Abstract
Many animals, including humans, select alternate forms of motion (gaits) to move efficiently in different environments. However, it is unclear whether primitive animals, such as nematodes, also use this strategy. We used a multifaceted approach to study how the nematode Caenorhabditis elegans freely moves into and out of water. We demonstrate that C. elegans uses biogenic amines to switch between distinct crawling and swimming gaits. Dopamine is necessary and sufficient to initiate and maintain crawling after swimming. Serotonin is necessary and sufficient to transition from crawling to swimming and to inhibit a set of crawl-specific behaviors. Further study of locomotory switching in C. elegans and its dependence on biogenic amines may provide insight into how gait transitions are performed in other animals.
Keywords: locomotion, optogenetics, caged amines, ablation, magnetic manipulation
Animals from widely diverse taxa (e.g., from annelids to chordates) move efficiently in different environments by selecting alternate forms of motion, sometimes referred to as gaits (1, 2). Gait transitions are often facilitated by dopamine and serotonin (3–10). One needs only look at individuals with disrupted aminergic systems (e.g., Parkinson's disease) to grasp the importance that bioamines have for motor transitions in particular and for survival in general (11). Despite recent progress, limited knowledge exists regarding when different motor gaits first evolved and what neural strategies are used to switch between them (2).
The nematode Caenorhabditis elegans lives in the soil and decaying vegetation, which offer land and water microniches (12). Although most research on C. elegans locomotion has focused on crawling, worms are nevertheless apt swimmers, easily orienting to chemical cues while immersed (13). Local variation in water availability likely necessitates C. elegans to routinely enter and exit aquatic environments.
C. elegans is a genetically tractable animal whose 302 neurons and ≈8,000 known synapses make it a promising model system for the study of transitions between motor patterns. On an agar substrate and in water, C. elegans moves forward with dorsoventral bending. At present, two opposing views exist regarding the nature of locomotory patterns in C. elegans. First, crawling and swimming may be at opposite extremes of a single gait and, as such, represent the output of a single neural circuit (14–18). Second, crawling and swimming may represent different gaits produced by functionally distinct neural circuits (3, 13). We used a multifaceted approach spanning in-depth behavioral assays, neuron ablations, optogenetics, and photolysis of caged amines to determine whether swimming and crawling are the product of one or two functionally distinct neuronal circuits and next investigated the roles played by dopamine and serotonin as worms naturally transition between water and land.
Results
Swimming and Crawling Have Distinct Kinematics.
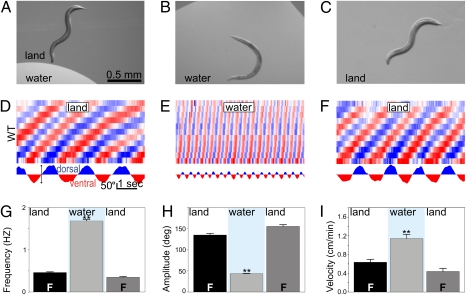
To study how C. elegans transitions between these environments, we used a video analysis system to quantify body curvature of individual WT worms as they crawled into and out of puddles on an agar surface (Fig. 1 A–C and Fig. S1). By dividing the length of the worm into 12 equal segments and measuring the angles between adjacent segments, our software extracted the changes in body curvature over time as the animals performed different behaviors in different environments (Fig. S1). Worms crawled on agar by propagating dorsoventral bends from head to tail. These traveling bends had an average amplitude of 135° and occurred at a frequency of 0.5 Hz (Fig. 1 D, G, and H). While crawling, worms often engaged in a foraging behavior (consisting of 10-Hz head oscillations) and occasional pumping of their feeding organ (the pharynx). After submersion, worms ceased crawling for 6 s before the onset of the first swimming cycle. After this delay (hereafter referred to as “swim onset”), worms swam continuously for extended periods (>45 min). Swimming was accomplished by dorsoventral bends that averaged 45° in amplitude and occurred at a frequency of 1.7 Hz (Fig. 1 E, G, and H). Foraging and pumping ceased during swimming. Worms swam with their characteristic alternation of dorsal and ventral “C”-shaped postures until the puddle was absorbed by the agar. Once the height of the puddle dropped below the thickness of the worm, they crawled away, dragging residual water (Fig. 1C). After emergence, crawling resumed with kinematics resembling those before submersion (including foraging and pumping; Fig. 1 D–I, Fig. S2, and Movie S1). As shorthand, we henceforth refer to behavior on an agar substrate exposed to air as behavior in the “land” condition and behavior in a puddle of water as behavior in the “water” condition.
Fig. 1.
Crawling and swimming have distinct kinematics. A crawling worm enters a puddle (A) and starts swimming (B) until the puddle is absorbed and crawling resumes (C). (D–F) Curvature matrices represent changes in body shape over time. Body position is represented on the y axis (with head at the bottom and tail at the top). Time is plotted on the x axis, and body curvature is represented by a trichromatic scale whereby blue = 120° dorsal, red = 120° ventral, and white = no curvature. For example, changes in neck curvature vs. time can be extracted from the second (from the bottom) row in the matrix and used to compare the slow and deep bends used in crawling with the fast and shallow bends observed during swimming (D, Lower). We performed this analysis as each individual worm crawled initially (D), swam in a puddle (E), and eventually began crawling again after emergence (F). Crawling and swimming bends are distinguishable by frequency (G) and angular excursion (H). We used velocity (obtained by tracking the centroid of animals) to report changes in the efficiency of locomotion (I). Here and henceforth, all bars report means with SEM, n > 12, blue background indicates water environment, F indicates foraging. *P < 0.05, and **P < 0.001.
Worms in Viscous Liquids Alternate Between Swim- and Crawl-Like Gaits.
The distinct kinematics and subbehaviors observed during crawling and swimming suggested that they may be generated by functionally distinct patterns of neural activity (gaits). Alternatively, the differences in crawl and swim kinematics may be entirely explained by the different mechanical constraints imposed by wet and dry environments (14–18). Behavioral evidence for gait-like forms of motion is obtained by examining locomotion over a range of speeds (2). For instance, on a treadmill, humans move with distinct kinematics at low (walking) and fast (running) speeds. Importantly, humans cannot display a walk–run hybrid and instead switch between bouts of fast walking and slow running at intermediate speeds (≈1.9 m/s) indicating two fundamental gaits (2).
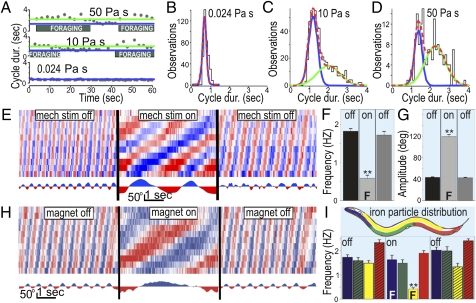
We performed gait analysis using viscous methylcellulose solutions to constrain speed. As previously found (16, 17), worms in low viscosity (0.024 Pa s) swam, slowing their swimming as viscosity was increased (10 and 50 Pa s; Fig. 2A). Unexpectedly, worms in higher viscosities repeatedly switched between distinct bouts of swim- and crawl-like motion (Fig. 2A and Movie S2). As in crawling (Fig. S2), the slower form of locomotion was more variable in cycle durations and coincided with foraging and pumping. All-points histograms of cycle durations for animals moving in three different viscosities confirmed that motion at low viscosity could be explained by one state, whereas motion at higher viscosities could only be explained by two states. This is evidenced by unimodal and bimodal Gaussian distribution fits for the low- and higher-viscosity conditions, respectively (n = 30; Fig. 2 B–D). Interestingly, a bimodal distribution was also found for the sensory cilia mutant che-3 at low viscosity [or in water (13)], suggesting that sensory input is required to maintain swimming and/or prevent switching to crawl-like motion (Fig. S3). Thus, although swimming frequency decreases with increasing viscosity, C. elegans shows distinct crawling and swimming gaits when its motion is constrained by viscous solutions.
Fig. 2.
Transition between crawling and swimming gaits is influenced by pressure. As viscosity was increased (by altering methylcellulose concentrations), worms alternated between bouts of swim- and crawl-like cycle durations (A). All-points cycle-duration histograms show one vs. two types of motion in lower (B) and higher viscosities, respectively (C and D). Swimming worms in water were reversibly induced to crawl by compression between glass slides (E), showing similar kinematics as those on “land” (agar) during stimulation (F and G). Swim-to-crawl transitions were also induced by feeding worms iron and pulling them to the substrate with an electromagnet (H). (I) Worms with iron particles in specific body regions (yellow bars) transitioned to crawling when stimulated, whereas worms with iron in other parts of their body (red, green) remained swimming even while pinned down to the substrate. Worms pinned down by the anterior portion of their bodies continued swimming but engaged in foraging while being pulled (blue).
Localized Mechanical Stimulation Causes Swim-to-Crawl Transition.
To test whether C. elegans selects crawling in response to mechanical pressure (such as experienced when contacting the substrate), we performed two additional experiments. For our first experiment, we slowly compressed worms in water between two glass slides. Worms displayed normal swimming until compressed to less than the thickness of their bodies (75–90 μm), whereupon they switched between bouts of swimming and crawling (Fig. 2 E–G). Further compression induced constant crawling accompanied by foraging and pumping. For our second experiment, we fed worms iron particles and placed them in water above an electromagnet. Worms with iron distributed throughout the gut exhibited normal swimming while the magnet was OFF (Fig. 2 H and I). Turning the magnet ON reversibly pulled these worms to the substrate, causing them to crawl and forage (Fig. 2H, yellow in Fig. 2I; Movie S3). Similar to a previous report on semiimmobilized preparations (19), worms with iron in more restricted portions of their body continued to swim while stuck in place (blue, green, and red in Fig. 2I). We conclude that contacting the substrate with specific areas of the body is sufficient to induce transition from swimming to crawling.
Dopaminergic Signaling Is Necessary for Swim-to-Crawl Transition.
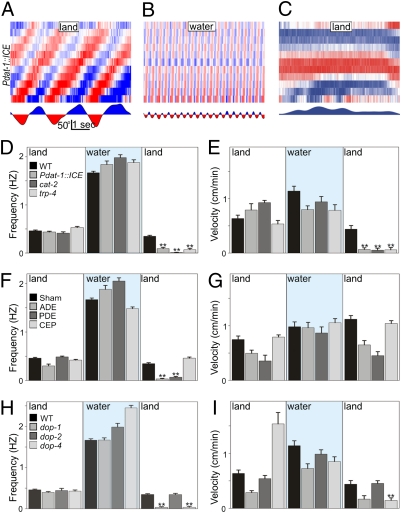
Our magnet experiment revealed that pressure on areas of known (mechanosensitive) dopaminergic innervation could induce crawling (20). Genetic ablation of all eight dopaminergic neurons [achieved in the Pdat-1::ICE strain (21)] resulted in severe defects in freely transitioning from swimming to crawling. Specifically, Pdat-1::ICE worms crawled as well as initiated and maintained swimming normally; however, once puddles receded, they became immobile for up to 30 min (Fig. 3 A–E and Movie S4). Only a few individuals succeeded initiating, but failed to maintain, crawling after emergence. Throughout this time, however, Pdat-1::ICE worms could crawl away when prodded, demonstrating that dopamine neurons are required for the swim–crawl transition rather than general crawl performance. This impairment was mirrored by the tyrosine hydroxylase mutant, cat-2, deficient in dopamine synthesis and by the trp-4 mutant, which lacks a mechanoreceptor expressed in dopaminergic neurons (22, 23) (Fig. 3 D and E). Although laser ablation of two of the three classes of dopaminergic neurons in C. elegans (ADE and PDE) recapitulated the defect reported above, ablation of the third class (the four CEPs) had no effect on the swim-to-crawl transition (Fig. 3 F and G). Downstream, we found that deletion of D1-like (but not D2-like) dopamine receptors (dop-1 or dop-4 vs. dop-2 and/or dop-3, respectively; Fig. S4) likewise perturbed the swim-to-crawl transition (Fig. 3 H and I).
Fig. 3.
Dopamine is necessary for swim-to-crawl transition. Animals lacking dopaminergic neurons (Pdat-1::ICE) can crawl (A), enter water, and swim normally (B), but become immobile upon emergence (C). Dopamine production and dopamine mechanoreceptor mutants are likewise impaired, as illustrated by their crawling frequencies (D) and linear velocities (E). Ablation of ADE and PDE dopamine neurons disrupts swim-to-crawl transition (F and G). D1-like dopamine receptors (dop-1 and dop-4) are required for swim-to-crawl transition (H and I).
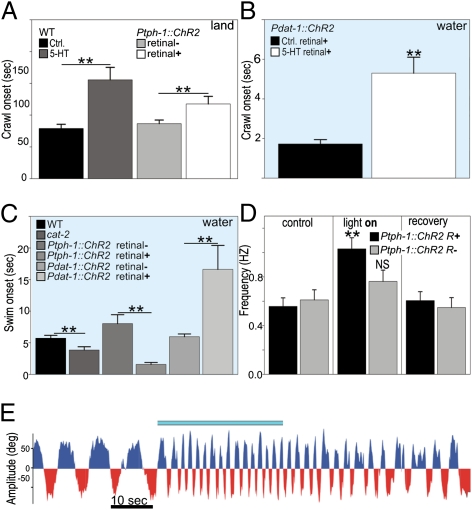
Dopamine Is Sufficient to Induce Swim-to-Crawl Transition in Water.
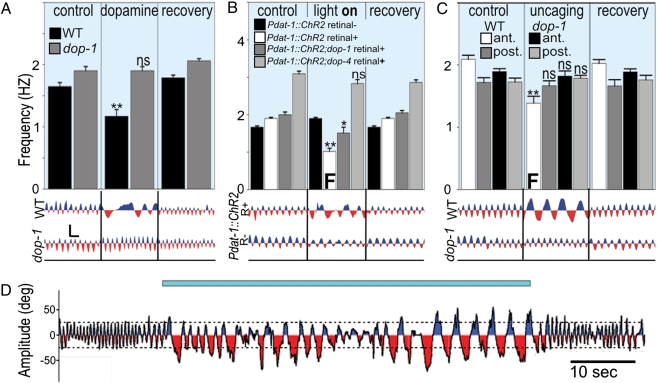
To test whether dopamine was sufficient to induce the swim-to-crawl transition, we exposed swimming worms to exogenous dopamine. This produced crawl-like bouts (with foraging and pumping) in swimming WT worms (but not in mutants lacking D1-like receptors; Fig. 4A). By expressing the photoactivatable cation-channel Channelrhodopsin-2 (ChR2) in all dopaminergic neurons (under the control of Pdat-1), we could trigger the swim-to-crawl transition in swimming worms (Fig. 4 B and D and Movie S5). Pdat-1::ChR2 worms continued to crawl and forage in water for the duration of light stimulation. Lack of D1-like receptors was sufficient to partially (dop-1) or completely (dop-4) prevent this effect (Fig. 4B).
Fig. 4.
Dopamine is sufficient for swim-to-crawl transition. (A) Exogenous dopamine caused WT (but not dop-1) worms to exhibit crawling bouts in water. (B) Photoactivation of ChR2 in dopamine neurons reversibly induced crawl-like behavior in WT but less so for D1-like receptor mutants. (C) Photo-uncaging of dopamine injected into the anterior (solid bars) but not posterior half of animals reversibly induced crawl-like behavior in WT but not in a dop-1 background. Representative neck curvature traces shown below. (Scale bar for A–C on left, 50° by 1 s.) (D) Time course of neck curvature before, during, and after photoactivation (indicated by blue bar) of ChR2 in dopamine neurons of a WT worm in water.
Dopamine has been previously implicated in reducing the crawling rate of worms entering a lawn of bacteria in the so-called basal-slowing response whereby crawling velocity decreases and the worm frequently moves backward to pivot in place while eating (24). Consistent with this result, we found that photo-activation of dopamine neurons in animals crawling on unseeded (blank) agar plates induced an immediate transition to a slower form of crawling with the same kinematics and increased reversal frequency observed in basal-slowing (Fig. S5). We conclude that dopamine can induce discrete, context-dependent changes in locomotory patterns: from swimming to crawling for worms in water, and from crawling to basal-slowing behavior on land.
Because dopamine released in the worm can act humorally in addition to synaptically, the complete wiring diagram of the nervous system of C. elegans cannot be easily used to determine which neurons are altered by dopamine to affect the observed transition (25). We therefore attempted to narrow down the sites of dopamine action by synthesizing and injecting light-sensitive, caged dopamine into the posterior and anterior regions of the worm (26). We found that worms thus treated swam normally in all cases but that only photo-uncaging of dopamine in their anterior section caused the worms to recapitulate the behaviors seen with stimulation of the dopaminergic neurons by ChR2 (Fig. 4C).
Together these results demonstrate that dopamine signaling is necessary and sufficient for worms to switch from swimming to crawling gaits. We propose that dopamine released by the ADE and PDE neurons may activate D1-like dopamine receptors located in the anterior half of the animal to trigger the transition from swimming to crawling gaits.
Serotonin Signaling Is Necessary for Crawl-to-Swim Transition.
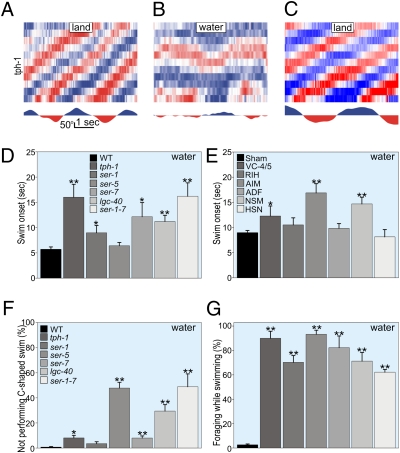
A test of available aminergic mutants for crawl-to-swim impairments revealed that the tryptophan hydroxylase mutant tph-1, which is unable to synthesize serotonin, crawled normally before and after submersion but was severely impaired in swim initiation (Fig. 5 A–C). Delayed swimming onsets were also observed for serotonin receptor mutants (Fig. 5D). C. elegans has six classes of serotonergic neurons (VC-4, -5, RIH, AIM, ADF, NSM, and HSN). Ablation of VC-4, -5, AIM, or NSM neurons delayed swim onset, implicating serotonin signaling in swim initiation (Fig. 5E).
Fig. 5.
Serotonin is necessary for crawl-to-swim transition. tph-1 mutants deficient in serotonin synthesis crawl normally (A) but are impaired in swim initiation and performance (B); after emergence, crawling resumes normally (C). Swim onset was delayed in serotonergic pathway mutants (D) and in worms with their AIM, NSM, or VC-4 and -5 serotonergic neuron classes ablated (E). Serotonergic pathway mutants showed defective swimming (F), including bouts of crawl-like behaviors (G).
Serotonin was also necessary to maintain normal swimming and to prevent crawling in water (Movie S6). Paralleling the effects of dopamine exposure in WT (and untreated che-3 mutants in low viscosity), tph-1 mutants displayed crawl-like bouts while submersed (Fig. S6 A and B). Ablation of the VC-4, -5, RIH, or AIM neurons recapitulated the tph-1 phenotype (Fig. 5 D and E). Although most ablated animals showed no change in swimming amplitudes (Fig. S7), some showed significant increases in swimming velocities (Fig. S6D) resulting from bouts of high-amplitude tail bends (Fig. S8). Among the seven described serotonin receptors in C. elegans, velocity was most impaired for mutants missing LGC-40 or both SER-1 and SER-7 (Fig. S6F). These serotonin-receptor mutants (and those lacking SER-5) exhibited poor swim coordination and showed crawl-like bouts accompanied by foraging and pumping (Fig. 5 F and G).
Serotonin Is Sufficient to Induce Crawl-to-Swim Transition on Land and to Delay Crawl Onset.
Exogenous serotonin was sufficient to delay crawl onset when puddles receded. It prolonged swim-like movement in shallow puddles, in a manner reminiscent of fish flailing on land, until all water had completely disappeared (Fig. 6A and Movie S7).
Fig. 6.
Serotonin is sufficient for crawl-to-swim transition. Exogenous and endogenous serotonin delayed onset of crawling after puddle absorption for WT worms on land (A), and onset of photostimulated crawling for Pdat-1::ChR2 worms in water (B). Altering the dopamine-serotonin balance toward serotonin either genetically via cat-2 or by photostimulation of serotonergic neurons caused faster than WT swim onset. The opposite effect was accomplished by photostimulation of dopaminergic neurons (C). Conversely, photostimulation of serotonergic neurons in crawling worms produced swim-like frequencies (D). (E) Time course of neck curvature of Ptph-1::ChR2 worm on land before, during, and after photoactivation of serotonergic neurons.
Consistent with a role in blocking crawl-initiation and maintaining swimming, exogenous serotonin delayed onset of photo-induced crawling of Pdat-1::ChR2 worms in water (Fig. 6B). Conversely, photoactivation of ChR2 in serotonergic neurons (via Ptph-1::ChR2 worms) drastically hastened swim onset (Fig. 6C) and dramatically increased frequency of bend propagation on land (Fig. 6 D and E and Movie S8). These findings suggested that the balance between serotonin and dopamine could bias gait transitions in C. elegans. We investigated this possibility and found supporting evidence in the dopamine synthesis mutant (cat-2) in which lower than normal dopamine levels could explain a faster than normal swim onset (Fig. 6D). Moreover, average swim onsets for mutants with impairments in production of both amines were similar to WT (bas-1, 4.47 ±1.89 s; cat-4, 5.98 ±1.65 s; bas-1;cat-4 double mutant, 5.46 ± 2.36 s). Taken together, these data lead us to conclude that serotonin signaling is necessary and sufficient for worms to switch from crawling to swimming gaits. Furthermore, it seems likely that the balance between serotonin and dopamine influences gait transitions in C. elegans.
Discussion
Swimming and Crawling as Distinct Motor Gaits.
Consistent with previous reports, we found that worms modulated their swimming gait as liquid viscosity was increased (15–17). However, the bimodal Gaussian distributions seen for their cycle durations at highest viscosities, and the associated foraging and pumping seen during only the slowest form, strongly suggest that these animals are alternating between two distinct gaits. Foraging and pharyngeal pumping are two (of a number of) behaviors present when worms crawl but absent when worms swim. This implies a discrete change in global behaviors occurring when worms transition between environments.
Previous studies have suggested that all of the kinematic differences between crawling and swimming are the product of changes in the physical environment and not due to discrete changes in neural activity (14–18). The transition from swimming to crawling seen for worms in our magnet experiment suggests that crawling can be induced in water by mechanical stimulation of specific areas of the worm's body. This is further supported by the observation that worms with iron in alternate areas of their bodies continued to swim when pulled (and pinned) against the substrate by a magnetic field (Fig. 2I). Additional evidence supporting swimming and crawling as distinct gaits came from the time required by unrestrained WT worms to begin swimming after entering water (6 s). During this time (swim onset), worms neither crawled nor swam. Furthermore, the existence of mutants with behavioral defects specific to transitions between these environments (as seen in strains deficient in dopamine and serotonin signaling; Fig. 7 A and C) can only be understood if the worms were transitioning between different behaviors. Together, these data indicate that crawling and swimming must be the outputs of (at least) partially distinguishable neural networks.
Recent studies of locomotion in C. elegans have made extensive use of modeling techniques to quantitatively describe the physical interactions between environment and this tiny organism (15, 17, 27–30). Indeed, any thorough understanding of locomotion must include knowledge of the physical properties of both the animal and its environment. These strategies, coupled with approaches focusing on the evolution, anatomy, physiology, and natural history make C. elegans a promising system for understanding the production and control of adaptive locomotory gaits.
Dopamine Mediates the Transition from Swimming to Crawling.
On land, dopamine has been shown to modulate C. elegans crawling behavior by decreasing velocity when worms enter a patch of food in the so-called basal-slowing behavior (24). We found that light activation of dopaminergic neurons in worms crawling on land (in the Pdat-1::ChR2 strain) recapitulated this finding (Fig. 4). In water, activation of dopamine neurons via D1-like signaling induced an immediate switch from swimming to a version of how the worm crawls off food (Fig. 4 B and D). Although activation of dopamine neurons decreases velocity in both contexts, loss of dopamine signaling produces opposite results. Namely, worms with reduced dopamine signaling continue crawling quickly as if food is absent upon entering a food patch (24), whereas the same dopamine deficient worms cease all movement upon transitioning from water to land (Fig. 3 C and E). Moreover, the basal-slowing response relies on D2-like dopamine signaling rather than the D1-like signaling pathway we observed to be required for the swim-to-crawl transition (24). Dopamine, therefore, seems to affect locomotion in a context-dependent way, slowing crawling on land through D2-like pathway and inducing crawling as animals exit water through a D1-like pathway.
Our results also provide an explanation for the swimming behavior of mutant worms that lack the dopamine reuptake transporter DAT-1 in previous studies. Worms lacking dat-1 become paralyzed after extended swimming (10 min) during which internal dopamine presumably accumulates to high concentration (SWIP, swim-induced paralysis) (31). Our findings are consistent with the SWIP phenotype because dopamine release is proposed to be down-regulated during swimming. As dopamine levels rise without reuptake, the dat-1 mutant may switch from swimming to crawling before eventual paralysis. This is consistent with our finding that worms submerged in water switch from swimming to crawl-like behavior and foraging after light activation of dopaminergic neurons (in the Pdat-1::ChR2 strain; Fig. 4 B and D).
Serotonin Mediates the Transition from Crawling to Swimming.
Like dopamine, the role of serotonin on behavior modulation also seems to be context-dependent. Worms deprived of food are proposed to release higher levels of serotonin when the animal crawls back onto a food patch (24). The extra serotonin dramatically decreases speed, which presumably ensures that the starved worm eats to satiation (24). By contrast, we found that photo-activation of serotonergic neurons increased bend frequency and speed to swim-like levels. We also found that exposure to serotonin hastened swim onset and prolonged swimming in worms emerging from puddles. Conversely, lack of serotonin resulted in dramatic delay in swim onset and in deficient swimming behavior. Therefore, although it remains to be seen whether other neuromodulators play a role in the transition from crawl to swim [analogous to the role of octopamine for gait switching in the leech (3)], serotonin is clearly important in the induction and maintenance of swimming in C. elegans.
Dopamine and Serotonin in Behavioral Transitions.
Dopamine and serotonin are emerging as universal switches turning behaviors ON or OFF. More than 30 y ago, serotonin was found to be involved in initiating and maintaining swimming and fictive swim rhythms in the nerve cord of the leech (32, 33). More recently, dopamine has been shown to switch a fictive swim rhythm to a fictive crawling rhythm in leech (3). The role of serotonin in rapid locomotory rhythms and dopamine in slow locomotory rhythms is surprisingly conserved across animals as evolutionarily diverse as leech (3, 9), sea-slugs (4, 7), lamprey (5, 8), and mouse (6). Even in humans, loss of dopamine neurons in Parkinson's disease leads to debilitating problems in initiating and switching between motor programs (analogous to the immobility of dopamine-deficient worms emerging from water) (11). The profound importance that selecting the correct motor pattern has on survival may explain how dopamine and serotonin have retained their roles as behavioral switches during 1.1 billion years of divergent evolution (34). Further study of crawl–swim switching in C. elegans has the potential to uncover the fundamental neural mechanisms underlying how dopamine enables the natural transitions between motor patterns, as well as how motor switching becomes dysfunctional in Parkinson's disease.
Materials and Methods
C. elegans were grown on nematode growth media (NGM) agar plates seeded with OP50 bacteria at 20 °C as previously described (35). Information on strains, viscosity, pressure and magnetic assays, statistical analysis, laser ablation of neurons, behavioral pharmacology, and optogenetics is in SI Materials and Methods.
Behavioral Assays.
Each assay was conducted on 10–30, never-starved, young adult worms. Worms were cleaned of bacteria by allowing them to crawl on an empty plate before each experiment. After transfer to assay plate, worms were allowed a 2-min acclimation period. Movie recordings were made at 30 frames/s, 344 pixels/mm using a Flea2 camera (Point Grey Research) and StreamPix software (NorPix). Data were analyzed blind to experimental treatment and genotype.
Single worms.
The behavior of a worm was video recorded for 2 min on a blank NGM agar plate (Movie S1) before a 3-μL drop of NGM buffer was placed in its path, allowing the worm to crawl into the puddle. After ≈5 min the puddle was absorbed by the agar enough that the worm could escape and resume crawling. Filming continued for 5 min after puddle emergence. Animal midlines (13 points) of single worms were derived as previously described using a custom image analysis algorithm available upon request (13) (ImagePro; Media Cybernetics). The series of 11 angles formed by the midline was represented in a color-coded “curvature column” (Fig. S1). A time series of curvature columns formed a “curvature matrix” (e.g., Fig. 1B) in which blue and red stripes represent the waves of dorsal and ventral curvature, respectively, passing along the body. Curvature matrices were divided into neck-bend cycles according to curvature of the second most anterior row in the curvature matrix using IgorPro (Wave Metrics). The quality of each digitized worm midline was manually checked, superimposing it on the original video frame, and corrected if necessary for every video frame used in this study (≈1,000,000 frames).
Groups of worms.
The behavior of 15 worms confined within a square copper frame (1.2 cm per side) on a blank NGM agar plate was recorded before, during, and after a 150-μL drop of NGM buffer was placed in the frame and wicked away with a Kimwipe tissue. Worm centroids were detected and tracked using ImagePro.
Supplementary Material
Acknowledgments
We thank Kurt Svoboda, Dorothy Paul, Hans Hofmann, Lauren O'Connell, and Serge Faumont for critical reading of manuscript drafts; and Villu Maricq, Robert Horvitz, Richard Komuniecki, and Shohei Mitani for worm strains. Additional strains were kindly provided by the Caenorhabditis Genetics Center, which is funded by the National Institutes of Health (NIH) National Center for Research Resources; the C. elegans Gene Knockout Project at the Oklahoma Medical Research Foundation, which is part of the International C. elegans Gene Knockout Consortium; and the National BioResource Project in Japan. This work was supported by the University of Texas at Austin, Alcohol Beverage Medical Research Foundation, NIH National Institute of Neurological Disorders and Stroke Grant R01NS075541 (to J.T.P.-S.), Deutsche Forschungsgemeinschaft Grant SFB807-TP11, and Cluster of Excellence Frankfurt (to A.G.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1108673108/-/DCSupplemental.
References
- 1.Bradford EH. Examination of human gait. J Boston Soc Med Sci. 1897;2:20–21. [PMC free article] [PubMed] [Google Scholar]
- 2.Alexander RM. Principles of Animal Locomotion. Princeton: Princeton Univ Press; 2003. [Google Scholar]
- 3.Mesce KA, Pierce-Shimomura JT. Shared strategies for behavioral switching: Understanding how locomotor patterns are turned on and off. Front Beh Neurosci. 2010;4:1–5. doi: 10.3389/fnbeh.2010.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Katz PS, Frost WN. Intrinsic neuromodulation in the Tritonia swim CPG: Serotonin mediates both neuromodulation and neurotransmission by the dorsal swim interneurons. J Neurophysiol. 1995;74:2281–2294. doi: 10.1152/jn.1995.74.6.2281. [DOI] [PubMed] [Google Scholar]
- 5.Wallén P, et al. Effects of 5-hydroxytryptamine on the afterhyperpolarization, spike frequency regulation, and oscillatory membrane properties in lamprey spinal cord neurons. J Neurophysiol. 1989;61:759–768. doi: 10.1152/jn.1989.61.4.759. [DOI] [PubMed] [Google Scholar]
- 6.Dunbar MJ, Tran MA, Whelan PJ. Endogenous extracellular serotonin modulates the spinal locomotor network of the neonatal mouse. J Physiol. 2010;588:139–156. doi: 10.1113/jphysiol.2009.177378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Woodward OM, Willows AOD. Dopamine modulation of Ca(2+) dependent Cl(-) current regulates ciliary beat frequency controlling locomotion in Tritonia diomedea. J Exp Biol. 2006;209:2749–2764. doi: 10.1242/jeb.02312. [DOI] [PubMed] [Google Scholar]
- 8.McPherson DR, Kemnitz CP. Modulation of lamprey fictive swimming and motoneuron physiology by dopamine, and its immunocytochemical localization in the spinal cord. Neurosci Lett. 1994;166:23–26. doi: 10.1016/0304-3940(94)90831-1. [DOI] [PubMed] [Google Scholar]
- 9.Esch T, Mesce KA, Kristan WB. Evidence for sequential decision making in the medicinal leech. J Neurosci. 2002;22:11045–11054. doi: 10.1523/JNEUROSCI.22-24-11045.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.El Manira A, Grillner S. Switching gears in the spinal cord. Nat Neurosci. 2008;11:1367–1368. doi: 10.1038/nn1208-1367. [DOI] [PubMed] [Google Scholar]
- 11.Voon VP, et al. Chronic dopaminergic stimulation in Parkinson's disease: From dyskinesias to impulse control disorders. Lancet Neurol. 2009;8:1140–1149. doi: 10.1016/S1474-4422(09)70287-X. [DOI] [PubMed] [Google Scholar]
- 12.Félix M-A, Braendle C. The natural history of Caenorhabditis elegans. Curr Biol. 2010;20:R965–R969. doi: 10.1016/j.cub.2010.09.050. [DOI] [PubMed] [Google Scholar]
- 13.Pierce-Shimomura JT, et al. Genetic analysis of crawling and swimming locomotory patterns in C. elegans. Proc Natl Acad Sci USA. 2008;105:20982–20987. doi: 10.1073/pnas.0810359105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gray J, Lissmann HW. The locomotion of nematodes. J Exp Biol. 1964;41:135–154. doi: 10.1242/jeb.41.1.135. [DOI] [PubMed] [Google Scholar]
- 15.Berri S, Boyle JH, Tassieri M, Hope IA, Cohen N. Forward locomotion of the nematode C. elegans is achieved through modulation of a single gait. HFSP J. 2009;3:186–193. doi: 10.2976/1.3082260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Korta J, Clark DA, Gabel CV, Mahadevan L, Samuel ADT. Mechanosensation and mechanical load modulate the locomotory gait of swimming C. elegans. J Exp Biol. 2007;210:2383–2389. doi: 10.1242/jeb.004572. [DOI] [PubMed] [Google Scholar]
- 17.Fang-Yen C, et al. Biomechanical analysis of gait adaptation in the nematode Caenorhabditis elegans. Proc Natl Acad Sci USA. 2010;107:20323–20328. doi: 10.1073/pnas.1003016107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boyle JH, Berri S, Tassieri M, Hope IA, Cohen N. Gait modulation in C. elegans: It's not a choice, it's a reflex! Front Beh Neur. 2011;5:1–3. doi: 10.3389/fnbeh.2011.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Faumont S, Miller AC, Lockery SR. Chemosensory behavior of semi-restrained Caenorhabditis elegans. J Neurobiol. 2005;65:171–178. doi: 10.1002/neu.20196. [DOI] [PubMed] [Google Scholar]
- 20.White JG, Southgate E, Thomson JN, Brenner S. The structure of the nervous system of the nematode Caenorhabditis elegans. Philos Trans R Soc Lond. 1986;314:1–340. doi: 10.1098/rstb.1986.0056. [DOI] [PubMed] [Google Scholar]
- 21.Hills T, Brockie PJ, Maricq AV. Dopamine and glutamate control area-restricted search behavior in Caenorhabditis elegans. J Neurosci. 2004;24:1217–1225. doi: 10.1523/JNEUROSCI.1569-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li W, Feng Z, Sternberg PW, Xu XZS. A C. elegans stretch receptor neuron revealed by a mechanosensitive TRP channel homologue. Nature. 2006;440:684–687. doi: 10.1038/nature04538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kang L, Gao J, Schafer WR, Xie Z, Xu XZS. C. elegans TRP family protein TRP-4 is a pore-forming subunit of a native mechanotransduction channel. Neuron. 2010;67:381–391. doi: 10.1016/j.neuron.2010.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sawin ER, Ranganathan R, Horvitz HR. C. elegans locomotory rate is modulated by the environment through a dopaminergic pathway and by experience through a serotonergic pathway. Neuron. 2000;26:619–631. doi: 10.1016/s0896-6273(00)81199-x. [DOI] [PubMed] [Google Scholar]
- 25.Chase DL, Pepper JS, Koelle MR. Mechanism of extrasynaptic dopamine signaling in Caenorhabditis elegans. Nat Neurosci. 2004;7:1096–1103. doi: 10.1038/nn1316. [DOI] [PubMed] [Google Scholar]
- 26.Lee TH, Gee KR, Ellinwood EH, Seidler FJ. Combining ‘caged-dopamine’ photolysis with fast-scan cyclic voltammetry to assess dopamine clearance and release autoinhibition in vitro. J Neurosci Methods. 1996;67:221–231. [PubMed] [Google Scholar]
- 27.Karbowski J, et al. Conservation rules, their breakdown, and optimality in Caenorhabditis sinusoidal locomotion. J Theor Biol. 2006;242:652–669. doi: 10.1016/j.jtbi.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 28.Sznitman J, Purohit PK, Krajacic P, Lamitina T, Arratia PE. Material properties of Caenorhabditis elegans swimming at low Reynolds number. Biophys J. 2010;98:617–626. doi: 10.1016/j.bpj.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park S-J, Goodman MB, Pruitt BL. Analysis of nematode mechanics by piezoresistive displacement clamp. Proc Natl Acad Sci USA. 2007;104:17376–17381. doi: 10.1073/pnas.0702138104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Petzold BC, et al. Caenorhabditis elegans body mechanics are regulated by body wall muscle tone. Biophys J. 2011;100:1977–1985. doi: 10.1016/j.bpj.2011.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McDonald PW, et al. Vigorous motor activity in Caenorhabditis elegans requires efficient clearance of dopamine mediated by synaptic localization of the dopamine transporter DAT-1. J Neurosci. 2007;27:14216–14227. doi: 10.1523/JNEUROSCI.2992-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Willard AL. Effects of serotonin on the generation of the motor program for swimming by the medicinal leech. J Neurosci. 1981;1:936–944. doi: 10.1523/JNEUROSCI.01-09-00936.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nusbaum MP, Kristan WB., Jr Swim initiation in the leech by serotonin-containing interneurones, cells 21 and 61. J Exp Biol. 1986;122:277–302. doi: 10.1242/jeb.122.1.277. [DOI] [PubMed] [Google Scholar]
- 34.Blair JE, Shah P, Hedges SB. Evolutionary sequence analysis of complete eukaryote genomes. BMC Bioinformatics. 2005;6:53. doi: 10.1186/1471-2105-6-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.