Abstract
Contrary to their reptilian ancestors, which had numerous dental generations, mammals are known to usually develop only two generations of teeth. However, a few mammal species have acquired the ability to continuously replace their dentition by the constant addition of supernumerary teeth moving secondarily toward the front of the jaw. The resulting treadmill-like replacement is thus horizontal, and differs completely from the vertical dental succession of other mammals and their extinct relatives. Despite the developmental implications and prospects regarding the origin of supernumerary teeth, this striking innovation remains poorly documented. Here we report another case of continuous dental replacement in an African rodent, Heliophobius argenteocinereus, which combines this dental system with the progressive eruption of high-crowned teeth. The escalator-like mechanism of Heliophobius constitutes an original adaptation to hyper-chisel tooth digging involving high dental wear. Comparisons between Heliophobius and the few mammals that convergently acquired continuous dental replacement reveal that shared inherited traits, including dental mesial drift, delayed eruption, and supernumerary molars, comprise essential prerequisites to setting up this dental mechanism. Interestingly, these dental traits are present to a lesser extent in humans but are absent in mouse, the usual biological model. Consequently, Heliophobius represents a suitable model to investigate the molecular processes leading to the development of supernumerary teeth in mammals, and the accurate description of these processes could be a significant advance for further applications in humans, such as the regeneration of dental tissues.
Keywords: mole-rats, intensive burrowing activity, hypsodonty, abrasion, attrition
Most nonmammalian vertebrates continuously replace their dentition, entailing many generations of identical teeth. In contrast, mammals develop a limited dentition composed at most of two generations of heteromorphous teeth, decreasing in number but generally increasing in shape complexity during evolution (1, 2). This has contributed to the acquisition of a more efficient masticatory apparatus than reptile jaws (3) that allowed mammals to diversify their feeding habits (4) and to include tough and abrasive plants in their diets. To withstand abrasive intakes, many mammals acquired a more durable dentition by way of diverse evolutionary trends (5). The most frequently observed is the increase in tooth height (i.e., hypsodonty), which leads in extreme cases to ever-growing teeth. Another trend to improve durability is continuous dental replacement (CDR), an extremely rare phenomenon in mammals. CDR corresponds to a progressive replacement of the dentition by regular development of additional teeth, which move from the rear to the front of the jaw throughout the animal's life. Among the ca. 5,500 species of mammals, only the three manatee species (Trichechus, Sirenia) and the pygmy-rock wallaby (Petrogale concinna, Macropodiformes) are known to display this striking innovation (6, 7). To date, this mechanism appears poorly documented. Furthermore, the genetic etiology and the developmental and molecular mechanisms leading to CDR, notably the occurrence of supernumerary teeth in mammals (8, 9), remain to be determined.
Here we report a case of CDR in a subterranean rodent, the silvery mole-rat Heliophobius argenteocinereus, belonging to African mole-rats (Bathyergidae, Rodentia), which could significantly contribute to the understanding of this rare phenomenon. The dental peculiarities of this species further underscore the interest of African mole-rats as models for examining topical issues, such as “eusociality” (10, 11), extreme longevity (12), and cancer resistance (13). We investigated Heliophobius dentition compared with Trichechus, P. concinna, and another African mole-rat (the Cape mole-rat, Georychus capensis) to appraise the main dental characteristics of this rodent on the one hand and to identify the specific traits relevant to improved knowledge of the CDR mechanism on the other.
Results and Discussion
Original System of Dental Replacement Among Mammals Combining Hypsodonty and Continuous Dental Replacement.
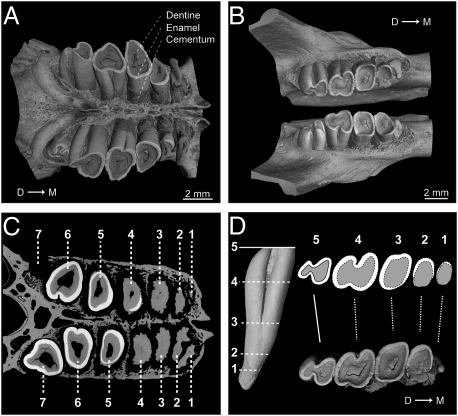
Heliophobius displays four to seven cheek teeth (Fig. 1 A–C and Tables S1 and S2), whereas the maximum cheek tooth number per dental quadrant does not usually exceed five in rodents and four in African mole-rats. It is thus the only known rodent displaying so many supernumerary cheek teeth. Although its abnormal tooth number has previously been reported (14), the underlying dental mechanism has never been properly interpreted. Heliophobius has single-rooted and bilophate hypsodont teeth. Tooth occlusal surfaces rapidly become flat and undergo a series of shape transformations, finally resulting in upper heart-shaped and lower pear-shaped teeth (Fig. 1 A–C) that lack enamel to varying degrees and are covered by cementum. Morphometric comparisons between occlusal crown shapes observed from rear to front and virtual transverse slices of an erupting tooth belonging to the same row lead to the conclusion that the various crown shapes result from an increasing degree of dental wear (Fig. 1D). All teeth have an overall identical cone-like shape and only their relative size is susceptible to change during life. We cannot say whether the first erupting tooth is a premolar (Fig. S1), as in most African mole-rats, but all subsequent teeth are molars.
Fig. 1.
Dental characteristics of H. argenteocinereus (RMCA96.036-M-5467). (A) X-ray synchrotron microtomographic 3D rendering of upper jugal tooth rows. (B) X-ray synchrotron microtomographic 3D rendering of lower jugal tooth rows. (C) Virtual cross-section of upper jugal tooth rows from synchrotron microtomographic data. (D) Morphological correspondences between virtual transverse slices of erupting lower molar and dental wear stages observed from the rear to front of the lower tooth row. D → M, distal-to-mesial point.
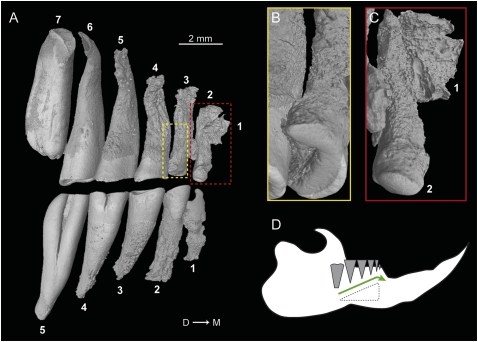
Most specimens of Heliophobius show either one erupting or freshly erupted molar behind the functional tooth row. After eruption, each tooth drifts from rear to front. Evidence of horizontal movements is demonstrated by continual remodeling of interalveolar bone septa (Fig. S2A). The stress is particularly intense along the mesiodistal axis, inducing extremely thin interalveolar bone and dental resorption (Fig. 1C). All teeth display more intense resorption on the distal root side because of the compressive stress of subsequent teeth (15) (Fig. 2 A and B). Teeth that have drifted to the mesial extremity of the row display crowns entirely worn by the combined action of wear and resorption (Fig. 2C). These molars, which are no longer occluding, have the highest rate of root resorption, which is probably induced by a hypofunctional periodontium coupled with a continuous dental drift (16). They are not truly shed, but their remains are pushed out from the row alignment before being completely resorbed inside the bone (Figs. 1C and 2C). Because the molar progression is not synchronized between each quadrant, asymmetric dental rows occur (Fig. 1 A and B), as do rare cases of misalignment (Fig. S2B). Teeth also continue to grow and erupt vertically while moving to the front (Fig. 2 A and D). The originality of the Heliophobius dental system relies on the superimposition of CDR over preexisting hypsodonty resulting in an escalator-like movement of teeth, constantly compensating for intensive dental wear. This dental replacement is not observed in other bathyergid rodents. Among other mammals, elephants present an analogous dental replacement, but their regular dentition develops sequentially, without the occurrence of any supplementary teeth.
Fig. 2.
Dental resorption and replacement in the silvery mole-rat. (A) Lateral view of X-ray synchrotron microtomographic 3D rendering of right upper and lower jugal tooth rows of Heliophobius (RMCA96.036-M-5467). (B and C) Focus on slightly and highly resorbed mesial upper teeth. (D) Dental replacement system in Heliophobius. The green arrow indicates dental drift.
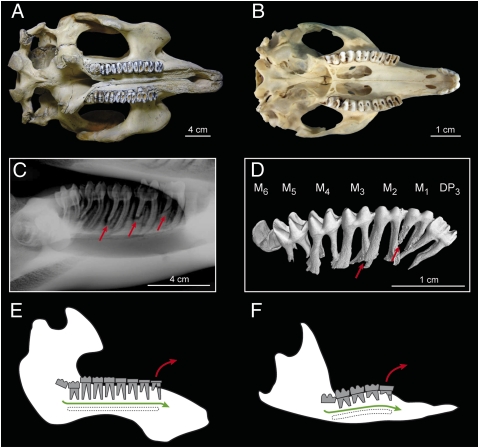
Unlike Heliophobius, Trichechus and P. concinna have low-crowned and bi- or trirooted cheek teeth but are bilophodont as well (Fig. 3 A and B). The replacement of their jugal dentition works like a simple treadmill in which each freshly developed molar erupts vertically at the rear of the row and then migrates horizontally toward the front (Fig. 3 C–F). A part of the regular dentition, including premolars (Fig. 3D), clearly develops before the system changes course, such that a single molar could become regularly ever-duplicated. Such an assumption relies on the fact that additional molars are identical to the second molars in manatees (6). The distal root is first intensively resorbed, and the crown breaks away from the mesial root when the tooth reaches the front of the row (Fig. 3 C–F). Even if the crown is severely eroded during the drift, it is not resorbed and vertical readjustments are not significant, contrary to what happens in Heliophobius.
Fig. 3.
Characteristics of the dental dynamics of manatees and the pygmy-rock wallaby. (A and B) Palatal view of skulls of T. manatus (NHM1985.6.30.2) and P. concinna (WAM-M4169). (C) Lateral X-ray radiograph of a left lower tooth row of T. senegalensis (Poulard's Coll. M201). (D) Lateral view of X-ray synchrotron microtomographic 3D rendering of the left lower tooth row of a juvenile specimen of P. concinna (WAM-M9346). DP, deciduous premolar; M, molar. Red arrows indicate root resorption. (E and F) Dental replacement systems of Trichechus and P. concinna. Green arrows indicate dental drift; red arrows indicate molar loss.
Dental Mechanism Adapted to High Dental Wear.
Manatees and the pygmy-rock wallaby consume, respectively, floating meadow grasses and specific ferns, which contain highly abrasive hard silica phytoliths (17, 18). Heliophobius feeds on underground parts of plants such as tubers and bulbs, which are by far less abrasive. At present, it is not clear why this dentition evolved in Heliophobius and not in other African mole-rats. Nonetheless, Heliophobius is the only solitary mole-rat living in mesic Afrotropics, where the soil is very hard and difficult to work during the dry season (19) and, contrary to social bathyergids, this chisel-tooth digger builds extended burrow systems alone to find food resources (20, 21). Such extensive burrowing activity could induce important attrition on its dentition and an ingestion of higher amounts of abrasive dust compared with other bathyergids. We therefore suppose that the dental system of Heliophobius might be a striking adaptation to hyper-chisel tooth digging, necessary to counterbalance the effects of severe friction (both attrition and abrasion) and to maintain the dentition functional throughout its life.
In manatees, tooth movement rate is correlated to food intake (6). Whereas growing tooth germs at the rear of the jaw principally generate the forward pressure, the dental movement is controlled by the quality and amount of ingested food, which impact on mechanical stress during mastication. As it mainly affects tooth wear, one can say that abrasion plays a major role in mechanical stress. In fact, the tough component of fibrous grasses mainly regulates the rate of displacement. For instance, a high exogenous grit intake, such as sand, can lead to severe wear and high loss of teeth coupled with a low tooth-movement rate in manatees. In the case of Heliophobius, where the ingestion of exogenous abrasive matter is inevitable during digging, and notably foraging, hypsodonty limits the effect of severe wear. This dental characteristic might comprise one of the components that regulate, even indirectly, the rate of dental regeneration of this mole-rat.
Three Dental Traits Are Essential for Continuous Dental Replacements in Mammals.
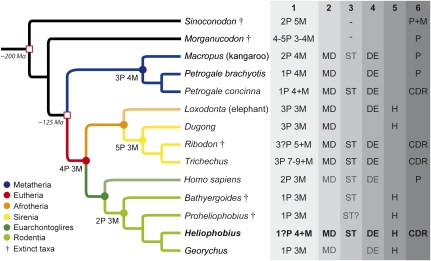
Trichechus, P. concinna, and Heliophobius have highly different ecological traits, and are very distant phylogenetically. CDR is clearly a convergent mechanism resulting in similar strategies closely related to high rates of dental wear. However, these mammals share three dental traits that we assume to be crucial prerequisites to setting up CDR: dental mesial drift, delayed eruption, and the occurrence of supernumerary molars. Interestingly, these dental characteristics have been identified in some of their extant or extinct relatives (Fig. 4).
Fig. 4.
Phylogenetic relationships and main dental characteristics of mammals developing CDR and some of their closest extant and extinct relatives. (1 and nodes) Jugal dental formula. (2) Presence of dental mesial drift (MD). (3) Presence of supernumerary teeth (ST). (4) Presence of delayed dental eruption (DE). (5) Presence of hypsodonty (H). (6) Jugal tooth replacement: premolar (P), molar (M), continuous dental replacement (CDR). In gray: occasional characteristic (for ST) or slightly manifested characteristic (for MD and DE).
Mesial drift corresponds to a forward movement of teeth as a result of distal pressure. It is well-known in elephants and exists in dugongs (22), the sister group of manatees. It also occurs in macropods, especially in grazing forms (e.g., kangaroos), including other Petrogale species (23). To date, mesial drift has never been described in African mole-rats. However, our analysis of some specimens of the bathyergid species G. capensis revealed that mesial drift, even slight, is not only confined to Heliophobius. The Cape mole-rat displays root resorption of the mesial teeth (notably the fourth premolars) as well as remodeling of interalveolar septa, as does the silvery mole-rat (Fig. S3 A–C). These features are due to both subsequent eruption and forward pressure of the last molars. The presence of large mesial diastemata in these rodents, as in other mammals showing mesial drift (e.g., elephants, sirenians, and macropods), is also essential to prevent orthodontic problems that sometimes occur in humans, inasmuch as diastemata can act as a buffer between jugal and frontal teeth.
The delayed dental eruption extends dentition longevity and corresponds to the acquisition of a definitive dentition during adulthood; this means that the last tooth erupts after the age of sexual maturity is reached. Three stages of eruption can be defined. As most mammals acquire a definitive dentition by sexual maturity, this stage is defined as “normal eruption.” A “slightly delayed eruption” corresponds to the completion of dentition before twice the age of sexual maturity is reached, as in humans (∼20 y for a permanent dentition and ∼13 y for sexual maturity) and many primates (24). After this limit, mammals can be considered as having a true “delayed eruption,” and this is the case for most of the afrotherian species, which spend the majority of their lifespan without a complete dentition (24). Such delayed eruption also occurs in macropods (23). Mammals with CDR can be considered as having a delayed eruption because their dentition is apparently endless. As with some subterranean rodents, Georychus displays a slightly delayed eruption of its dentition according to its pronounced dental wear gradient (Fig. S3 D and E) compared with other rodents. For instance, the last permanent teeth of Georychus generally erupt after its sexual maturity [∼15 mo for a permanent dentition and ∼10 mo for sexual maturity (25)], whereas the whole adult dentition of the mouse becomes functional well before sexual maturity (∼4 wk for a permanent dentition and ∼6 wk for sexual maturity), as in most of rodents (24).
Supernumerary teeth are rare in mammals because they generally cause functional pathologies. For instance, they are frequently associated with diseases caused by gene mutations in humans (9), but they can sometimes be related to nonsyndromic cases transmitted between generations (26). More generally, permanent supernumerary teeth are often associated with a lengthening of the jaws, as with simplified and nonreplaced teeth observed in odontocete cetaceans. The earliest true manatee, the Miocene Potamosiren, did not have supernumerary molars, whereas the Pliocene form, Ribodon, already had CDR (17). Additional molars frequently occur in kangaroos (23), even if these molars appear later in life history. A fourth molar probably occurred in the earliest known bathyergid Proheliophobius as in their extinct sister Bathyergoides (27), but actually cases of supernumerary molars remain scarce in bathyergid rodents.
Developmental Implications and Prospects Concerning Supernumerary Teeth in Mammals.
Such shared biological traits suggest that analogous molecular processes probably control CDR and were independently activated at least three times in recent mammalian history. The fossil record indicates the capacities to vertically replace molars and to possess more than three or four molars were lost since nearly 200 Mya in mammals [e.g., Sinoconodon (1)]. CDR could not involve the same developmental pathways, given that the pathways concerned were probably lost well over the time lapse generally assumed to coincide with irreversible losses of gene function [i.e., from 16 to 24 Mya (28)]. Although the developmental mechanisms remain unknown, it is likely that epithelial odontogenic tissues contain a permanent dental lamina permitting continual replacement of the previously erupted teeth, as documented in dental families of nonmammalian vertebrates (29, 30). Dental lamina and associated stem cells could indeed play a major role in CDR because of their deep involvement in vertebrate tooth renewal (31), notably in mammals (32). Considering that supernumerary molars are the result of a putative duplication of one molar (6), they probably derive from an extension of the dental lamina arising from first molars. This assumption needs further investigation, particularly focusing on the potential genes that have a bearing on such an extension.
A complex network of signaling pathways drives tooth development. Among all these actors, Runx2, Il11, Apc, and some master genes from the Wnt/β-catenin signaling pathway appear to be suitable candidates to explain the origin of supernumerary teeth (9). Indeed, they have been demonstrated to be involved in the development of such abnormalities in humans and, sometimes, mutant mice (33–36). However, when their expression is affected, the occurrence of supernumerary teeth is generally accompanied by disorders or diseases: Cleidocranial dysplasia (CCD) is related to heterozygous mutations (haploinsufficiency) in Runx2 (33); craniosynostosis is linked to Il11 loss of function (34); and adenomatous polyposis, tumors, and odontomes can be related to Apc loss of function (35) or activation of Wnt/β-catenin signaling (35, 36). Runx2 seems to be the best candidate given that delayed eruption is also one of the symptoms of CCD, in addition to supernumerary teeth. Even if a comparison has already been drawn between some afrotherian anatomical traits and CCD symptoms (24), the presence of addition molars in manatees has not been discussed in relation to Runx2. To the extent that mutations in Runx2 are extremely variable and sporadically induce isolated dental anomalies (9), implications of this gene in CDR are likely for the three concerned mammalian genera, even if its accurate role remains to be tested. Il11 could also be involved in CDR, because delayed eruption is also associated with Il11 loss of function. However, additional molars were never noticed among supernumerary teeth, and dental anomalies cannot be dissociated from bone disorders (34), contrary to some cases of haploinsufficiency for Runx2.
Our study demonstrates that Heliophobius presents striking dental characteristics related to CDR, including supernumerary molars. These characteristics are partially present in humans, but are absent in mice. Heliophobius therefore represents a model more appropriate than mice to explore the genetic etiology of supernumerary teeth in mammals. Maintaining a perennial dentition in a context of hyperabrasive intakes mainly involves two radically different adaptations in mammals. Although hypsodonty is inappropriate for the human dental system, the understanding of both CDR and the molecular processes leading to supernumerary molars could constitute a basis for further studies on regeneration of dental tissues and their applications in tooth engineering (37).
Materials and Methods
Skulls.
Fifty-five skulls of H. argenteocinereus were investigated. These specimens are mainly housed in the Royal Museum for Central Africa (RMCA) of Tervuren (Belgium), and others come from the Museum National d'Histoire Naturelle (MNHN) of Paris. The skulls of T. senegalensis from Poulard's Collection (Poulard's Coll. M201) of Lyon (France), T. manatus (NHM1985.6.30.2) from the National History Museum (NHM) of London, and P. concinna (WAM-M4169 and WAM-M9346) from the Western Australian Museum (WAM) of Perth (Australia) were also used here for comparison and illustrations. Fifteen skulls of G. capensis from the NHM and MNHN were studied to draw comparisons. The main interest of this bathyergid species concerns its dental eruption sequence, which is already known and accurately detailed (25).
Two- and Three-Dimensional Data Acquisition of Dentitions.
Dentitions of H. argenteocinereus were digitized using a stereomicroscope (Leica; M165C) connected to a spot CCD camera (Leica; DFC420) at 7.3× magnification. Different X-ray methods were performed to more accurately depict and analyze tooth morphologies, including roots and osteological characteristics. X-ray radiography from the Ecole Nationale Vétérinaire of Lyon was used to image a skull of Trichechus. High-quality images of two skulls of Heliophobius, including a young specimen (Fig. S2), were obtained using X-ray synchrotron microtomography at the European Synchrotron Radiation Facility (ESRF; Grenoble, France), beamlines ID19 and BM5, with a monochromatic beam at an energy of 25 keV and using a cubic voxel of 5.06 μm. A pink beam at an energy of 60 keV, using a cubic voxel of 7.46 μm (beamline ID19; ESRF), was also used to scan the skull of P. concinna. This method has been proven to be very useful for accurate imaging of small elements (38, 39) such as teeth (40). Noninvasive virtual extraction of entire teeth (i.e., crown and roots) is also permitted (41). Three-dimensional renderings and virtual slices were then performed using VGStudio Max 2.0 software (Volume Graphics).
Statistics.
Dentitions of Heliophobius are described according to the number of erupting teeth, functional teeth, and worn-out teeth; skull lengths were also measured (Table S1). Percentages were then calculated for the whole sample (Table S2). Similar descriptions were realized on Georychus specimens (Table S3).
Supplementary Material
Acknowledgments
We acknowledge the curators J. Cuisin (MNHN, Paris), R. How and C. Stevenson (WAM, Perth), and R. Portela Miguez (NHM, London), who allowed access to their mammal collections. We are grateful to the ESRF (Grenoble) and the team of beamlines ID19 and BM5 for support of this research project by giving access to their experimental stations. Thanks to all the members of Team “Evo-Devo of Vertebrate Dentition,”and to V. Laudet, H. Magloire, M.-L. Couble, and J. Burden (Institut de Génomique Fonctionnelle de Lyon), for both technical assistance and comments on the manuscript. We also thank two anonymous reviewers for manuscript improvements. This work was supported by the ANR “Quenottes” Program (L.V.), National Science Foundation/Revealing Hominid Origins Initiative “Small Mammals” Award BCS-0321893 (to L.V.), a Centre National de la Recherche Scientifique postdoctoral grant (to H.G.R.), and Project Ministerstvo Školství, Mládeže a Tělovýchovy 600766580 (to R.Š.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1109615108/-/DCSupplemental.
References
- 1.Luo Z-X, Kielan-Jaworowska Z, Cifelli RL. Evolution of dental replacement in mammals. Bull Carnegie Mus Nat Hist. 2004;36:159–175. [Google Scholar]
- 2.Koussoulakou DS, Margaritis LH, Koussoulakos SL. A curriculum vitae of teeth: Evolution, generation, regeneration. Int J Biol Sci. 2009;5:226–243. doi: 10.7150/ijbs.5.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ross CF, et al. Modulation of intra-oral processing in mammals and lepidosaurs. Integr Comp Biol. 2007;47:118–136. doi: 10.1093/icb/icm044. [DOI] [PubMed] [Google Scholar]
- 4.Luo Z-X. Transformation and diversification in early mammal evolution. Nature. 2007;450:1011–1019. doi: 10.1038/nature06277. [DOI] [PubMed] [Google Scholar]
- 5.Janis CM, Fortelius M. On the means whereby mammals achieve increased functional durability of their dentitions, with special reference to limiting factors. Biol Rev Camb Philos Soc. 1988;63:197–230. doi: 10.1111/j.1469-185x.1988.tb00630.x. [DOI] [PubMed] [Google Scholar]
- 6.Domning DP, Hayek L-AC. Horizontal tooth replacement in the Amazonian manatee (Trichechus inunguis) Mammalia. 1984;48:105–127. [Google Scholar]
- 7.Sanson GD. Morphological adaptations of teeth to diets and feeding in the Macropodoidea. In: Grigg G, Jarman P, Hume I, editors. Kangaroos, Wallabies and Rat-Kangaroos. Sydney: Surrey Beatty & Sons; 1989. pp. 151–168. [Google Scholar]
- 8.D'Souza RN, Klein OD. Unraveling the molecular mechanisms that lead to supernumerary teeth in mice and men: Current concepts and novel approaches. Cells Tissues Organs. 2007;186:60–69. doi: 10.1159/000102681. [DOI] [PubMed] [Google Scholar]
- 9.Wang X-P, Fan J. Molecular genetics of supernumerary tooth formation. Genesis. 2011;49:261–277. doi: 10.1002/dvg.20715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jarvis JUM. Eusociality in a mammal: Cooperative breeding in naked mole-rat colonies. Science. 1981;212:571–573. doi: 10.1126/science.7209555. [DOI] [PubMed] [Google Scholar]
- 11.Jarvis JUM, O'Riain MJ, Bennett NC, Sherman PW. Mammalian eusociality: A family affair. Trends Ecol Evol. 1994;9:47–51. doi: 10.1016/0169-5347(94)90267-4. [DOI] [PubMed] [Google Scholar]
- 12.Pérez VI, et al. Protein stability and resistance to oxidative stress are determinants of longevity in the longest-living rodent, the naked mole-rat. Proc Natl Acad Sci USA. 2009;106:3059–3064. doi: 10.1073/pnas.0809620106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seluanov A, et al. Hypersensitivity to contact inhibition provides a clue to cancer resistance of naked mole-rat. Proc Natl Acad Sci USA. 2009;106:19352–19357. doi: 10.1073/pnas.0905252106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Landry SC. The interrelationships of the New and Old World hystricomorph rodents. Univ Calif Publ Zool. 1957;56:1–118. [Google Scholar]
- 15.Wise GE, King GJ. Mechanisms of tooth eruption and orthodontic tooth movement. J Dent Res. 2008;87:414–434. doi: 10.1177/154405910808700509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sringkarnboriboon S, Matsumoto Y, Soma K. Root resorption related to hypofunctional periodontium in experimental tooth movement. J Dent Res. 2003;82:486–490. doi: 10.1177/154405910308200616. [DOI] [PubMed] [Google Scholar]
- 17.Domning DP. Evolution of manatees; a speculative history. J Paleontol. 1982;56:599–619. [Google Scholar]
- 18.Sanson GD, Nelson JE, Fell P. Ecology of Peradorcas concinna in Arnhem land in the wet and dry season. Proc Ecol Soc Aust. 1985;13:69–72. [Google Scholar]
- 19.Šklíba J, Šumbera R, Chitaukali WN, Burda H. Home-range dynamics in a solitary subterranean rodent. Ethology. 2009;115:217–226. [Google Scholar]
- 20.Šumbera R, Burda H, Chitaukali WN, Kubová J. Silvery mole-rats (Heliophobius argenteocinereus, Bathyergidae) change their burrow architecture seasonally. Naturwissenschaften. 2003;90:370–373. doi: 10.1007/s00114-003-0439-y. [DOI] [PubMed] [Google Scholar]
- 21.Šklíba J, Šumbera R, Vitámvás M. Resource characteristics and foraging adaptations in the silvery mole-rat (Heliophobius argenteocinereus), a solitary Afrotropical bathyergid. Ecol Res. 2011 10.1007/s11284-011-0860-1. [Google Scholar]
- 22.Lanyon JM, Sanson GD. Degenerate dentition of the dugong (Dugong dugon), or why a grazer does not need teeth: Morphology, occlusion and wear of mouthparts. J Zool (Lond) 2006;268:133–152. [Google Scholar]
- 23.Jackson SM. Australian Mammals: Biology and Captive Management. Melbourne: CSIRO; 2003. [Google Scholar]
- 24.Asher RJ, Lehmann T. Dental eruption in afrotherian mammals. BMC Biol. 2008;6:14. doi: 10.1186/1741-7007-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taylor PJ, Jarvis JUM, Crowe TM. Age determination in the Cape mole rat Georychus capensis. S Afr J Zool. 1985;20:261–267. [Google Scholar]
- 26.Batra P, Duggal R, Parkash H. Non-syndromic multiple supernumerary teeth transmitted as an autosomal dominant trait. J Oral Pathol Med. 2005;34:621–625. doi: 10.1111/j.1600-0714.2005.00271.x. [DOI] [PubMed] [Google Scholar]
- 27.Lavocat R. [East African Miocene rodents I. Early Miocene.] Mém Trav EPHE Inst Montpellier. 1973;1:1–284. French. [Google Scholar]
- 28.Collin R, Miglietta MP. Reversing opinions on Dollo's Law. Trends Ecol Evol. 2008;23:602–609. doi: 10.1016/j.tree.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 29.Smith MM, Fraser GJ, Mitsiadis TA. Dental lamina as source of odontogenic stem cells: Evolutionary origins and developmental control of tooth generation in gnathostomes. J Exp Zoolog B Mol Dev Evol. 2009;312B:260–280. doi: 10.1002/jez.b.21272. [DOI] [PubMed] [Google Scholar]
- 30.Moriyama K, Watanabe S, Iida M, Sahara N. Plate-like permanent dental laminae of upper jaw dentition in adult gobiid fish, Sicyopterus japonicus. Cell Tissue Res. 2010;340:189–200. doi: 10.1007/s00441-010-0935-2. [DOI] [PubMed] [Google Scholar]
- 31.Huysseune A, Thesleff I. Continuous tooth replacement: The possible involvement of epithelial stem cells. Bioessays. 2004;26:665–671. doi: 10.1002/bies.20039. [DOI] [PubMed] [Google Scholar]
- 32.Järvinen E, Tummers M, Thesleff I. The role of the dental lamina in mammalian tooth replacement. J Exp Zoolog B Mol Dev Evol. 2009;312B:281–291. doi: 10.1002/jez.b.21275. [DOI] [PubMed] [Google Scholar]
- 33.Wang X-P, et al. Runx2 (Cbfa1) inhibits Shh signaling in the lower but not upper molars of mouse embryos and prevents the budding of putative successional teeth. J Dent Res. 2005;84:138–143. doi: 10.1177/154405910508400206. [DOI] [PubMed] [Google Scholar]
- 34.Nieminen P, et al. Inactivation of IL11 signaling causes craniosynostosis, delayed tooth eruption, and supernumerary teeth. Am J Hum Genet. 2011;89:67–81. doi: 10.1016/j.ajhg.2011.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang X-P, et al. Apc inhibition of Wnt signaling regulates supernumerary tooth formation during embryogenesis and throughout adulthood. Development. 2009;136:1939–1949. doi: 10.1242/dev.033803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Järvinen E, et al. Continuous tooth generation in mouse is induced by activated epithelial Wnt/β-catenin signaling. Proc Natl Acad Sci USA. 2006;103:18627–18632. doi: 10.1073/pnas.0607289103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bluteau G, Luder H-U, De Bari C, Mitsiadis TA. Stem cells for tooth engineering. Eur Cell Mater. 2008;16:1–9. doi: 10.22203/ecm.v016a01. [DOI] [PubMed] [Google Scholar]
- 38.Tafforeau P, et al. Applications of X-ray synchrotron microtomography for non-destructive 3D studies of paleontological specimens. Appl Phys A-Mater. 2006;83:195–202. [Google Scholar]
- 39.Kruta I, Landman N, Rouget I, Cecca F, Tafforeau P. The role of ammonites in the Mesozoic marine food web revealed by jaw preservation. Science. 2011;331:70–72. doi: 10.1126/science.1198793. [DOI] [PubMed] [Google Scholar]
- 40.Charles C, et al. Modulation of Fgf3 dosage in mouse and men mirrors evolution of mammalian dentition. Proc Natl Acad Sci USA. 2009;106:22364–22368. doi: 10.1073/pnas.0910086106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tabuce R, et al. Anthropoid versus strepsirhine status of the African Eocene primates Algeripithecus and Azibius: Craniodental evidence. Proc Biol Sci. 2009;276:4087–4094. doi: 10.1098/rspb.2009.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.