Abstract
Dorsolateral prefrontal cortex (DLPFC) is recruited during visual working memory (WM) when relevant information must be maintained in the presence of distracting information. The mechanism by which DLPFC might ensure successful maintenance of the contents of WM is, however, unclear; it might enhance neural maintenance of memory targets or suppress processing of distracters. To adjudicate between these possibilities, we applied time-locked transcranial magnetic stimulation (TMS) during functional MRI, an approach that permits causal assessment of a stimulated brain region's influence on connected brain regions, and evaluated how this influence may change under different task conditions. Participants performed a visual WM task requiring retention of visual stimuli (faces or houses) across a delay during which visual distracters could be present or absent. When distracters were present, they were always from the opposite stimulus category, so that targets and distracters were represented in distinct posterior cortical areas. We then measured whether DLPFC-TMS, administered in the delay at the time point when distracters could appear, would modulate posterior regions representing memory targets or distracters. We found that DLPFC-TMS influenced posterior areas only when distracters were present and, critically, that this influence consisted of increased activity in regions representing the current memory targets. DLPFC-TMS did not affect regions representing current distracters. These results provide a new line of causal evidence for a top-down DLPFC-based control mechanism that promotes successful maintenance of relevant information in WM in the presence of distraction.
Keywords: external interference, top-down control
Dorsolateral prefrontal cortex (DLPFC) has long been associated with visual working memory (WM) function, with current models suggesting that it is a source of top-down control over posterior regions maintaining information across the short term (1, 2). One such control process is that of resistance to external distraction, when the appearance of irrelevant information in the visual scene could potentially interfere with maintenance of visual memoranda (3). Moreover, recent studies suggest that filtering of irrelevant information is one important determinant of WM capacity (4, 5), as irrelevant information may occupy limited storage resources that could otherwise be devoted to maintaining relevant information (4). Human lesion (6) and functional magnetic resonance imaging (fMRI) studies (7–9) indicate a specific role for the DLPFC when external distraction must be overcome during the maintenance phase of WM. However, little causal, temporally specific evidence exists on whether DLPFC acts during delays to suppress further processing of distracters (6, 8, 9) or whether it instead enhances neural representations of the maintained targets to protect them (7, 10, 11). One striking aspect of DLPFC responses is that they typically concern relevant stimuli (targets) but not irrelevant information (distracters) (11), including during WM (12). Such observations appear more consistent with target-protection accounts of DLPFC function, but a decisive causal test is nevertheless required.
Two recent studies combined transcranial magnetic stimulation (TMS) over frontal cortex with neural measures of brain activity during visual WM, as assessed via electroencephalography (EEG) (13) or fMRI (14), to examine the impact of causal TMS manipulations. However, both studies used TMS over the inferior frontal junction, unlike our focus on DLPFC here; they also used prolonged offline TMS and hence could not time-lock TMS to specific task events, unlike in the present study. We return to these results in relation to our own in Discussion.
To study DLPFC influences on posterior WM-related areas during distraction in a causal and time-locked manner, we applied event-related TMS concurrently with fMRI. Applying TMS online during fMRI scanning has revealed blood oxygen level-dependent (BOLD) changes that occur not only at the site of stimulation, but also in anatomically remote but functionally coupled regions. Such observations suggest that TMS induces local activity, which propagates to areas communicating with the stimulated site in particular task conditions (15–18). Moreover, such communication (or functional coupling) of the stimulated region with other brain areas can change in a dynamic fashion, thereby permitting assessment of task context-dependent activity propagation within brain networks (19). Recent studies with TMS-fMRI (20, 21) or TMS-EEG (22, 23) have validated the approach of using TMS as a causal “physiological probe” during behavior, when stimulation modulates remote BOLD responses (or evoked potentials) in a condition-dependent manner, yet does not disrupt task performance. Such an approach also permits study of functional coupling patterns under behaviorally “normal” conditions (thereby avoiding the complications of interpreting neural changes associated with behavioral disruption). Here we adopted this approach to investigate the role of DLPFC under conditions of distraction during WM maintenance. By using fMRI to image how specific posterior brain regions were affected by DLPFC stimulation under different task conditions, we sought to determine how communication between DLPFC and those posterior regions might vary as a function of task context.
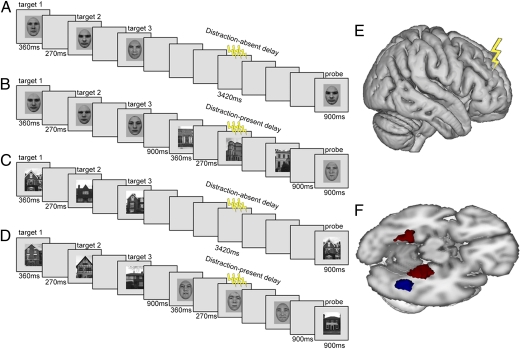
Our use of event-related DLPFC-TMS concurrent with fMRI during WM therefore permitted a unique test of: (i) whether DLPFC causally has an impact on posterior brain areas during visual WM; (ii) if this depends on the presence of distracters; and (iii) whether DLPFC influences target- or distracter-representing posterior brain regions during WM maintenance in the face of such distraction. We used a visual delayed recognition paradigm in which participants retained faces or houses over a short delay period (Fig. 1 A–D). This task has been shown to produce a sustained delay period response (taken as an index of short-term storage; ref. 24) in posterior category-selective extrastriate regions [fusiform face area (FFA) and parahippocampal place area (PPA) for faces and houses, respectively] (25–27). Additionally, during the delay period, external distraction could arise as visual stimuli from the opposite category to the memory targets (i.e., for face memory trials, any distracters were house stimuli, whereas for house memory trials, any distracters were face stimuli). The presence of distracting stimuli during the delay period has been shown to result in additional activation of DLPFC (8, 9, 28), indicating a role for this particular region in dealing with distracters; however, whether this DLPFC role operates via target protection or distracter suppression has remained unclear.
Fig. 1.
(A–D). Schematic of trial events for four example conditions. Each trial began with presentation of three targets from one category. In half of trials, the targets were faces (A and C); in the other half, they were houses (B and D). After a short delay, a probe stimulus from the target category appeared; participants judged whether or not it had been one of the three preceding targets. In the middle of the delay period (indicated by the lightening flashes in A–D), three high-intensity or three low-intensity TMS pulses were applied, at timepoints when three distracters from the opposite category to the targets could also be presented (C and D). E illustrates application of TMS over right DLPFC (see Methods and Fig. 3 for localization of this region in each participant) during fMRI. This approach allowed assessment of any impact from stimulation there on activity in posterior FFA or PPA ROIs (in F, see blue and red clusters, respectively, for a representative participant).
On each trial we applied three high-frequency TMS pulses at either a high intensity, intended to produce significant modulations of the BOLD response, or at a low intensity, intended to act as a control (for any nonspecific effects of TMS, e.g., the acoustic artifact from coil discharge and scalp tactile sensation) without producing significant BOLD response changes. The specific TMS parameters and intensities were adopted from several previous concurrent TMS-fMRI studies (20, 21). Importantly, in the present experiment, the TMS pulses were time-locked to the middle of the unfilled delay period, at the point when distracters could appear. We anticipated that these TMS parameters would not disrupt behavior but would be sufficient to modulate BOLD responses in those regions functionally coupled with DLPFC at the time of stimulation. By measuring the remote effects of high- vs. low-intensity TMS in the FFA and PPA of each participant, we could then assess whether any remote TMS effects were dependent on the presence/absence of distraction. Moreover, because memory targets and distracters were always of the opposite category within each trial, we could test whether DLPFC-TMS influenced the category-sensitive posterior areas representing current memory targets or those representing current distracters, thereby allowing us to infer whether remote causal influences of DLPFC during the delay period of a WM task are related to successful maintenance of target information or suppression of distracter information.
Results
Behavior.
High- vs. low-intensity TMS did not alter behavioral performance, consistent with our intended use of TMS as a physiological probe (19, 20). For each participant, mean proportion correct and median reaction times (milliseconds) were calculated for each condition (Table 1). A three-way repeated-measures analyses of variance (ANOVA) was performed on each of these dependent variables with factors of memory target (face, house), distraction (present, absent), and TMS intensity (high, low). For mean proportion correct, there were no significant main effects or interactions (all F values < 3), but a substantial trend for a main effect of distraction (regardless of TMS) with less accuracy overall when distraction was present [F(1, 15) = 2.99; P = 0.10]. This trend reached significance on a one-tailed t test [t(15) = 2.12; P = 0.05] that can be justified by the directional expectation that distracters should hinder rather than help, despite being of the opposite category to targets. For the reaction time data, there were no significant main effects or interactions (all F values < 1.7).
Table 1.
Mean accuracy and reaction times for each condition
| Distraction condition | Mean proportion correct | Mean median RT, ms |
| Absent | ||
| Face | ||
| Low | 0.76 (0.10) | 1,019.25 (252.68) |
| High | 0.73 (0.11) | 1,009.06 (231.13) |
| House | ||
| Low | 0.72 (0.15) | 1,015.84 (238.90) |
| High | 0.72 (0.11) | 1,035.94 (259.14) |
| Present | ||
| Face | ||
| Low | 0.74 (0.10) | 988.63 (272.40) |
| High | 0.71 (0.11) | 1,017.28 (277.75) |
| House | ||
| Low | 0.69 (0.09) | 1,017.04 (246.62) |
| High | 0.72 (0.10) | 996.09 (235.10) |
SDs are in parentheses.
fMRI Data.
We first investigated the effect of distraction present vs. absent for right DLPFC under the TMS coil [see Methods for localization of this region of interest (ROI)]. As expected (7–9, 28), higher activity was observed for right DLPFC in the presence of distraction [t(15) = 2.55; P < 0.05; two-tailed, as for all subsequent t tests reported here]. Also, as expected, activity in this region was greater for high- vs. low-intensity TMS [t(15) = 2.63; P < 0.05], indicating that our TMS parameters were successful in significantly modulating the targeted region. The left DLPFC homolog showed a similar but marginal trend for higher activity with distraction present vs. absent [t(15) = 1.94; P = 0.07]. However, unlike (stimulated) right DLPFC, there was no significant impact of right DLPFC TMS intensity on (unstimulated) left DLPFC activity [t < 1.1; not significant (n.s.)].
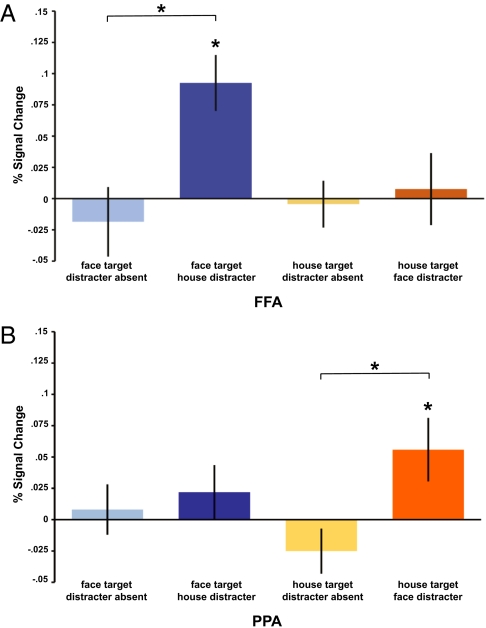
Next, we assessed whether high- vs. low-intensity DLPFC TMS affected activity in the posterior FFA and PPA ROIs. (For each participant, we could reliably identify right hemisphere FFA and bilateral PPA; see Methods.) A three-way ANOVA on (high > low) TMS-induced differences of BOLD percent signal change, with factors of ROI (right FFA, bilateral PPA), memory-target category (face, house), and distraction (present, absent) yielded a significant three-way interaction [F(1,15) = 11.26; P < 0.01]. This interaction was due to the FFA response being increased by (high) DLPFC-TMS specifically during face memory with house distracters [t(15) = 4.04; P < 0.01; Fig. 2A], whereas analogously in PPA responses were increased by TMS specifically during house memory with face distracters [t(15) = 2.19; P < 0.05; Fig. 2B]. When the preferred category for the corresponding posterior ROI functioned as a distracter instead of a memory target, significant TMS-induced effects were no longer observed. Specifically, TMS did not modulate FFA responses for house memory with face distracters [t(15) = 0.28; n.s.], and analogously, in PPA, face memory trials with house distracters were not modulated by TMS [t(15) = 1.01; n.s.]. The significant TMS effects on distraction-present trials differed from distraction-absent trials within FFA during face memory [t(15) = 3.74; P < 0.01] but not house memory [t(15) = 0.51; n.s.], and within PPA for house memory [t(15) = 2.57; P < 0.05 but not face memory [t(15) = 0.45; n.s.] (Fig. 2).
Fig. 2.
Interparticipant mean BOLD percent signal changes (±1 SEM) due to high-minus low-intensity DLPFC-TMS (i.e., the differential change in BOLD due to an increase in TMS intensity) are shown for FFA (A) and PPA (B). Asterisks indicate significant effects of TMS. Note that high-intensity TMS increased BOLD in FFA specifically when faces served as memory targets in the presence of house distracters. Analogously, high-intensity TMS increased activity in PPA specifically when houses were memory targets in the presence of face distracters. Thus, DLPFC stimulation had an impact on the posterior region representing the current target (rather than the current distracter), but only in the presence of distraction.
For completeness, we also report the results for bilateral FFA when pooling across hemispheres in those 11 participants for whom both right and left FFA could be readily identified. Despite the reduced n, the same pattern of results was found. Three-way ANOVA on the TMS-induced differences in FFA, with right vs. left FFA now as an additional factor, found no interaction of hemisphere × target category × distraction (F < 1.5; n.s.). The interaction between memory target and distraction still reached significance [F(1, 10) = 5.65; P < 0.05] with a pairwise comparison showing a marginal effect of TMS on face targets in the presence of house distracters [t(10) = 2.02; P = 0.07]. Similarly, when the TMS-induced effects for the PPA data were separated by right vs. left PPA, which was possible for all participants, there were no interactions involving hemisphere (all F values < 1). Thus, our critical findings for the posterior ROIs did not depend on hemisphere (except for the general aspect that, consistent with previous studies, FFA was more readily identified in the right hemisphere in more subjects; refs. 29 and 30). Finally, we investigated whether the critical fMRI results in FFA/PPA due to DLPFC-TMS remain significant when considering just those participants for whom the behavioral effect of distracter presence was largest; for the corresponding subset of 9/16 participants, we confirmed it to be so.
Discussion
Current evidence suggests that DLPFC becomes involved in visual WM when distracters might disrupt the short-term maintenance of visual target information (7–9, 28). DLPFC might then exert modulating influences on posterior visual regions involved in WM storage. However, directly causal evidence for such DLPFC influences over posterior regions had remained lacking (although see one patient study in ref. 6). To provide such causal evidence, our approach applied time-locked TMS to DLPFC during the delay period and studied whether and how posterior regions (FFA and PPA) would be influenced by TMS, thus revealing which “gates of communication” were open between these regions and DLPFC when TMS was administered. In this way, we could determine whether DLPFC communicates primarily with distracter processing areas to suppress their activity or with target representing regions to protect ongoing target maintenance during a delay with distraction.
Our online TMS-fMRI data provide a unique form of evidence for the latter target-protection account of DPLFC-based control. When distracters were presented during the task delay period, we observed an increase in both right and left DLPFC activity (regardless of TMS intensity), implying that this region is involved during distraction. Importantly, DLPFC-TMS time-locked to the presentation of these distracters led to activity increases in posterior stimulus category-sensitive visual regions (FFA or PPA), but only when distracters were present. Finally, this remote influence was evident as an increase in BOLD response in the region representing the current memory targets but not the distracters.
These results provide compelling evidence that control signals from DLPFC propagate to posterior target-representing regions to help overcome the deleterious effects of distraction during WM maintenance, consistent with the emerging view that a key role for prefrontal cortex is selective processing of target-specific information (10, 31–34). Additionally, the lack of any TMS effects in the absence of distraction supports the view that the DLPFC is not necessary for “simple” maintenance (35–37), but rather becomes key when distraction must be overcome (8, 38). Our results show that the influence of DLPFC on posterior regions is not static and fixed (which should have led to equivalent remote effects of TMS in all our conditions) but instead can change in a highly dynamic way with different task conditions. This dynamic role for DLPFC may accord with an emerging “adaptive coding” account for DLPFC function (3), which emphasizes that the tuning of DLPFC neurons can alter dynamically with task conditions. Such adaptive coding implies changes in communication with posterior regions, as also implied by the remote effects of DLPFC-TMS we observed. Moreover, the adaptive coding framework puts particular emphasis on DLPFC representation of target information (10, 39), which could accord with the present target protection findings.
But other findings on visual WM have been taken to emphasize delay period distracter suppression rather than target enhancement (6, 8, 9). It should, however, be noted that standard fMRI and EEG studies cannot, on their own, provide truly causal evidence (in an interventional sense) for the role of specific frontal regions in modulating posterior regions. A more interventional approach, such as TMS over a frontal region combined with neural measures of the impact on posterior regions, is required.
Some recent work by Zanto et al. (13) did use TMS, albeit combining it with EEG rather than fMRI and using TMS in an offline manner (i.e., a long train of low-frequency repetitive TMS, intended to reduce excitability of the targeted site for a prolonged period afterward). Such prolonged TMS cannot be time-locked to task events, unlike the online TMS used here during fMRI. Moreover, Zanto et al. (13) targeted the inferior frontal junction with TMS, unlike the DPLFC-TMS site targeted here, which we had chosen because the latter is specifically known to activate when dealing with distracters during a WM delay period (7–9, 28), a result we also replicated here. Zanto et al. (13) found that EEG signatures from posterior electrodes for distinguishing task-relevant vs. task-irrelevant stimuli during encoding were diminished after offline TMS over the inferior frontal junction. Specifically, the enhancement of response amplitude for task-relevant stimuli observed under sham stimulation was decreased after real TMS, and the associated diminution in response normally shown for task-irrelevant stimuli was also less evident after real TMS.
In a related study, Higo et al. (14) also applied the same offline TMS protocol again over the inferior frontal junction, followed by fMRI scanning in which participants had to retain only a subset of previously encoded information and discard what became task-irrelevant information. In stimulus-sensitive posterior regions, they, too, observed a TMS-induced loss of effects depending on task relevance/irrelevance. Their findings complement those of Zanto et al. (13) in highlighting the role of the inferior frontal junction (as distinct from DLPFC) in modulating task-relevant vs. task-irrelevant stimulus processing. Despite the many considerable differences between the present study and this previous work, we think the results can be reconciled. We suggest that DLPFC plays a role in target protection during the delay period, as shown here, whereas the inferior frontal junction plays a role in distinguishing relevant from irrelevant information for encoding, as the other offline TMS studies have shown. This finding would be consistent with growing evidence for functional specialization within different frontal regions (34, 40, 41).
In sum, our study provides a new line of causal evidence that DLPFC is a source of top-down cognitive control signals during WM maintenance, specifically engaged when distracting information is present in the visual scene. These control signals vary dynamically with task context, being found here to increase activity in posterior brain regions related to maintained WM targets. These results are consistent with the view that DLPFC protects relevant information in the face of irrelevant distracting information.
Methods
Participants.
Sixteen right-handed participants (9 male) with no history of neurological or psychiatric abnormality had a mean age of 25 y (SD = 5.7). All were screened to confirm suitability for receiving TMS and gave written informed consent in accord with local ethics approval.
Stimuli.
Ninety grayscale male and female face stimuli were drawn from the Karolinska face database (http://webscript.princeton.edu/∼tlab/databases/database-2-karolinska-dataset/) and the XM2VTS database of the University of Surrey (http://www.ee.surrey.ac.uk/CVSSP/xm2vtsdb/). Ninety grayscale house stimuli were from an in-house database. All stimuli were 220 × 290 pixels and subtended 12.7° × 16.8° of visual angle. Face stimuli were cropped to an oval shape, removing the hair, and the resulting outer contours were blurred by using the Adobe Photoshop (CS4) blur tool. All stimuli were presented at display center against a gray background. Given that 120 stimuli from each category (faces, houses) were required per functional run (see below), and there were 90 from each category available, 30 stimuli from each category were repeated within each run with the constraint that stimuli that were presented as memory targets could not be presented as distracters within the same run. Also, to avoid possible effects of proactive interference, there were at least three trials kept between stimuli that were memory targets in one trial and nonmatching probes in another. For each participant, the stimuli to be repeated within one scanning run were randomly selected across runs to prevent systematic repetition of specific stimuli within the experiment.
Delayed Recognition WM Task.
Each trial began with sequential presentation of either three face or three house memory target stimuli, each presented for 360 ms separated by 270 ms. For distraction-absent trials, this presentation was followed by an unfilled delay period of 3,420 ms. For distraction-present trials, there was an initial unfilled delay period of 900 ms, followed by sequential presentation of three distracter stimuli (each for 360 ms separated by 270 ms) from the opposite category, then a further 900 ms of unfilled delay. For both trial types (distraction present or absent), the delay period of 3,420 ms was followed by a single probe stimulus from the same category as the memory target, presented for 900 ms. Participants judged via two-choice right-handed button press whether or not this probe matched one of the memory target stimuli that had appeared earlier in the trial (50% probability of match). The intertrial interval (ITI) was 1,700–4,600 ms, varied along 600-ms steps, resulting in six ITI lengths that occurred with equal probability eight times within each run.
fMRI.
Whole brain images were acquired on a 1.5-T scanner (Sonata; Siemens). High-resolution T1-weighted images (176 sagittal slices; 1 × 1 × 1 mm within a 256 × 240 matrix) were obtained in all participants. A multislice gradient echo echoplanar imaging sequence was used to acquire the BOLD signal (repetition time of 3,000 ms, echo spacing of 50 ms; 64 × 96 matrix; 33 axial slices, 3 × 3 × 2.5 mm, 1.25-mm gap; 50% phase oversampling in the phase encoding direction was applied to shift any Nyquist ghost artifact due to presence of the TMS coil outside the volume of interest) (16, 17, 20, 21, 42). Each participant completed five functional runs, each comprising 184 volumes, including 5 initial volumes discarded to allow for steady-state tissue magnetization. Before functional scans for the WM task, a fieldmap scan [double echo FLASH (gradient recalled echo) sequence with TE1 = 10 ms, TE2 = 12.46 ms; 3 × 3 × 2-mm resolution with 1-mm gap] was acquired to allow for correction of magnetic field inhomogeneities during preprocessing.
TMS.
TMS was delivered via a Magstim Rapid2 stimulator by using an MRI-compatible 70-mm figure-of-eight coil. The stimulator was placed inside an RF-shielded cabinet in the scanner room. The TMS coil cable was passed although a ferrite sleeve and filter box (Magstim). To prevent leakage current through the TMS coil, a high-voltage relay was connected to the stimulator (42), and during non-TMS-stimulation periods, this relay was closed to prevent current flow through the TMS coil. The relay was opened 90 ms before the first TMS pulse and closed 90 ms after the last pulse on each trial.
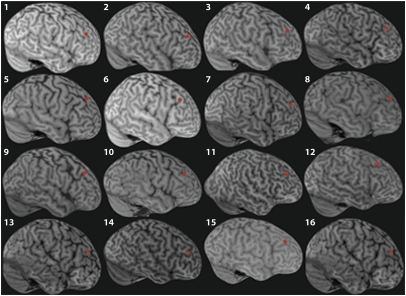
The TMS site over right DLPFC was selected based on a combination of individual anatomy and Montreal Neurological Institute (MNI) coordinates from a previous fMRI study [40, 46, and 22; as per an activation in right DLPFC reported by Sakai et al. (7) for a WM task with distraction]. See ref. 43 on the rationale for taking individual anatomy into account as well as published group activation coordinates. For each participant's native-space T1 scan, the midpoint along the middle frontal gyrus was identified. The coordinates of this midpoint were converted to MNI space (via rigid body transformation) and compared with the Sakai et al. (7) coordinates. If the Euclidean distance between these coordinates was >2 cm, the target was adjusted by linearly shifting the target in the direction of the Sakai et al. (7) coordinates until it was at a distance of not more than 2 cm. The mean MNI coordinates across participants for the resulting stimulation target were 37, 36, 34 (SD for x = 3.1, y = 3.1, z = 4.2]. For each participant, the TMS target in MNI space was then converted back into native space (Fig. 3), and this location was marked on their scalp after neuronavigation with Brainsight infrared frameless stereotaxy (Rogue Research), performed outside the scanner.
Fig. 3.
Location of right DLPFC-TMS site. For each participant, the location of the right DLPFC targeted with TMS is indicated by a red marker on its own 3D-rendered native space structural scan.
In the scanner, the TMS coil was held in position over the marked location with a custom nonferromagnetic holder. The coil was oriented at ∼45° from the midline with the handle of the coil pointing in a posterior direction to induce an initially anteroposterior current. Three TMS pulses were applied over the right DLPFC on each trial—either three at 110% (”high” intensity) or three at 40% (”low” intensity) of each participant's resting motor threshold—with parameters adopted from previous TMS-fMRI studies (20, 21). The first pulse was applied 1,710 ms after memory target offset, coinciding with the second distracter for distraction-present trials or at the same timepoint in the unfilled delay period of distraction-absent trials. The three brief pulses were not expected to disrupt behavior (which could otherwise have complicated interpretation of the concurrent fMRI data). Instead, as in other recent TMS-fMRI studies (20, 21), we applied brief TMS pulses here as a physiological probe to determine any propagation of influences to brain regions remote from the stimulated site (here specific extrastriate regions; see below). Each pulse was timed to coincide with the slice readout phase of slice acquisition, resulting in a stimulation rate of 11 Hz. This procedure resulted in the loss of only one MR slice per TMS pulse. For further details on the setup and implementation of TMS-fMRI in this scanning environment with similar parameters, see refs. 16, 17, 20, 21, and 42.
Design and Experimental Procedure.
We used a fully factorial, balanced, event-related design with three factors: memory target category (face or house), distraction (present or absent), and TMS intensity (high or low), resulting in a total of eight conditions. Participants performed 48 trials per run, randomly ordered, resulting in 30 trials per condition across 5 runs. Stimuli were viewed through a projection mirror. Before scanning, participants received training on up to 48 trials to familiarize them with the task.
fMRI Preprocessing and Analyses.
After reconstruction, slices spoiled by TMS pulses (one slice per pulse; see above) were removed by means of an in-house routine, in which slices with a signal magnitude of >3 SD from the mean were replaced by the mean of the same slices from the preceding and proceeding volumes (17, 21). Next, the first five volumes were discarded, and images were realigned to the middle of the series. The images were then spatially smoothed by using a 5-mm full-width-at-half-maximum Gaussian kernel, and slice-timing correction was applied. The final step of preprocessing was correction of magnetic field inhomogeneities (as measured by the acquired fieldmap scans) via geometric unwarping.
The voxel-wise effects of the eight experimental conditions were estimated by multiple linear regression of the high-pass filtered (cut off 128 s), prewhitened voxel time series onto a model specifying each condition. Each condition-specific trial was specified by a delta function placed at the start of a trial, which was then convolved with the synthetic hemodynamic response function, resulting in each trial being modeled as one epoch.
Individually Defined ROIs.
Our a priori ROIs were the stimulated right DLPFC (for completeness, we also looked at the symmetric left DLPFC; see below) plus the FFA and PPA, defined in each individual by their functional preference for faces or houses on distraction-absent trials regardless of TMS intensity (see below).
For DLPFC ROIs, we centered spheres with a radius of 10 mm at each participant's TMS site in right DLPFC. Parameter estimates scaled to reflect percent signal change relative to voxel baseline were then averaged across all voxels within the sphere for each individual participant.
For each participant, to identify posterior ROIs in inferior temporal cortex sensitive to stimulus category (27, 29, 30), the following standard contrasts were computed for distraction-absent trials regardless of TMS intensity: face > house for FFA; house > face for PPA. (Note that the factorial experimental design rendered these contrasts orthogonal to those from which further effects of interest were computed, for which TMS intensity was a factor.) The posterior ROIs were then derived from significant clusters for these contrasts, as determined by Z > 2.3 with a cluster-corrected threshold of P = 0.05, which also conformed to the anatomical constraints described by Ranganath et al. (27). Specifically, FFA was constrained to the fusiform gyrus, and PPA was constrained to parahippocampal gyrus extending into collateral sulcus. In this way we were able to identify bilateral PPA and right FFA in every participant, and accordingly the results are reported for these ROIs (i.e., pooling over PPA in both hemispheres, while scoring FFA for the right hemisphere, in which it was more consistently found across participants) (see refs. 29 and 30) regarding the tendency for right lateralization of FFA in participant groups). In 11 participants, we could also identify face-sensitive clusters in the left FFA. For this subset, we computed TMS-evoked responses pooled across both hemispheres for further analysis.
Acknowledgments
We thank Andy Anderson, Sven Bestmann, and Oliver Josephs for their technical assistance. E.F. was supported by an FP7 Marie Curie fellowship. K.H. and J.D. were supported by European Union BrainSynch Network FP7 Grant 200728. C.R. was supported by the University of Zurich. E.F., J.D., and the Centre for Neuroimaging are funded by the Wellcome Trust. J.D. holds a Royal Society Anniversary Research Professorship.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Curtis CE, D'Esposito M. Persistent activity in the prefrontal cortex during working memory. Trends Cogn Sci. 2003;7:415–423. doi: 10.1016/s1364-6613(03)00197-9. [DOI] [PubMed] [Google Scholar]
- 2.Postle BR. Working memory as an emergent property of the mind and brain. Neuroscience. 2006;139:23–38. doi: 10.1016/j.neuroscience.2005.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duncan J. An adaptive coding model of neural function in prefrontal cortex. Nat Rev Neurosci. 2001;2:820–829. doi: 10.1038/35097575. [DOI] [PubMed] [Google Scholar]
- 4.McNab F, Klingberg T. Prefrontal cortex and basal ganglia control access to working memory. Nat Neurosci. 2008;11:103–107. doi: 10.1038/nn2024. [DOI] [PubMed] [Google Scholar]
- 5.Vogel EK, McCollough AW, Machizawa MG. Neural measures reveal individual differences in controlling access to working memory. Nature. 2005;438:500–503. doi: 10.1038/nature04171. [DOI] [PubMed] [Google Scholar]
- 6.Chao LL, Knight RT. Contribution of human prefrontal cortex to delay performance. J Cogn Neurosci. 1998;10:167–177. doi: 10.1162/089892998562636. [DOI] [PubMed] [Google Scholar]
- 7.Sakai K, Rowe JB, Passingham RE. Active maintenance in prefrontal area 46 creates distractor-resistant memory. Nat Neurosci. 2002;5:479–484. doi: 10.1038/nn846. [DOI] [PubMed] [Google Scholar]
- 8.Postle BR. Delay-period activity in the prefrontal cortex: One function is sensory gating. J Cogn Neurosci. 2005;17:1679–1690. doi: 10.1162/089892905774589208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clapp WC, Rubens MT, Gazzaley A. Mechanisms of working memory disruption by external interference. Cereb Cortex. 2010;20:859–872. doi: 10.1093/cercor/bhp150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Egner T, Hirsch J. Cognitive control mechanisms resolve conflict through cortical amplification of task-relevant information. Nat Neurosci. 2005;8:1784–1790. doi: 10.1038/nn1594. [DOI] [PubMed] [Google Scholar]
- 11.Rainer G, Asaad WF, Miller EK. Selective representation of relevant information by neurons in the primate prefrontal cortex. Nature. 1998;393:577–579. doi: 10.1038/31235. [DOI] [PubMed] [Google Scholar]
- 12.Miller EK, Erickson CA, Desimone R. Neural mechanisms of visual working memory in prefrontal cortex of the macaque. J Neurosci. 1996;16:5154–5167. doi: 10.1523/JNEUROSCI.16-16-05154.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zanto TP, Rubens MT, Thangavel A, Gazzaley A. Causal role of the prefrontal cortex in top-down modulation of visual processing and working memory. Nat Neurosci. 2011;14:656–661. doi: 10.1038/nn.2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Higo T, Mars RB, Boorman ED, Buch ER, Rushworth MFS. Distributed and causal influence of frontal operculum in task control. Proc Natl Acad Sci USA. 2011;108:4230–4235. doi: 10.1073/pnas.1013361108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bestmann S, Baudewig J, Siebner HR, Rothwell JC, Frahm J. BOLD MRI responses to repetitive TMS over human dorsal premotor cortex. Neuroimage. 2005;28:22–29. doi: 10.1016/j.neuroimage.2005.05.027. [DOI] [PubMed] [Google Scholar]
- 16.Ruff CC, et al. Concurrent TMS-fMRI and psychophysics reveal frontal influences on human retinotopic visual cortex. Curr Biol. 2006;16:1479–1488. doi: 10.1016/j.cub.2006.06.057. [DOI] [PubMed] [Google Scholar]
- 17.Blankenburg F, et al. Interhemispheric effect of parietal TMS on somatosensory response confirmed directly with concurrent TMS-fMRI. J Neurosci. 2008;28:13202–13208. doi: 10.1523/JNEUROSCI.3043-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ruff CC, et al. Distinct causal influences of parietal versus frontal areas on human visual cortex: Evidence from concurrent TMS-fMRI. Cereb Cortex. 2008;18:817–827. doi: 10.1093/cercor/bhm128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Driver J, Blankenburg F, Bestmann S, Vanduffel W, Ruff CC. Concurrent brain-stimulation and neuroimaging for studies of cognition. Trends Cogn Sci. 2009;13:319–327. doi: 10.1016/j.tics.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 20.Blankenburg F, et al. Studying the role of human parietal cortex in visuospatial attention with concurrent TMS-fMRI. Cereb Cortex. 2010;20:2702–2711. doi: 10.1093/cercor/bhq015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bestmann S, et al. Dorsal premotor cortex exerts state-dependent causal influences on activity in contralateral primary motor and dorsal premotor cortex. Cereb Cortex. 2008;18:1281–1291. doi: 10.1093/cercor/bhm159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morishima Y, et al. Task-specific signal transmission from prefrontal cortex in visual selective attention. Nat Neurosci. 2009;12:85–91. doi: 10.1038/nn.2237. [DOI] [PubMed] [Google Scholar]
- 23.Taylor PCJ, Nobre AC, Rushworth MFS. Subsecond changes in top down control exerted by human medial frontal cortex during conflict and action selection: A combined transcranial magnetic stimulation electroencephalography study. J Neurosci. 2007;27:11343–11353. doi: 10.1523/JNEUROSCI.2877-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feredoes E, Tononi G, Postle BR. The neural bases of the short-term storage of verbal information are anatomically variable across individuals. J Neurosci. 2007;27:11003–11008. doi: 10.1523/JNEUROSCI.1573-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Druzgal TJ, D'Esposito M. Dissecting contributions of prefrontal cortex and fusiform face area to face working memory. J Cogn Neurosci. 2003;15:771–784. doi: 10.1162/089892903322370708. [DOI] [PubMed] [Google Scholar]
- 26.Sala JB, Rämä P, Courtney SM. Functional topography of a distributed neural system for spatial and nonspatial information maintenance in working memory. Neuropsychologia. 2003;41:341–356. doi: 10.1016/s0028-3932(02)00166-5. [DOI] [PubMed] [Google Scholar]
- 27.Ranganath C, DeGutis J, D'Esposito M. Category-specific modulation of inferior temporal activity during working memory encoding and maintenance. Brain Res Cogn Brain Res. 2004;20:37–45. doi: 10.1016/j.cogbrainres.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 28.Dolcos F, Miller B, Kragel P, Jha A, McCarthy G. Regional brain differences in the effect of distraction during the delay interval of a working memory task. Brain Res. 2007;1152:171–181. doi: 10.1016/j.brainres.2007.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kanwisher N, McDermott J, Chun MM. The fusiform face area: A module in human extrastriate cortex specialized for face perception. J Neurosci. 1997;17:4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Downing PE, Chan AW, Peelen MV, Dodds CM, Kanwisher NG. Domain specificity in visual cortex. Cereb Cortex. 2006;16:1453–1461. doi: 10.1093/cercor/bhj086. [DOI] [PubMed] [Google Scholar]
- 31.Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annu Rev Neurosci. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- 32.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 33.Everling S, Tinsley CJ, Gaffan D, Duncan J. Filtering of neural signals by focused attention in the monkey prefrontal cortex. Nat Neurosci. 2002;5:671–676. doi: 10.1038/nn874. [DOI] [PubMed] [Google Scholar]
- 34.Hampshire A, Thompson R, Duncan J, Owen AM. The target selective neural response—similarity, ambiguity, and learning effects. PLoS ONE. 2008;3:e2520. doi: 10.1371/journal.pone.0002520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Postle BR, et al. Repetitive transcranial magnetic stimulation dissociates working memory manipulation from retention functions in the prefrontal, but not posterior parietal, cortex. J Cogn Neurosci. 2006;18:1712–1722. doi: 10.1162/jocn.2006.18.10.1712. [DOI] [PubMed] [Google Scholar]
- 36.Postle BR, Berger JS, D'Esposito M. Functional neuroanatomical double dissociation of mnemonic and executive control processes contributing to working memory performance. Proc Natl Acad Sci USA. 1999;96:12959–12964. doi: 10.1073/pnas.96.22.12959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.D'Esposito M, Postle BR. Neural correlates of processes contributing to working memory function: Evidence from neuropsychological and pharmacological studies. In: Monsell S, Driver J, editors. Control of Cognitive Processes: Attention and Performance XVIII. Cambridge, MA: MIT Press; 2000. pp. 579–602. [Google Scholar]
- 38.Malmo RB. Interference factors in delayed response in monkey after removal of the frontal lobes. J Neurophysiol. 1942;5:295–308. [Google Scholar]
- 39.Hillyard SA, Vogel EK, Luck SJ. Sensory gain control (amplification) as a mechanism of selective attention: Electrophysiological and neuroimaging evidence. Philos Trans R Soc Lond B Biol Sci. 1998;353:1257–1270. doi: 10.1098/rstb.1998.0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hampshire A, Duncan J, Owen AM. Selective tuning of the blood oxygenation level-dependent response during simple target detection dissociates human frontoparietal subregions. J Neurosci. 2007;27:6219–6223. doi: 10.1523/JNEUROSCI.0851-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Owen AM. The functional organization of working memory processes within human lateral frontal cortex: The contribution of functional neuroimaging. Eur J Neurosci. 1997;9:1329–1339. doi: 10.1111/j.1460-9568.1997.tb01487.x. [DOI] [PubMed] [Google Scholar]
- 42.Weiskopf N, et al. Image artifacts in concurrent transcranial magnetic stimulation (TMS) and fMRI caused by leakage currents: Modeling and compensation. J Magn Reson Imaging. 2009;29:1211–1217. doi: 10.1002/jmri.21749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sack AT, et al. Optimizing functional accuracy of TMS in cognitive studies: A comparison of methods. J Cogn Neurosci. 2009;21:207–221. doi: 10.1162/jocn.2009.21126. [DOI] [PubMed] [Google Scholar]