, leaving a hole within the IJ-density (red arrow in B, E, and F); when the N-DRC base plate is absent, however, as in the drc mutant pf3, the IJ-density is uninterrupted [i.e., no hole is observed (red dashed circle in G and H)]. (Scale bar: 20 nm.)
Fig. 2.
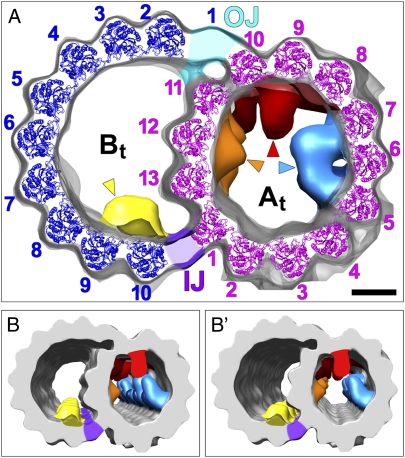
3D structure of DMTs reveals several MT-associated electron densities. Isosurface renderings of the averaged axonemal repeat from Chlamydomonas pseudo-WT show the DMT in cross-sectional views. (A) Crystal structures of the α/β-tubulin dimer were placed into the electron density of the DMT PFs, except for PF B1 (details are provided in the main text). No obvious differences were observed between the 13 A-tubule PFs; however, this does not exclude the possibility that one of them is composed of tektin instead of tubulin (69). Tubulin dimers and PF numbers are both colored pink in the A-tubule (At) and dark blue in the B-tubule (Bt). Note the channel that passes through the trimeric (PF A10-A11-B1) outer junction (OJ). The connecting density between PFs A1 and B10 at the IJ (purple-colored density) is relatively thin, suggesting that it is not a tubulin PF. Four additional features are present inside the DMT lumen (arrowheads and colored density): MIP1 (light blue), MIP2 (red), MIP3 (yellow), and MIP4 (orange). (Scale bar: 5 nm.) (B and B′) Stereo images provide a 3D view of the DMT and its associated inner structures.
The inner AB-junction (closest to the radial spokes) is built differently from the outer junction. It is formed by a density, previously referred to as “B-11th density” (reviewed in 16), which bridges a distance of ∼8 nm between PFs A1 and B10. This density is only ∼3 nm thick in cross-sectional views, and thus is clearly thinner than a tubulin PF across the MT wall (Figs. 1 A and D and 2). It also lacks the prominent ridge on the MT outer surface, and thus appears relatively smooth on both inner and outer surfaces (Figs. 1F and 2). Most markedly, longitudinal tomographic slices (along the MT axis) through the PFs A1 and B10 do not show a continuous filamentous density at the inner AB-junction, as would be expected for longitudinally associated tubulin subunits. Instead, the arrangement of the inner AB-junction has a ladder-like appearance, with somewhat globular rungs connecting the A- and B-tubules with an axial periodicity of 8 nm (Fig. 3 E–H). To differentiate the nonfilamentous density of the inner AB-junction from the tubulin PFs, we will refer to this density as the IJ-proteins. The shapes of the α- and β-tubulins are very similar to each other, and the resolution of our data is not sufficient to discriminate whether the IJ-proteins bind to the α- and/or β-tubulin subunits of the A- and B-tubules.
Tomographic slices through the outer and inner AB-junctions of averaged sea urchin sperm flagella and isolated DMTs show the same architecture of a trimeric outer junction with a channel that lacks density (Fig. S2 D′′, E′′, and H) and an IJ that contains rungs with 8-nm axial periodicity connecting PFs A1 and B10 (Fig. S2 I and J). In WT Chlamydomonas axonemes and sea urchin sperm flagella, one of the IJ-rungs near the nexin–dynein regulatory complex (N-DRC) is missing, giving the appearance of a “hole” in the B-tubule with 96-nm periodicity (14) (Fig. 3 B, E, and F and Fig. S2 H and J). The similarities between the Chlamydomonas and sea urchin images indicate that the fundamental features of the AB-junctions in DMTs are conserved across species. Interestingly, in drc mutants like pf3 (Table 1), which lack the N-DRC base plate normally attached to PFs A2–A4, the hole in the inner AB-junction is filled (14) (Fig. 3 G and H), presumably by regular IJ-protein(s).
A-Tubule MIP1, MIP2, and MIP4.
Classic EM studies of chemically fixed flagella have occasionally observed electron-dense material in the lumens of axonemal MTs, especially in insect sperm tails (20, 21). Two recent reports have described MIPs that bind periodically to the luminal side of the DMT walls (8, 10) (Fig. S2 A–F). Meanwhile, other studies have described MIPs associated with the inner surface of subpellicular MTs in some protozoans (25), as well as periodic luminal particles in cytoplasmic MTs of various cells and tissues (26, 27). The subpellicular particles appear to be periodically attached to the MT walls in a fashion similar to the axonemal MIPs, whereas cytoplasmic MT particles seem to be suspended within the MT lumen with less obvious interactions with the MT walls.
We previously reported that the A-tubule contains two sets of MIPs, MIP1 and MIP2, that attach to the luminal side of PF A5 and between PFs A9/A10, respectively (10) (Fig. S2E). Additional material appears along the A-luminal side of PFs A11–A13 of the partition; in sea urchin sperm flagella, this material is relatively thick, whereas it is less noticeable in Chlamydomonas doublets (10). In our previous study, both MIP1 and MIP2 appeared as rows of globular densities that seemed identical within their respective row. MIP1 was reported to repeat at 8-nm intervals, and MIP2 was reported to repeat at 16-nm intervals (10) (Fig. S2 A and B). These results were based on averaging only 120 axonemal 96-nm-long repeats, limiting the resolution to 4.3 nm (10).
We have now obtained significantly improved averages with a better signal-to-noise ratio and resolution (Fig. 1C and Table 1), revealing previously undescribed details about the axonemal MIPs (Fig. 4). Our averages reveal that the structure of MIP1 is more complex than previously thought. The row of MIP1 consists of two different types of subunits: a longer subunit, MIP1a, arches from PF A5 to A7 and repeats every 16 nm, with intervening shorter subunits, MIP1b, that are attached only to PF A5 (Figs. 2 and 4 C and D). The long and short MIP1 particles seem to alternate “a-b-,” whereby each “dash” represents an 8-nm center-to-center spacing, generating an apparent 16-nm periodicity with an 8-nm subperiodicity (Fig. 4 C and D). Because the short 8-nm repeat matches the tubulin dimer repeat, each MIP1 must bind consistently to the same location on the tubulin dimer. Although the short subunits, MIP1b, are highly uniform, the long subunits, MIP1a, seem to be more heterogeneous, where some appear thinner and only partially connected between the densities on PFs A5 and A7 (Fig. 4D). Higher resolution data will be required to determine whether these small differences between the MIP1a units are significant, which would suggest a 48-nm rather than a 16-nm periodicity.
The recently obtained averages corroborate that most of the MIP2 densities bind to PFs A9/A10 but that MIP2 is more complex, with an overall periodicity of 48 nm. MIP2a reaches further into the A-tubule lumen and is attached only to PFs A9/A10, whereas the MIP2b subunit does not reach as far into the lumen but runs along the MT wall from PF A8–A12 of the partition (Figs. 2 and 4 E and F). Two longer and one shorter subunit generate the sequence “a-a-b-,” whereby each dash represents a 16-nm center-to-center spacing, effectively generating a 48-nm periodicity with a 16-nm subperiodicity (Fig. 4 F and J). Apart from the MIP2b densities, three additional shallow densities per 48-nm repeat, named MIP4, are distributed along the inner surface of PFs A12 and A13 of the partition (Figs. 2 and 4 F, I, and J).
Averages of sea urchin sperm flagella reveal the same general organization of MIPs in the DMTs as described for Chlamydomonas. Features that are conserved between species include the “a-b-” alternating MIP1a/MIP1b densities with an overall 16-nm periodicity (Fig. S2A′′) and the 16-nm spaced MIP2 densities that might have different lengths (Fig. S2B′′), but our resolution of sea urchin flagella is insufficient to confirm a 48-nm periodicity. A major difference between the Chlamydomonas and sea urchin flagellar DMTs is the structure of the partition. In Chlamydomonas, PF A11–A13 appear to be typical PFs with bump-shaped densities, MIP2b and MIP4 with 48-nm periodicities, attached to the inside wall of the A-tubule (Figs. 1 A and D–F; 2; and 4 I and J and Fig. S2 D′ and E′). In contrast, in the sea urchin, the center of the partition is twice as thick in cross-sectional views and seems to be built of two nearly equally thick walls that are separated by a narrow gap (Fig. S2 D′′ and E′′). The part of the partition wall facing the lumen of the B-tubule overlays well with the densities of the PFs seen in Chlamydomonas DMTs, whereas the density facing the lumen of the A-tubule in sea urchin DMTs seems to arise from additional material with a remarkably smooth surface (Fig. S2 D′′–G).
B-tubule MIP3 and Beak Structure.
MIP3 was previously described as a globular structure positioned between PFs B9 and B10 on the inside of the B-tubule and with an axial repeat of 16 nm (10). Our data provide more detailed views of MIP3, showing that the large densities, MIP3a, form arches connecting PFs B9 and B10 (Figs. 2 and 4 G and H). These arches appear to follow the left-handed helix of the B-tubule (28, 29), indicating that MIP3a binds to the same region of the tubulin-dimer on both PFs; however, greater resolution would be required to distinguish the actual binding site. MIP3a is arranged with a 16-nm axial periodicity, as described previously (10); however, between each 16-nm repeat, we found additional smaller spike-shaped densities, MIP3b (Fig. 4 F–H). This alternating pattern of “a-b-,” whereby each dash represents an 8-nm center-to-center spacing, generating an apparent 16-nm periodicity with an 8-nm subperiodicity, was also found for MIP3 in sea urchin sperm flagella (Fig. S2C′′).
Cilia and flagella of different species possess unique structural features that relate to motility and/or sensory regulation. In the proximal portion of Chlamydomonas axonemes, the B-tubules of DMT1, DMT5, and DMT6 contain a structural feature referred to as the “beak” (30). These had only been described using EM cross-sections of fixed and embedded material, and the 3D arrangement of the beak material was unclear. Because only DMT1, DMT5, and DMT6 contain beaks, it is not possible to average all nine DMTs together without obscuring these doublet-specific differences. Recently, subtomographic averages of only DMT1, DMT5, and DMT6 have shown that the beak has a fiber-like structure running along PFs B5 and B6 (13). After determining the DMT numbering for each averaged tomogram (using the 1–2 bridge as a reference), we could calculate doublet-specific averages and subgroup averages of our tomographic data. The subgroup average containing DMT1, DMT5, and DMT6 allowed us to visualize the beak, here called beak-MIP, at higher resolution than previous studies (Table 1), corroborating the main features and revealing previously undescribed details about its structure. The beak-MIP differs from MIP1–4; it forms a narrow ∼3-nm-thick band attached to the inner MT wall at the border between PFs B5 and B6. The band is oriented perpendicular to the MT wall and reaches ∼6 nm into the B-tubule lumen (Fig. 4 K and L). In longitudinal tomographic slices, the beak-MIP appears to form a continuous band, but the electron density within this band varies along its length. The more prominent densities are embedded in a somewhat amorphous matrix, but they appear to repeat with a 16-nm periodicity connecting end-to-end (Fig. 4 M and N). The band also appears to have two attachment sites per 16-nm particle to the luminal side of the B-tubule wall (Fig. 4N).
Discussion
Number of PFs in the B-Tubule and Inner AB-Junction.
Although the A-tubules, and indeed most native MTs, are formed by 13 PFs (15), there remained a controversy as to whether the B-tubules contain 10 or 11 PFs (reviewed in 16). The data presented here convincingly settle this controversy: The B-tubule consists of 10 PFs and an inner “8-nm ladder” junction between PFs A1 and B10. The B-tubule provides the steric interface for the ATP-sensitive interactions between the DMT and the dynein motors that drive cilia and flagellar motility. Therefore, the B-tubule architecture consisting of 10 PFs is a crucial and evolutionarily conserved feature of DMTs, similar to native cytoplasmic MTs that consistently contain 13 PFs. The periodicity and small size of the IJ-proteins could easily explain the variable number of PFs in the B-tubule reported in previous publications, because the IJ-protein clearly looks thinner than regular tubulin PFs in cross-sectional views (Figs. 1 A and D and 2), or it might not be visible at all, depending on the quality of preservation and thickness of sections through this nonfilamentous density.
Assembly of the A-Tubule.
In most species, axonemes assemble in vivo from a basal body template, whereas basal bodies and centrioles assemble from a nontubulin organizing complex of SAS5, SAS6, and BALD10 (31). Assembly of the axoneme begins with the polymerization of tubulin and other proteins to form the A-tubules, extending directly from the plus-ends of the basal body A-tubules (32–34). Assembly of the A-tubule is complex and involves the following components: (i) αβ-tubulin with and without posttranslational modifications (6); (ii) the ribbon proteins tektins, Rib43a, Rib72/Efhc1, and Efhc2 (35–38); (iii) the structural components MIP1a/MIP1b and MIP2a/MIP2b, for which the protein composition has not yet been identified (10); and (iv) possibly other unidentified proteins. It is not known whether all these various proteins coassemble with tubulin to form the A-tubule or are added later. Although the assembly of the axoneme will involve simple self-assembly of protein subunits, enzymatic steps and posttranslational modifications are likely to be required. Future functional studies of the many nontubulin components of DMTs will require identification and localization of specific components, which would then allow targeted manipulations, such as the generation of mutants. It has been proposed that these components could be important for assembly, formation of the specific periodicities of associated complexes, axoneme bending properties, and motility.
Assembly of the B-Tubule and Outer AB-Junction.
The next step in the formation of DMTs is the assembly of the B-tubule by means of the addition of tubulin onto the A-tubules at their outer junctions, starting with PF B1 (reviewed in 16).This initial step is consistently shown by classic papers on ciliogenesis and centriole/basal body replication from protozoa to mammals (32–34), where the C-tubules also form first from their outer junctions. Our results demonstrate that the outer AB-junction is formed by the PF B1 binding close or directly to the ridges of PFs A10 and A11 (Figs. 1A, 2A, and 3C). This trimeric interface A10-A11-B1 is unique for PF interactions, because tubulin subunits normally only interact along their lateral sides to form the MT cylinder. However, at the outer junction, it seems that the lateral side of the tubulin PF B1 interacts with the outer surfaces of tubulin of PFs A10 and A11. The only other structures, apart from DMTs and triplet MTs, known to form PF interactions similar to this trimeric junction are the artificial MT hooks that were formed in vitro on cellular MTs in the presence of Pipes buffer and excess tubulin to determine MT polarity (39–41).
The resolution of our EM density map is sufficient to visualize individual PFs and the different appearances of the smooth inner surfaces vs. the ridged outer surfaces of the MT walls clearly. This is caused by the slightly triangular-shaped tubulin subunits in cross-sectional views of the MT, whereby the inner MT surface is formed by flat triangle faces and the outside ridges are formed by the triangle vertexes (42). The X-ray structures of tubulin dimers can easily be fitted inside the electron densities of all DMT PFs in the orientation typical for 13 PF MTs, with the exception of PF B1. However, when we tried to place the crystal structure of tubulin into the PF B1 density, we found that the expected orientation was highly unfavorable because the tubulin dimer would occupy significant volumes outside of the observed EM density, especially in the central channel of the outer junction, whereas a relatively large density between B1 and A10 is not filled (Fig. 3C). Future studies will be required to show whether this discrepancy between the observed EM density and conventional pseudoatomic models is attributable to a unique orientation or conformation of the tubulin in the B1 PF or if small polypeptides are present that might participate in forming the outer junction, for example, in the groove between PFs A10 and B1. Given that the formation of PF B1 is the initial step of B-tubule assembly (32–34), this unique interaction of PF B1 with the A-tubule is key not only for DMT assembly, but also for axoneme stability, intraflagellar transport and ciliary motility.
The posttranslational modification of tubulin is critical to the formation of axonemal MTs in general (21), and in particular for the outer junction. For example, the failure in the formation or closure of the outer junction has been observed in the zebrafish fleer mutant (19). This defect was linked to mutations in tubulin that prevent it from being posttranslationally modified by polyglutamylation and/or polyglycylation, specifically in cilia (18). Furthermore, knockdown of a tubulin polyglutamylase in zebrafish altered tubulin polyglutamylation, cilia formation, and motility in a manner similar to that in the fleer mutant phenotype (19).
In other species, treatment with antibodies against polyglutamylated tubulin affected the dynein-tubulin interactions and reduced the amplitude but not the frequency of reactivated ciliary beating (43, 44). Taken together, these published results and our recent findings suggest that the polyglutamylation and/or polyglycylation of tubulin allows tubulin PF B1 to bind strongly to PFs A10 and A11 to initiate the assembly of the B-tubule or to stabilize it after the initial B-tubule polymerization.
Closure of the B-Tubule and Inner AB-Junction.
After initiation of B-tubule formation on the outer AB-junction, polymerization of tubulin continues (in the plus-end direction) to form additional PFs of the B-tubule in the direction of B1→B10, ending with 10 PFs (32–34, 45–47). We note that the polymerization of tubulin in this direction means that it follows the left-handed 11/2-start helix of tubulin dimers (28, 29). These steps so far closely resemble the formation of artificial tubulin “hooks” when soluble brain tubulin is assembled in vitro onto preexisting axonemal and mitotic spindle MTs in the presence of Pipes buffer (48). By observing that these tubulin hooks form on stable cellular MTs with 98% probability in a counter-clockwise direction, as viewed from the minus-end looking toward the plus-end of the axoneme, McIntosh and colleagues (40, 41) cleverly used this method to determine the polarity of MTs in the mitotic spindle. Presumably, in the cytoplasmic milieu of these in vitro experiments, there was either sufficient polyglutamylated or polyglycylated tubulin to permit the formation of tubulin hooks, or the negatively charged Pipes anion provided the charges on tubulin to facilitate the formation of this junction. Moreover, the fact that these artificial hooks initiate by forming “outer-like junctions” but do not close by forming “inner-like junctions” implies the absence of available or functional IJ-proteins in solutions of soluble brain tubulin and cytoplasm.
After the addition of PF B10, the B-tubule is joined to the A-tubule by the formation of the inner AB-junction consisting of the IJ-proteins. Our results demonstrate that the IJ-proteins connect PF B10 to PF A1 with an 8-nm axial periodicity (i.e., consistently at the same tubulin-dimer location). Compared with the outer AB-junction, the IJ is extremely stable. When DMTs from sea urchin sperm flagella are warmed to 40 °C, over a period of a few minutes, the B-tubule begins to melt off the A-tubule, beginning at the outer AB-junction (49, 50). Presumably, the thermal treatment weakens or breaks the polyglutamylated or polyglycylated tubulin connections. Near the end of the melting procedure, PFs B9 and B10 remain connected, possibly mediated by MIP3 (10), and PF B10 remains connected to PF A1 of the A-tubule via the IJ-proteins (10, 49).
Despite the failure to close the outer AB-junction in fleer mutants, a portion of the B-tubule remains attached to the A-tubule through the inner AB-junction (18, 19). These results indicate one of two possibilities: B-tubule assembly began in the fleer mutants with the formation of the outer AB-junction, which became unstable after formation of the IJ, or (perhaps more unlikely) B-tubule assembly was forced to reverse its direction of assembly, beginning at the inner AB-junction but failing to form the outer junction and close the B-tubule.
Functional Relationships of the Inner AB-Junction and the N-DRC.
In addition to forming the tight inner AB-junction, the IJ-proteins have a curious relationship with the N-DRC, and possibly with Hedgehog signaling components. In WT Chlamydomonas, the IJ-protein(s) closest to each N-DRC base plate is missing, generating a hole or missing rung along the IJ at 96-nm intervals. In contrast, in drc mutants lacking the N-DRC base plate, the hole is filled, presumably with regular IJ-protein(s). Perhaps when the N-DRC is present, it interferes with the assembly of its nearest IJ-protein(s), or one of the N-DRC components actually reaches through the MT wall to interact with, for example, MIP3. All the known drc mutants with defects in the N-DRC base plate and filled IJ-hole have severe motility defects (10); however, because multiple N-DRC subunits fail to assemble into the axoneme of these mutants, it cannot be determined if changes in the structure of the IJ specifically contribute to the observed motility defects.
Another striking phenotype occurs in the mouse hennin mutant that disrupts the gene encoding an ARL13b GTPase of the ARF/ARL family. Defects in this protein disrupt the Sonic Hedgehog signaling pathway, leading to multiple embryonic developmental defects (17). An EM examination of hennin cilia revealed that the inner AB-junctions failed to form and the B-tubules remained open at the point of the IJ-proteins. More recent studies demonstrated that the ARL13b GTPase is membrane-associated along regions of cilia containing DMTs and is associated with intraflagellar transport (51, 52). The role of ARL13b GTPase in forming the inner AB-junction is not yet clear. Possibilities range from ARL13b having only membrane-associated and signaling functions, where failure to close the IJ is an indirect (e.g., enzymatic) effect of the hennin mutation, to ARL13b ultimately becoming associated with the DMT (e.g., as IJ-protein). Additional work is needed to establish more precisely the location of ARL13b in cilia.
MIPs.
Our results clarify the structure of the beak-MIP present in only the proximal portion of the B-tubules of DMT1, DMT5, and DMT6 of the Chlamydomonas axoneme (30). The beak-MIP material is formed by a chain of subunits with 16-nm axial periodicity that attaches to the B-tubule wall between PFs B5 and B6 and projects into the B-tubule lumen. Investigators originally suggested a relationship between the beak-MIPs and the ability of Chlamydomonas to switch from a ciliary to flagellar waveform in response to photostimulation and changes in intracellular calcium (53, 54): The phenotype of the mbo mutants (that move backward only with a flagellar waveform) suggested that the beak-MIPs might function in waveform reversal because they are missing from DMT5 and DMT6 (but not DMT1) of mbo mutants. However, one of the MBO gene products, MBO2, was subsequently reported to localize along the entire length of the axoneme (55). Thus, the relationship between MBO2 protein, the beak-MIPs, and their function remains a mystery.
MIP3a forms arches that appear to “staple” PFs B9 and B10 together; similarly, MIP1a bridges between PFs A5 and A7. The fact that heat-treated DMTs lose most of the B-tubule PFs but often retain PF B9/B10 and MIP3 suggests that all or a subset of the MIP proteins play a role in DMT stability (10). Interestingly, some MIPs are found in close relation to structures that attach to the outside surface of the DMT, such as an MIP1b on the inside of the DMT close to the inner dynein I1 attachment and an MIP3a density close to the base plate of the N-DRC and the hole in the IJ (14).
Previous studies have identified homologs for many DMT proteins (10, 35–38); here, we show that this evolutionary conservation continues on a structural level. One exception is the strikingly different partition wall between Chlamydomonas and sea urchin flagella. In Chlamydomonas DMTs, the shape and thickness of the partition resemble a typical MT wall with attached bump-shaped MIP4 densities on the inner surface of the A-tubule (Figs. 1F, 2A, and 4J). In sea urchin DMTs, the center of the partition is twice as thick with a smooth inner surface, whereas associated bump-shaped MIP4 densities are not obvious (Fig. S2F′′); this is in contrast to the description (8) of filamentous densities running along the center of the partition on the inner surface of the A-tubule of sea urchin DMTs (Table S1).
Our observations demonstrate that the spatial arrangements of MIP1–3, and thus their protein compositions, are more complex than previously thought (10). However, essentially nothing is known about their composition or functions, even though MIPs are spatially conserved in flagella of protists (Chlamydomonas) and metazoans (sea urchin) and are likely to be important. A number of possible functions come to mind, which might help to guide future investigations. Generally, MIPs might facilitate the anchoring of axonemal complexes to the outside of DMTs and/or reinforce DMTs during flagellar bending; for example, MIP3 and the IJ-proteins might stabilize the IJ junction, and MIP1b might anchor inner dynein I1. MIPs might also function in the regulation of motility and/or in signaling; for example, the relationship of the signaling molecule ARL13b GTPase and the IJ-protein has yet to be resolved. Additional work is also needed to understand the relationship between MIPs and the various ciliary proteins that have been implicated in human disease; these include ARL13b and diseases associated with disruptions in the Sonic Hedgehog signaling pathway (17, 56) and the EF-Hand-Ca2+–binding proteins, Efhc1 and Efhc2, that have been implicated in juvenile myoclonic epilepsy (57). The structural findings described here, together with published biochemical and genetic data, provide a useful foundation for future work on the molecular assembly of the DMTs and axoneme, how the different specific periodicities are established, the mechanism of DMT stability, and the molecular mechanisms of axoneme motility and signal transduction.
Materials and Methods
Flagella and Axoneme Preparation.
Strains used in this study and details on the obtained 3D structures are summarized in Table 1. Axonemes were obtained from WT and mutant Chlamydomonas reinhardtii cells as previously described (14, 58, 59). The final axoneme pellet was resuspended in 10 mM Hepes (pH 7.4), 25 mM NaCl, 4 mM MgSO4, 1 mM EGTA, 0.1 mM EDTA, and 0.1 mM DTT and was processed for cryo-ET within 24 h.
Sea urchins (Strongylocentrotus purpuratus) were obtained from Marinus Scientific LLC and Santa Barbara Marine Biologicals. Live sperm were isolated according to Gibbons (60) and Nicastro et al. (9) and kept on ice until used. Isolated DMTs were purified according to Linck and Stephens (61) by dialyzing axonemes against 1 mM Tris (pH 8) with 0.1 mM EDTA for 48 h.
Cryosample Preparation and Cryo-ET.
Cryosamples were prepared and imaged as previously described (10, 14, 62). In brief, 10 nm of colloidal gold solution (Sigma–Aldrich) was applied to Quantifoil grids (Quantifoil MicroTools GmbH) and allowed to air-dry. Three microliters of sample and 1 μL of 10-fold concentrated 10-nm colloidal gold solution were added to the grid, and excess fluid was blotted with filter paper before the grid was rapidly frozen in liquid ethane with a homemade manual plunge freezing device. Frozen grids were stored in liquid nitrogen until used.
For cryo-ET, frozen grids were loaded into a cryoholder (Gatan, Inc.) and inserted into a Tecnai F30 transmission electron microscope (FEI, Inc.) equipped with a field emission gun, high tilt stage, postcolumn energy filter (Gatan, Inc.), and a 2,000 × 2,000 pixel CCD camera (Gatan, Inc.). Single-axis tilt series were recorded using the microscope control software SerialEM (63) at 300 kV in low-dose mode by rotating the sample from −65 to 65° in increments of 1.5–2.5°. The total accumulated electron dose was limited to ∼100 e/Å2. The defocus was −6 to −8 μm, and the energy filter was operated in zero loss mode with a 20 eV slit width. The magnification was 30,000 with a final pixel size of 1 nm.
Image Processing.
The software program IMOD (64) was used for fiducial alignment and tomogram reconstruction using weighted backprojection. Subtomogram averaging was performed using PEET software (10, 14) to average the 96-nm axonemal repeat units from reconstructed sea urchin and Chlamydomonas axonemes. The resolution of the DMT averages was estimated using the 0.5 criterion of the Fourier shell correlation method (65) and is listed in Table 1, together with the number of axonemal repeat units included in each average. Contrast transfer function and modulation transfer function corrections were calculated using a module of IMOD software (66), and the University of California San Francisco Chimera package (67) was used for 3D visualization and isosurface rendering. A pseudoatomic model for a tubulin PF of a 13 PF MTs was calculated using the tubulin crystal structure (PDB ID code 1JFF; 68), placed as a whole into the 23 PFs in our averaged EM density map, and displayed either as backbone atoms (Fig. 2A) or as density derived by filtering to 2 nm and thresholding by Chimera (Fig. 3C).
Supplementary Material
Acknowledgments
We thank Chen Xu for maintaining the Brandeis University EM facility, Charles Sindelar for assistance with calculating a pseudoatomic MT model, David DeRosier for helpful discussions, Raqual Bower and Douglas Tritschler (University of Minnesota), as well as Erin Dymek and Elizabeth Smith (Dartmouth College) for the construction and characterization of Chlamydomonas mutant strains used in this study. This work was supported by National Institutes of Health Grants GM083122 (to D.N.) and GM55667 (to M.E.P.), National Science Foundation Grants 1024963 (to R.W.L.) and DMR-MRSEC-0820492 (partially supporting T.H.), a Pew Scholar Award (to D.N.), and funding of X.F. by the Howard Hughes Medical Institute.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
*Axonemal MIP has so far been abbreviated “MIP” (10), and this nomenclature is continued in the present paper. However, should cytoplasmic or subpellicular MIP be confirmed in the future, a more specific nomenclature using axMIP, cMIP, and spMIP, respectively, might be in order.
†The PFs were numbered according to the most widely accepted PF numbering system (16) (Figs. 1D and 2A and Fig. S1).
See Author Summary on page 17249.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1106178108/-/DCSupplemental.
References
- 1.Afzelius BA. Cilia-related diseases. J Pathol. 2004;204:470–477. doi: 10.1002/path.1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fliegauf M, Benzing T, Omran H. When cilia go bad: Cilia defects and ciliopathies. Nat Rev Mol Cell Biol. 2007;8:880–893. doi: 10.1038/nrm2278. [DOI] [PubMed] [Google Scholar]
- 3.Griffiths GM, Tsun A, Stinchcombe JC. The immunological synapse: A focal point for endocytosis and exocytosis. J Cell Biol. 2010;189:399–406. doi: 10.1083/jcb.201002027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cavalier-Smith T. The phagotrophic origin of eukaryotes and phylogenetic classification of Protozoa. Int J Syst Evol Microbiol. 2002;52:297–354. doi: 10.1099/00207713-52-2-297. [DOI] [PubMed] [Google Scholar]
- 5.Kubo T, Yanagisawa HA, Yagi T, Hirono M, Kamiya R. Tubulin polyglutamylation regulates axonemal motility by modulating activities of inner-arm dyneins. Curr Biol. 2010;20:441–445. doi: 10.1016/j.cub.2009.12.058. [DOI] [PubMed] [Google Scholar]
- 6.Wloga D, Gaertig J. Post-translational modifications of microtubules. J Cell Sci. 2010;123:3447–3455. doi: 10.1242/jcs.063727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pazour GJ, Agrin N, Leszyk J, Witman GB. Proteomic analysis of a eukaryotic cilium. J Cell Biol. 2005;170:103–113. doi: 10.1083/jcb.200504008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sui H, Downing KH. Molecular architecture of axonemal microtubule doublets revealed by cryo-electron tomography. Nature. 2006;442:475–478. doi: 10.1038/nature04816. [DOI] [PubMed] [Google Scholar]
- 9.Nicastro D, McIntosh JR, Baumeister W. 3D structure of eukaryotic flagella in a quiescent state revealed by cryo-electron tomography. Proc Natl Acad Sci USA. 2005;102:15889–15894. doi: 10.1073/pnas.0508274102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nicastro D, et al. The molecular architecture of axonemes revealed by cryoelectron tomography. Science. 2006;313:944–948. doi: 10.1126/science.1128618. [DOI] [PubMed] [Google Scholar]
- 11.Ishikawa T, Sakakibara H, Oiwa K. The architecture of outer dynein arms in situ. J Mol Biol. 2007;368:1249–1258. doi: 10.1016/j.jmb.2007.02.072. [DOI] [PubMed] [Google Scholar]
- 12.Bui KH, Sakakibara H, Movassagh T, Oiwa K, Ishikawa T. Molecular architecture of inner dynein arms in situ in Chlamydomonas reinhardtii flagella. J Cell Biol. 2008;183:923–932. doi: 10.1083/jcb.200808050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bui KH, Sakakibara H, Movassagh T, Oiwa K, Ishikawa T. Asymmetry of inner dynein arms and inter-doublet links in Chlamydomonas flagella. J Cell Biol. 2009;186:437–446. doi: 10.1083/jcb.200903082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heuser T, Raytchev M, Krell J, Porter ME, Nicastro D. The dynein regulatory complex is the nexin link and a major regulatory node in cilia and flagella. J Cell Biol. 2009;187:921–933. doi: 10.1083/jcb.200908067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tilney LG, et al. Microtubules: Evidence for 13 protofilaments. J Cell Biol. 1973;59:267–275. doi: 10.1083/jcb.59.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Linck RW, Stephens RE. Functional protofilament numbering of ciliary, flagellar, and centriolar microtubules. Cell Motil Cytoskeleton. 2007;64:489–495. doi: 10.1002/cm.20202. [DOI] [PubMed] [Google Scholar]
- 17.Caspary T, Larkins CE, Anderson KV. The graded response to Sonic Hedgehog depends on cilia architecture. Dev Cell. 2007;12:767–778. doi: 10.1016/j.devcel.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 18.Redeker V, et al. Mutations of tubulin glycylation sites reveal cross-talk between the C termini of alpha- and beta-tubulin and affect the ciliary matrix in Tetrahymena. J Biol Chem. 2005;280:596–606. doi: 10.1074/jbc.M408324200. [DOI] [PubMed] [Google Scholar]
- 19.Pathak N, Obara T, Mangos S, Liu Y, Drummond IA. The zebrafish fleer gene encodes an essential regulator of cilia tubulin polyglutamylation. Mol Biol Cell. 2007;18:4353–4364. doi: 10.1091/mbc.E07-06-0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dallai R, Machida R, Uchifune T, Lupetti P, Frati F. The sperm structure of Galloisiana yuasai (Insecta, Grylloblattodea) and implications for the phylogenetic position of Grylloblattodea. Zoomorphology. 2005;124:205–212. [Google Scholar]
- 21.Popodi EM, Hoyle HD, Turner FR, Raff EC. Cooperativity between the beta-tubulin carboxy tail and the body of the molecule is required for microtubule function. Cell Motil Cytoskeleton. 2008;65:955–963. doi: 10.1002/cm.20318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gardner LC, O'Toole E, Perrone CA, Giddings T, Porter ME. Components of a “dynein regulatory complex” are located at the junction between the radial spokes and the dynein arms in Chlamydomonas flagella. J Cell Biol. 1994;127:1311–1325. doi: 10.1083/jcb.127.5.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kato T, Kagami O, Yagi T, Kamiya R. Isolation of two species of Chlamydomonas reinhardtii flagellar mutants, ida5 and ida6, that lack a newly identified heavy chain of the inner dynein arm. Cell Struct Funct. 1993;18:371–377. doi: 10.1247/csf.18.371. [DOI] [PubMed] [Google Scholar]
- 24.Dymek EE, Heuser T, Nicastro D, Smith EF. The CSC is required for complete radial spoke assembly and wild-type ciliary motility. Mol Biol Cell. 2011;22:2520–2531. doi: 10.1091/mbc.E11-03-0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cyrklaff M, et al. Cryoelectron tomography reveals periodic material at the inner side of subpellicular microtubules in apicomplexan parasites. J Exp Med. 2007;204:1281–1287. doi: 10.1084/jem.20062405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garvalov BK, et al. Luminal particles within cellular microtubules. J Cell Biol. 2006;174:759–765. doi: 10.1083/jcb.200606074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schwartz CL, Sarbash VI, Ataullakhanov FI, McIntosh JR, Nicastro D. Cryo-fluorescence microscopy facilitates correlations between light and cryo-electron microscopy and reduces the rate of photobleaching. J Microsc. 2007;227:98–109. doi: 10.1111/j.1365-2818.2007.01794.x. [DOI] [PubMed] [Google Scholar]
- 28.Amos L, Klug A. Arrangement of subunits in flagellar microtubules. J Cell Sci. 1974;14:523–549. doi: 10.1242/jcs.14.3.523. [DOI] [PubMed] [Google Scholar]
- 29.Linck RW, Amos LA. The hands of helical lattices in flagellar doublet microtubules. J Cell Sci. 1974;14:551–559. doi: 10.1242/jcs.14.3.551. [DOI] [PubMed] [Google Scholar]
- 30.Hoops HJ, Witman GB. Outer doublet heterogeneity reveals structural polarity related to beat direction in Chlamydomonas flagella. J Cell Biol. 1983;97:902–908. doi: 10.1083/jcb.97.3.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carvalho-Santos Z, et al. Stepwise evolution of the centriole-assembly pathway. J Cell Sci. 2010;123:1414–1426. doi: 10.1242/jcs.064931. [DOI] [PubMed] [Google Scholar]
- 32.Anderson RG, Brenner RM. The formation of basal bodies (centrioles) in the Rhesus monkey oviduct. J Cell Biol. 1971;50:10–34. doi: 10.1083/jcb.50.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dippell RV. The development of basal bodies in paramecium. Proc Natl Acad Sci USA. 1968;61:461–468. doi: 10.1073/pnas.61.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tamm S, Tamm SL. Origin and development of free kinetosomes in the flagellates Deltotrichonympha and Koruga. J Cell Sci. 1980;42:189–205. doi: 10.1242/jcs.42.1.189. [DOI] [PubMed] [Google Scholar]
- 35.Ikeda K, et al. Rib72, a conserved protein associated with the ribbon compartment of flagellar A-microtubules and potentially involved in the linkage between outer doublet microtubules. J Biol Chem. 2003;278:7725–7734. doi: 10.1074/jbc.M210751200. [DOI] [PubMed] [Google Scholar]
- 36.Norrander JM, deCathelineau AM, Brown JA, Porter ME, Linck RW. The Rib43a protein is associated with forming the specialized protofilament ribbons of flagellar microtubules in Chlamydomonas. Mol Biol Cell. 2000;11:201–215. doi: 10.1091/mbc.11.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stephens RE. Preferential incorporation of tubulin into the junctional region of ciliary outer doublet microtubules: A model for treadmilling by lattice dislocation. Cell Motil Cytoskeleton. 2000;47:130–140. doi: 10.1002/1097-0169(200010)47:2<130::AID-CM4>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 38.Stephens RE. Ciliogenesis, ciliary function, and selective isolation. ACS Chem Biol. 2008;3:84–86. doi: 10.1021/cb8000217. [DOI] [PubMed] [Google Scholar]
- 39.Heidemann SR, McIntosh JR. Visualization of the structural polarity of microtubules. Nature. 1980;286:517–519. doi: 10.1038/286517a0. [DOI] [PubMed] [Google Scholar]
- 40.Euteneuer U, McIntosh JR. Polarity of some motility-related microtubules. Proc Natl Acad Sci USA. 1981;78:372–376. doi: 10.1073/pnas.78.1.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McIntosh JR, Euteneuer U. Tubulin hooks as probes for microtubule polarity: An analysis of the method and an evaluation of data on microtubule polarity in the mitotic spindle. J Cell Biol. 1984;98:525–533. doi: 10.1083/jcb.98.2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li H, DeRosier DJ, Nicholson WV, Nogales E, Downing KH. Microtubule structure at 8 A resolution. Structure. 2002;10:1317–1328. doi: 10.1016/s0969-2126(02)00827-4. [DOI] [PubMed] [Google Scholar]
- 43.Gagnon C, et al. The polyglutamylated lateral chain of alpha-tubulin plays a key role in flagellar motility. J Cell Sci. 1996;109:1545–1553. doi: 10.1242/jcs.109.6.1545. [DOI] [PubMed] [Google Scholar]
- 44.Vent J, et al. Direct involvement of the isotype-specific C-terminus of beta tubulin in ciliary beating. J Cell Sci. 2005;118:4333–4341. doi: 10.1242/jcs.02550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kalnins VI, Porter KR. Centriole replication during ciliogenesis in the chick tracheal epithelium. Z Zellforsch Mikrosk Anat. 1969;100:1–30. doi: 10.1007/BF00343818. [DOI] [PubMed] [Google Scholar]
- 46.Sorokin SP. Reconstructions of centriole formation and ciliogenesis in mammalian lungs. J Cell Sci. 1968;3:207–230. doi: 10.1242/jcs.3.2.207. [DOI] [PubMed] [Google Scholar]
- 47.Steinman RM. An electron microscopic study of ciliogenesis in developing epidermis and trachea in the embryo of Xenopus laevis. Am J Anat. 1968;122:19–55. doi: 10.1002/aja.1001220103. [DOI] [PubMed] [Google Scholar]
- 48.Euteneuer U, McIntosh JR. Structural polarity of kinetochore microtubules in PtK1 cells. J Cell Biol. 1981;89:338–345. doi: 10.1083/jcb.89.2.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Linck RW, Langevin GL. Reassembly of flagellar B (alpha beta) tubulin into singlet microtubules: Consequences for cytoplasmic microtubule structure and assembly. J Cell Biol. 1981;89:323–337. doi: 10.1083/jcb.89.2.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stephens RE. Thermal fractionation of outer fiber doublet microtubules into A- and B-subfiber components. A- and B-tubulin. J Mol Biol. 1970;47:353–363. doi: 10.1016/0022-2836(70)90307-4. [DOI] [PubMed] [Google Scholar]
- 51.Cevik S, et al. Joubert syndrome Arl13b functions at ciliary membranes and stabilizes protein transport in Caenorhabditis elegans. J Cell Biol. 2010;188:953–969. doi: 10.1083/jcb.200908133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li Y, Wei Q, Zhang Y, Ling K, Hu J. The small GTPases ARL-13 and ARL-3 coordinate intraflagellar transport and ciliogenesis. J Cell Biol. 2010;189:1039–1051. doi: 10.1083/jcb.200912001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Segal RA, Huang B, Ramanis Z, Luck DJ. Mutant strains of Chlamydomonas reinhardtii that move backwards only. J Cell Biol. 1984;98:2026–2034. doi: 10.1083/jcb.98.6.2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tam LW, Lefebvre PA. Cloning of flagellar genes in Chlamydomonas reinhardtii by DNA insertional mutagenesis. Genetics. 1993;135:375–384. doi: 10.1093/genetics/135.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tam LW, Lefebvre PA. The Chlamydomonas MBO2 locus encodes a conserved coiled-coil protein important for flagellar waveform conversion. Cell Motil Cytoskeleton. 2002;51:197–212. doi: 10.1002/cm.10023. [DOI] [PubMed] [Google Scholar]
- 56.Lim YS, Chua CE, Tang BL. Rabs and other small GTPases in ciliary transport. Biol Cell. 2011;103:209–221. doi: 10.1042/BC20100150. [DOI] [PubMed] [Google Scholar]
- 57.Ikeda T, et al. The mouse ortholog of EFHC1 implicated in juvenile myoclonic epilepsy is an axonemal protein widely conserved among organisms with motile cilia and flagella. FEBS Lett. 2005;579:819–822. doi: 10.1016/j.febslet.2004.12.070. [DOI] [PubMed] [Google Scholar]
- 58.Rupp G, Porter ME. A subunit of the dynein regulatory complex in Chlamydomonas is a homologue of a growth arrest-specific gene product. J Cell Biol. 2003;162:47–57. doi: 10.1083/jcb.200303019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bower R, et al. IC138 defines a subdomain at the base of the I1 dynein that regulates microtubule sliding and flagellar motility. Mol Biol Cell. 2009;20:3055–3063. doi: 10.1091/mbc.E09-04-0277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gibbons BH. Reactivation of sperm flagella: Properties of microtubules-mediated motility. Methods Cell Biol. 1982;25(Pt B):253–271. [PubMed] [Google Scholar]
- 61.Linck RW, Stephens RE. Biochemical characterization of tektins from sperm flagellar doublet microtubules. J Cell Biol. 1987;104:1069–1075. doi: 10.1083/jcb.104.4.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nicastro D. Cryo-electron microscope tomography to study axonemal organization. Methods Cell Biol. 2009;91:1–39. doi: 10.1016/S0091-679X(08)91001-3. [DOI] [PubMed] [Google Scholar]
- 63.Mastronarde DN. Automated electron microscope tomography using robust prediction of specimen movements. J Struct Biol. 2005;152:36–51. doi: 10.1016/j.jsb.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 64.Kremer JR, Mastronarde DN, McIntosh JR. Computer visualization of three-dimensional image data using IMOD. J Struct Biol. 1996;116:71–76. doi: 10.1006/jsbi.1996.0013. [DOI] [PubMed] [Google Scholar]
- 65.Harauz G, van Heel M. Exact filters for general geometry three-dimensional reconstruction. Optik (Stuttg) 1986;73:146–156. [Google Scholar]
- 66.Xiong Q, Morphew MK, Schwartz CL, Hoenger AH, Mastronarde DN. CTF determination and correction for low dose tomographic tilt series. J Struct Biol. 2009;168:378–387. doi: 10.1016/j.jsb.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pettersen EF, et al. UCSF Chimera—A visualization system for exploratory research and analysis. J Comput Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 68.Löwe J, Li H, Downing KH, Nogales E. Refined structure of alpha beta-tubulin at 3.5 A resolution. J Mol Biol. 2001;313:1045–1057. doi: 10.1006/jmbi.2001.5077. [DOI] [PubMed] [Google Scholar]
- 69.Nojima D, Linck RW, Egelman EH. At least one of the protofilaments in flagellar microtubules is not composed of tubulin. Curr Biol. 1995;5:158–167. doi: 10.1016/s0960-9822(95)00037-6. [DOI] [PubMed] [Google Scholar]