Abstract
Dendritic cells (DC) are highly specialized antigen-presenting cells characterized by the ability to prime T-cell responses. Mesenchymal stem cells (MSC) are adult stromal progenitor cells displaying immunomodulatory activities including inhibition of DC maturation in vitro. However, the specific impact of MSC on DC functions, upon in vivo administration, has never been elucidated. Here we show that murine MSC impair Toll-like receptor-4 induced activation of DC resulting in the inhibition of cytokines secretion, down-regulation of molecules involved in the migration to the lymph nodes, antigen presentation to CD4+ T cells, and cross-presentation to CD8+ T cells. These effects are associated with the inhibition of phosphorylation of intracellular mitogen-activated protein kinases. Intravenous administration of MSC decreased the number of CCR7 and CD49dβ1 expressing CFSE-labeled DC in the draining lymph nodes and hindered local antigen priming of DO11.10 ovalbumin-specific CD4+ T cells. Upon labeling of DC with technetium-99m hexamethylpropylene amine oxime to follow their in vivo biodistribution, we demonstrated that intravenous injection of MSC blocks, almost instantaneously, the migration of subcutaneously administered ovalbumin-pulsed DC to the draining lymph nodes. These findings indicate that MSC significantly affect DC ability to prime T cells in vivo because of their inability to home to the draining lymph nodes and further confirm MSC potentiality as therapy for immune-mediated diseases.
Keywords: immunomodulation, tolerance
Stromal progenitors of mesodermal cells, referred to as mesenchymal stem cells (MSC) or multipotent mesenchymal stromal cells, are a heterogeneous population of self-renewing and multipotent cells isolated from the bone marrow (BM) (1). MSC raised hopes for their clinical exploitation for tissue-repair strategies and increasing experimental evidence supports their use also for immune-mediated diseases (2). In fact, MSC display a striking capacity of modulating the immune response (3). Despite a large body of experimental studies addressing the in vitro effects of MSC on immune cells, little is known about the mechanisms of MSC-mediated inhibition of the in vivo immune response. Dendritic cells (DC) are unique antigen-presenting cells (APCs) endowed with the ability of acquiring and processing antigens, up-regulating costimulatory molecules and therefore priming naive T cells. To present antigens to naive T cells, CCR7-expressing DC must migrate through lymphatic vessels from sites of inflammation to the closest draining lymph node (4). Activation of DC via Toll-like receptors (TLRs) up-regulates the expression of chemokine receptors involved in DC migration to the lymph nodes and enhances their in vivo mobilization properties (5). As a consequence, the total number of DC migrating in the draining lymph nodes deeply affects naive T-cell priming (6). Here we show that murine MSC inhibit in vitro DC effector properties, including antigen processing and presentation to T cells through the inhibition of the activation of mitogen-activated protein kinases (MAPKs) occurring upon TLR4 stimulation. Most important, we report that in vitro exposure of DC to MSC as well as in vivo intravenous administration of MSC results in a significant down-regulation of CCR7 and CD49dβ1, two molecules involved in DC homing to lymphoid organs. This event leads to inhibition of migration to the draining lymph nodes and subsequent impairment of priming of antigen-specific naive T cells.
Results
MSC Prevent Maturation of BM-Derived DC.
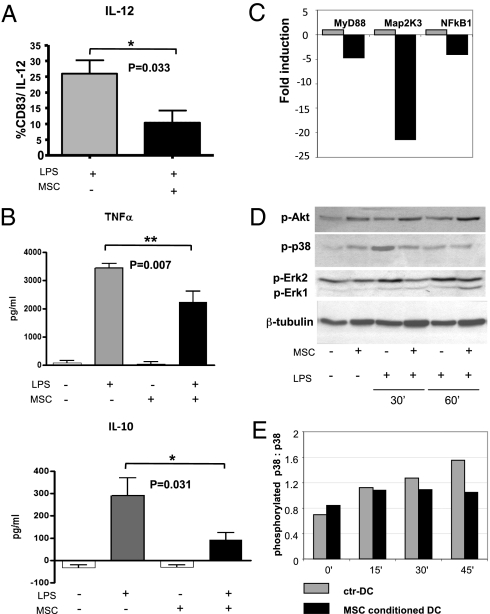
To evaluate the effect of MSC on TLR4-mediated activation of DC, we cocultured DC with MSC at a 1:3 ratio during LPS-induced maturation (MSC-conditioned DC). MSC-conditioned DC showed decreased levels of the pan-DC and activation surface markers CD11c, CD86, CD80, CD40, MHC class I, and MHC class II compared with DC activated without MSC (ctr-DC), suggesting a significant impairment of DC maturation (Fig. S1). Moreover, MSC impaired the ability of LPS to induce the up-regulation of both CCR7 and CD49dβ1, two molecules crucial for DC migration to lymph nodes (Fig. S1). We also observed a consistent impairment of IL-12 intracellular production by CD83+ MSC-conditioned DC compared with ctr-DC (Fig. 1A). Moreover, MSC exposure reduced levels of TNF-α and IL-10 in the supernatants of LPS-activated DC compared with controls (Fig. 1B). The significant inhibition of cytokine production by DC activated by LPS in the presence of MSC was confirmed by real-time PCR (Fig. S2). We also observed a significant decrease, in MSC-conditioned DC, of the expression of indoleamine 2,3-dioxygenase (IDO) (Fig. S2).
Fig. 1.
MSC inhibit LPS-induced cytokine production and modulate gene expression and phosphorylation of kinases involved in the TLR4 pathway. (A) Levels of intracellular IL-12 produced by CD83+ control DC (gray bars) and DC exposed to MSC during activation with 10 μg/mL of LPS overnight (black bars; P = 0.033). Results are representative of five independent experiments (mean ± SD). (B) TNF-α and IL-10 levels by ctr-DC (gray bars) and DC exposed to MSC during LPS activation (black bars), were quantified by ELISA (**P = 0.007 and *P = 0.03, respectively). Data are expressed as picogram per milliliter. Results are representative of four and seven independent experiments, respectively (mean ± SD). (C) After TLR4 stimulation, DC exposed to MSC (black bars) significantly down-modulated MyD88, MAP2k3, and NFkB1 compared with control DC (gray bars), as demonstrated using the RT2 Profiler PCR Array mouse Toll-Like Pathway. One of three independent experiments is shown. Gene expression is represented as relative mRNA amount (fold-induction) compared with the control sample. (D) Western blots of intracellular Akt, ERK1/2, p38 proteins from ctr-DC and MSC-conditioned DC, stimulated and not stimulated with LPS for 30 and 60 min, are shown. Anti–β-tubulin was used as control. One of three independent experiments are shown. (E) Histograms show the ratio between the activated phosphorylated p38 MAPK (Thr180/Tyr182) and total unphosphorylated p38 MAPK in ctr-DC (gray bars) and in MSC-conditioned DC (black bars) after 0, 15, 30, and 45 min of LPS stimulation. Data shown are representative of one of three independent experiments.
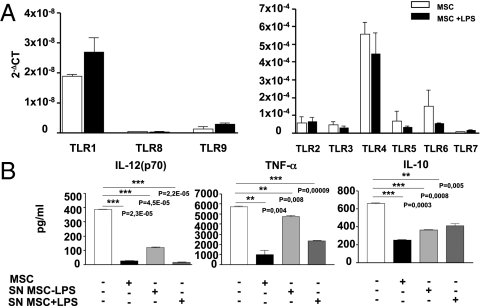
Because other groups have demonstrated that mouse MSC express the full array of TLRs (7, 8), we sought to analyze by quantitative real-time PCR the expression of TLRs from 1 to 9 on the MSC used in our experimental conditions. Similar to what has been previously reported, MSC express all TLRs at comparable levels, except for TLR8 and -9, which display much lower expression (Fig. 2A, Left). Interestingly, the exposure of MSC to LPS for 12 h did not significantly affect MSC TLR expression (Fig. 2A). Next, we addressed the possibility that LPS may affect the behavior of TLR4-bearing MSC during the coculture with DC. We repeated the experiments exposing DC to the supernatants from MSC cultured with or without LPS, thus avoiding direct contact of the two cell types. We observed that supernatants of LPS-stimulated MSC were able to inhibit IL-12p70, TNF-α, and IL-10 secretion by DC (Fig. 2B), similar to what we observed in DC cultured in the presence of MSC, thus suggesting that the inhibition of the release of cytokines by DC is not because of competition for LPS by the two cell types. These findings strongly support the role of soluble factors as mediators of such inhibition. Of note, supernatants from MSC not stimulated in vitro with LPS exhibited a lower ability to inhibit cytokines release by activated DC (Fig. 2B), confirming that inflammatory cues license MSC immunomodulatory activity (9, 10). Taken together, these findings demonstrate that MSC impair LPS-induced activation of DC through the release of soluble factors.
Fig. 2.
Gene expression of TLRs in MSC and inhibition of cytokines production by DC with MSC supernatants. (A) TLRs expression was assessed by quantitative RT-PCR on unstimulated (white bar) and LPS-stimulated MSC (10 μg/mL) (black bar). Mean ± SD from three independent experiments with unstimulated and LPS-stimulated MSC is shown. To magnify the differences among the expression of different TLRs, particularly in the case of TLR1, TLR8, and TLR9, two different scales have been depicted in the left and right graphs. (B) Secreted levels of IL12p70, IL-10, and TNF-α by ctr-DC (white bars), DC exposed to MSC (black bars), DC exposed to supernatants of MSC (light gray bar), and DC exposed to supernatants of LPS-stimulated MSC (dark gray bar), were quantified by ELISA (IL-12p70: ***P = 2.30931E-05, ***P = 4.53805E-05, ***P = 2.18932E-05, respectively; TNF-α: **P = 0.004, **P = 0.008, ***P = 9,42621E-05, respectively; IL-10: ***P = 0.0003, ***P = 0.0005, **P = 0.005, respectively). Data are expressed as picogram per milliliter. Results are the mean of three independent experiments (mean ± SD).
MSC Down-Modulate Key Molecules Involved in DC TLR4 Signaling.
To further investigate the effect of MSC on DC activation by LPS, we analyzed gene-expression profiles of DC activated by LPS in the presence or absence of MSC through the use of a mouse Toll-Like Receptor Signaling Pathway PCR Array (see the Gene Table list at SABiosciences, available at http://sabiosciences.com). Among the genes analyzed, 5 of 84 genes were significantly modified in MSC-conditioned DC (Table S1). Remarkably, myeloid differentiation primary response gene 88 (MyD88), MAP2K3, and NF-κB1 genes were significantly down-modulated (>4-fold) in MSC-conditioned DC compared with ctr-DC (Fig. 1C). We next studied their effect on two members of the MAPK superfamily, the extracellular signal-regulated kinases (ERK1/2) and the p38 MAPKinase (p38). As shown in Fig. 1D, we observed a time-dependent decrease in phosphorylated ERK1/2 and p38 being higher 30 min after LPS activation. Accordingly, the ratio between phosporylated p38 and unphosphorylated p38 in control DC increases over time. In contrast, in MSC-conditioned DC this ratio did not change upon prolonged exposure to LPS as the result of the impairment of p38 phosphorylation (Fig. 1E). We addressed the effect of MSC on Akt, a serine/threonine protein kinase involved in the inhibition of apoptosis. MSC increased phophorylation of Akt compared with ctr-DC mainly at 60 min (Fig. 1D). These findings confirm that MSC inhibit the MAPK cascade involved in the pathway leading to the phosphorylation of cytoplasmic components and nuclear transcription factors that promote cytokines production by mouse DC following TLR4 stimulation. On the other side, they can promote cell survival acting on the Akt pathway.
MSC Impair Class I-Associated Antigen Processing in DC and Cross-Presentation to CD8+ T Cells.
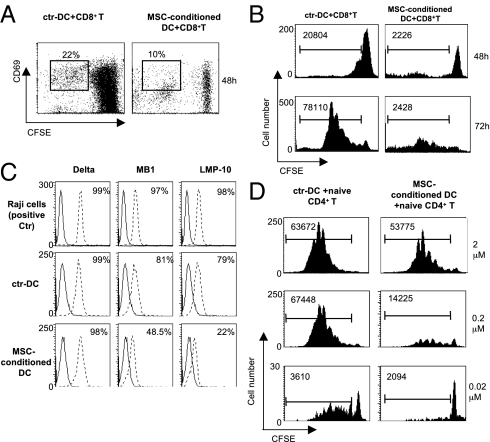
As MSC induced down-regulation of class I molecules, we investigated their effects on class I restricted cross-presentation of ovalbumin (OVA) to CD8+ T cells by DC. After LPS exposure, ctr-DC and MSC-conditioned DC were pulsed with OVA and cultured with 5,6-carboxyfluorescein diacetate succinimidyl ester (CFSE)-labeled OT-I CD8+ T cells (ratio 1:5). MSC-conditioned DC failed to induce the expression of the early activation marker CD69 on CD8+ T cells compared with control DC (Fig. 3A). Furthermore, T-cell proliferation was strongly inhibited by OVA-pulsed MSC-conditioned DC compared with OVA-pulsed ctr-DC as depicted by cell division profile on CFSE labeled cells analyzed at 48 and 72 h (Fig. 3B). We also measured cell death in the CD8+ subset by propidium iodide staining and we did not observe any significant increase of dead cells. Thus, MSC impair antigen cross-presentation by DC.
Fig. 3.
MSC interfere with antigen presentation to CD8+ and CD4+ T cells. (A) Percentage, at 48 h, of CD69+ CFSE-gated CD8+ T cells primed by ctr-DC (Left) or MSC-conditioned DC (Right) (DC:CD8 = 1:5). (B) Proliferating CD8+ T cells primed by ctr-DC (Left) or MSC-conditioned DC (Right), as depicted by CFSE dilution at 48 (Upper) and at 72 h (Lower); numbers indicate the absolute values of cells recovered in standardized acquisitions. One of three independent experiments is shown. (C) The expression of three APM-associated molecules, Delta, MB1, and LMP-10 on Raji cells (Top), control DC (Middle), and MSC-conditioned DC (Lower) is depicted. One three independent experiments is shown. (D) Inhibition of naive CD4+ OVA-specific transgenic DO11.10 T cells cultured with ctr-DC (Left) or MSC-conditioned DC (Right) pulsed with increasing concentrations of pOVA323–339 (0.02 μM; 0.2 μM; 2 μM) (DC:CD4 = 1:8). Absolute numbers indicate the absolute values of cells recovered in standardized acquisitions (shown at the top of the histograms) as described in Materials and Methods. One of three independent experiments is shown.
The processing and presentation of HLA class I antigen-derived peptides is accomplished through a complex series of intracellular events involving constituents of the constitutive proteasome, as well of the IFN-γ–inducible proteasome (immunoproteasome). Thus, we asked whether the inhibition of DC cross-presentation by MSC to CD8+ T cells associated with an impairment of some of the constituents of the intracellular antigen processing machinery (APM). As shown in Fig. 3C, DC activated in the presence of MSC showed a striking down-regulation of MB-1 and LMP-10 but not of Delta. These results suggest that the impaired capacity of MSC-treated DC to prime CD8+ antigen-specific T cells leads to a deranged antigen processing involving both proteasome- and immunoproteasome-associated molecules in the cytosol compartment.
MSC Inhibit DC Ability to Prime CD4+ T Cells.
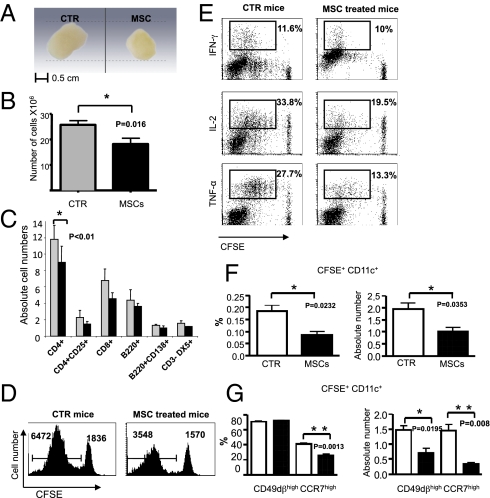
To evaluate the influence of MSC on MHC class II restricted antigen presentation, we used naive DO11.10 CD4+ T cells carrying a transgenic T-cell receptor (TCR) specific for the OVA peptide323-339. LPS-treated ctr-DC and MSC-conditioned DC, pulsed with increasing doses of OVA peptide, were cocultured with CFSE-labeled DO11.10 CD4+ T cells. We observed that DC activated in the presence of MSC showed an impaired capacity to prime CD4+ pOVA323–339-specific T cells in a dose-dependent manner (Fig. 3D). Therefore, we established a mouse model based on the adoptive transfer of purified CFSE-labeled, OVA-specific naive T cells from DO11.10 transgenic mice (H-2d) into syngeneic BALB/c recipient mice (H-2d) followed, 24 h later, by subcutaneous immunization with LPS-activated, OVA-pulsed DC. Three hours later, one group of animals was injected intravenously with PBS and another group with 1.25 × 106 MSC. On day 3, draining lymph nodes of adoptively transferred mice from the two groups were isolated and the size, cell number, phenotype, CFSE dilution, and cytokine secretion of injected transgenic CD4+ T cells were analyzed. Upon macroscopic inspection, we observed a remarkable decrease in size of draining lymph node from mice injected with MSC compared with the draining lymph node of control mice, where a more prominent swelling was observed (Fig. 4A). Notably, we observed a significant difference in size between the draining and the controlateral lymph nodes (not draining) in the control group, which was not observed in the MSC-injected mice. Such difference in size was not exclusively a result of the inhibition of local swelling, but mainly because of a significant decrease of the draining lymph node cells number in the MSC-injected mice compared with controls (cellular number: ctr-DC mice: 25 ± 3.49 vs. MSC-treated mice: 16 ± 3.29, *P = 0.016) (Fig. 4B). Analysis of the lymphocyte cellular component showed a significant decrease of absolute numbers of CD4+ T cells (*P < 0.05) (Fig. 4C). CD8+ T cells, CD4+CD25bright T cells, B220+ B cells, B220−CD138+ plasma cells, and CD3−DX5+ NK cells were decreased compared with control mice, albeit not reaching a statistical significance (Fig. 4C). When we compared, by CFSE dilution, priming of OVA-specific CD4+ DO11.10 cells from draining lymph nodes, we observed an impairment of proliferation in the treated mice compared with controls (Fig. 4D). OVA-specific T cells from MSC-treated mice displayed a remarkably decreased IL-2 and TNF-α production with respect to control mice, but IFN-γ production was unaltered (Fig. 4E). Taken together, these results indicate that MSC intravenous administration leads to an impairment of priming of antigen-specific naive T cells in the draining lymph nodes.
Fig. 4.
MSC impair in vivo CD4+ T-cell priming by DC. (A) The draining lymph nodes from a control (Left) and a MSC-treated mouse (Right) are shown. (Scale bar, 0.5 cm.) One of three representative experiments is depicted. (B) Histograms show the difference in the absolute cell number (×106) of the draining lymph node from control mice (gray bars) and MSC-treated mice (black bars); *P = 0.016. Results are representative of three independent experiments (mean ± SD). (C) The histograms show the percentage of positive cells (CD4+, CD4+/CD25+, CD8+, B220+, B220+/CD138+, CD3−/DX5+) expressed as absolute cell number for each cell subset in the draining lymph node of control mice (gray bars) and MSC-treated mice (black bars), *P < 0.05 for CD4+ T cells. Results are representative of three independent experiments (mean ± SD). (D) CFSE profiles of KJ1-26-gated transgenic DO11.10 CD4+ T cells demonstrate the absolute numbers of proliferating cells in the draining lymph node of control mice (Left) and MSC-treated mice (Right). Absolute numbers indicate the absolute values of cells recovered in standardized acquisitions (shown at the top of the histograms). One of three representative experiments is depicted. (E) Intracellular staining for IFN-γ, IL-2, and TNF-α on proliferating CD4+DO11.10 T cells from the draining lymph node of control mice (Left) and MSC-treated mice (Right) are depicted. The percentage of transgenic DO11.10 CD4+ T cells producing cytokines is shown at the top of plots. One of three representative experiments is displayed. (F) The percentage (Left) and the absolute number (Right) of CFSE+CD11c+ DC recovered from draining lymph nodes are shown. *P = 0.0232 (Left); *P = 0.0353 (Right) (G) The percentage (Left) and the absolute number (Right) of CFSE+CD11c+ DC expressing CCR7 and CD49dβ1 recovered from draining lymph nodes are depicted. Control mice (white bars) and MSC-treated mice (black bars). **P = 0.0013 (Left); *P = 0.0195, **P = 0.008 (Right).
MSC Inhibit DC Migration to Lymph Nodes.
To study the effects of MSC on the migratory properties of DC, we labeled LPS-activated OVA-pulsed DC with CFSE and injected them subcutaneously in naive animals that previously received transgenic DO11.10 naive T cells, as previously described. Following intravenous administration of MSC, we observed a significant decrease of the absolute number of CD11chigh CFSE+ DC in the lymph nodes of MSC-treated animals compared with PBS-injected controls, suggesting a reduced recruitment of activated DC to the draining lymph nodes (Fig. 4F). More importantly, the percentage, as well as the absolute number of CCR7high CFSE+ CD11c+ DC, was significantly decreased in the lymph nodes of MSC-injected animals compared with PBS-treated controls. Similarly, the absolute number of CD49dβ1high DC was significantly lower in MSC-treated animals compared with controls, but the percentage was unaltered (Fig. 4G and Fig. S3). Overall, these results provide strong experimental evidence that MSC are able to in vivo affect the expression of surface molecules implicated in DC migration to the lymph nodes.
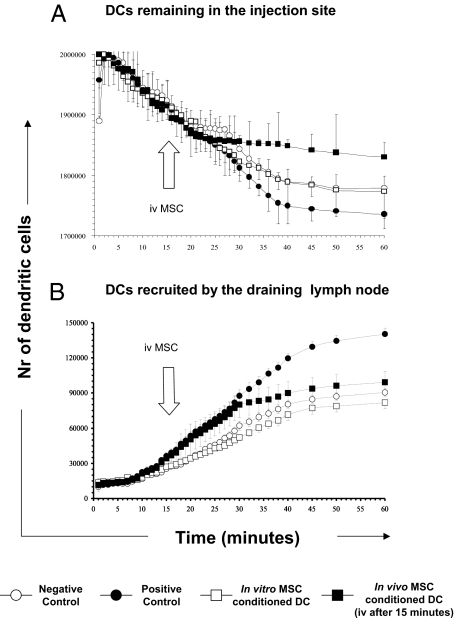
To further confirm these data, we labeled DC with technetium-99m hexamethylpropylene amine oxime (99mTc-HMPAO) and followed their in vivo bio-distribution and trafficking by scintigraphic imaging of their migratory pattern in the 60 min following their administration. In the negative control group, naive mice were subcutaneously immunized with 2 × 106 LPS-activated, OVA-pulsed DC. In the positive control group, intravenous injection of purified pOVA323–339-specific naive DO11.10 T cells was followed, 24 h later, by subcutaneous immunization with LPS-activated, OVA-pulsed DC. We immunized BALB/c mice, previously injected with CD4+ DO11.10 transgenic T cells, either with LPS-activated, OVA-pulsed DC in vitro exposed to MSC (in vitro MSC-conditioned DC group) or with LPS-activated, OVA-pulsed DC followed by the injection, 15 min later, with 1.25 × 106 MSC (in vivo MSC-conditioned DC group). As depicted in Fig. 5A, the number of DC released by the site of injection in mice pretreated with OVA-specific transgenic T cells (positive control) was significantly higher than in the negative-control mice lacking Ag-specific T cells (**P < 0.004). Strikingly, in mice immunized with in vitro MSC-conditioned DC, we observed a significantly decreased migration of DC from the site of injection (**P < 0.004 compared with the positive-control group, respectively). Intravenous MSC administration virtually blocked the escape of OVA-pulsed DC (in vivo MSC-conditioned DC group) from the injection site demonstrated by the sudden change of the slope of the curve within few minutes from MSC injection (Fig. 5A, closed square line). The relevance and the rapidity of this effect were confirmed by the fact that the number of DC still stuck in the injection site was significantly higher in these animals with respect to the positive-control group (**P < 0.001).
Fig. 5.
MSC impair DC migratory features. Graphs represent the number of DC released by the site of injection (A) and recruited to the draining lymph nodes (B) as measured by the detection of radioactivity released by 99mTc-HMPAO–labeled DC. Labeled DC was followed for 60 min after injection by scintigraphic imaging. Four different experimental conditions were studied: (i) negative control group represents mice exclusively subcutaneously immunized with OVA-pulsed DC (○, 6 mice); (ii) positive control group (•, 7 mice) indicates mice intravenously injected with DO11.10 T cells and subsequently immunized with OVA-pulsed DC; (iii) in vitro MSC-conditioned DC group (□, 6 mice) represents mice intravenously injected with DO11.10 T cells and, subsequently immunized with OVA-pulsed in vitro MSC conditioned-DC; (iv) in vivo MSC–conditioned DC group (■, 4 mice) designate mice intravenously injected with DO11.10 T cells, subsequently immunized with OVA-pulsed DC and intravenously injected with MSC 15 min later (arrow).
The analysis of the draining lymph node uptake showed complementary data to these results. Positive-control mice, undergoing active immunization with OVA-pulsed DC after intravenous injection of DO11.10 transgenic T cells, showed the largest and fastest recruitment of DC in the draining lymph nodes (Fig. 5B and Fig. S4) (*P < 0.03 with respect to negative control). In vitro MSC-conditioned mice groups displayed a significant reduction in the uptake of labeled OVA-pulsed DC similar to that one of negative controls (*P < 0.01 with respect to positive control). Remarkably, we noticed a sudden change in the slope of the curve of in vivo MSC-conditioned DC group (closed square line) occurring immediately after MSC intravenous injection, suggesting that in vivo MSC administration can completely abrogate migration of DC to the draining lymph nodes (Fig. 5B). These results indicate that MSC alter significantly the migration of antigen-bearing DC in the draining lymph nodes where T-cell priming occurs. Of relevance, injection of MSC affects DC migration patterns within few minutes, suggesting that in vivo MSC-mediated inhibition of DC–T-cell interactions is an extremely rapid event.
Discussion
In this study, we are unique in providing evidence that the in vivo immunomodulatory activity of MSC is recapitulated by their ability to directly inhibit DC effector functions. Several reports have demonstrated that MSC have a profound impact on immune functions both in humans and mice (3). For example, MSC have been shown to inhibit T-cell proliferation through the induction of cell division arrest (11), as well as to inhibit the differentiation, maturation, and functions of monocyte-derived DC (12–18). However, most studies reporting on the immunomodulatory features of MSC have been carried out in vitro. Despite some reports addressing the effect of MSC in experimental models of immune-mediated diseases, little information about the in vivo mechanisms has been provided. In particular MSC in vivo administration resulted mainly in the inhibition of pathogenic antigen-specific T cells, as in a mouse model of allograft rejection (19), experimental autoimmune encephalomyelitis (20), graft-versus-host disease (21), and collagen-induced arthritis (22). Despite these findings, it is not known whether T-cell tolerance induced in vivo by MSC is the consequence of the direct interaction between MSC and T cells or the result of an impaired T-cell priming by APC. Here we confirm that MSC halt maturation of BM-derived DC, leading to an inefficient antigen presentation. MSC affect LPS-induced activation, leading to a decreased production of cytokines, such as TNF-α and IL-12, and this effect is caused by soluble factors, as demonstrated by experiments with MSC supernatants. Interestingly we confirmed that MSC expressed all sets of TLRs, including TLR4, the triggering of which by LPS enhances the inhibitory activity of MSC supernatants. In contrast to reports by others (16), we could not detect an increased production of IL-10, suggesting that the DC-mediated tolerogenic effect is mainly because of inefficient T-cell priming. We also observed that MSC inhibit LPS-activated DC production of IDO, a key enzyme involved in NF-κB activation, leading to DC maturation (23).
To evaluate at the molecular level the effect of MSC on TLR-induced DC activation, we investigated the effect of MSC on MAPKs involved in the phosphorylation of cytoplasmic components and nuclear transcription factors promoting cytokine production. We demonstrated that MSC inhibited phosphorylation of MAPKs downstream of MyD88, whose activation following TLR4 stimulation leads to the NF-κB–mediated production of IL-12, a key cytokine driving the Th1 response (24). We also observed an enhanced activation of Akt on DC exposed to MSC. These findings indicate that activation of Akt and impairment of NF-κB are responsible for the impaired activation/maturation state of LPS-activated MSC-exposed DC, a molecular mechanism recently associated with the immunogenicity subversion of Leishmania infantum-infected DC (25).
We have also shown that MSC affect DC ability to process soluble OVA and subsequently present them in the context of MHC class I molecules to OVA-specific CD8+ T cells, a process know as “cross-priming” (26). These observations have focused our attention on modulation of the APM, the defects in expression and function of which may lead to abnormalities in the formation of complexes transported to the cell surface. The processing and presentation of HLA class I antigen-derived peptides is accomplished through a complex series of intracellular events involving multiple molecular species. These species include the constitutive proteasome subunits δ, MB1, and ζ, as well as the immunoproteasome β-type subunits LMP2, LMP7, and LMP10. Down-regulation of key components of the constitutive proteasome, as well as of the immunoproteasome, has been reported by several studies following exposure to different stimuli. For example, it has been demonstrated that tumor cells can inhibit the expression of several APM components, including MB1 and LMP10, and that such diminished expression occurs also in immature DC (27). A similar profile of change in catalytic subunit profiles has been recently reported to occur in plasma cells from patients with myeloma (28). The results reported in our study are in lines with these observations concerning a paired down-regulation of MB1 a-catalytic β-subunits located in the inner rings of the constitutive proteasome (29) and LMP10, an IFN-γ–inducible proteasomal subunit (30), and are consistent with the proposed effect of MSC on DC maturation, resulting in an accumulation of immature DC and an impaired capability to present MHC class I-associated antigens to CD8 T cells.
The most striking result of this study arises from the demonstration that MSC impair in vivo naive T-cell priming by DC. Naive T cells are primed in the lymph nodes by antigen-presenting DC, the number and activation state of which affects the efficiency and quality of the developing T-cell response (6). We first demonstrated that activated CD11chigh CFSE+ DC are significantly reduced in the draining lymph nodes of MSC-treated animals compared with controls, and that this effect is the result of an in vivo decrease of CCR7 and CD49dβ1 expression on recruited DC. These results are unique in suggesting that MSC are able to affect in vivo the expression of surface molecules implicated in DC migration to the lymph nodes. To further confirm these findings, we used an experimental model where subcutaneous injection of OVA-pulsed DC and subsequent migration to the draining lymph nodes results in the activation of OVA-specific DO.11.10 transgenic naive CD4+ T cells (31).
We showed that the intravenous injection of MSC in BALB/c mice previously immunized with LPS-activated DC pulsed with OVA results in a decrease in cell number of the draining lymph nodes compared with that of the control mice, suggestive of an antiproliferative effect on immune cells, probably caused by inhibition of cell division (11). Of relevance, MSC administration led in vivo to an impaired priming of OVA-specific transgenic CD4+ naive T cells. In line with these findings, we demonstrated that impairment of T-cell priming is because of the inability of in vitro DC-conditioned MSC to efficiently leave the site of injection and reach the draining lymph nodes. Even more important, we observed that MSC intravenous injection induces an almost immediate arrest of the otherwise rapid migration of LPS-activated OVA-pulsed DC toward the draining lymph nodes. This inhibition of migration is likely to be the result of an impaired up-regulation of molecules involved in DC trafficking, such as CCR7 and CD49dβ1, as suggested by a significant decrease of the expression of these molecules on CFSE-labeled activated DC detected in the draining lymph nodes. If such inhibition occurring in vivo is because of a direct interaction between DC and MSC at the site of DC injection, or is mediated by the release of soluble factors, has yet to be determined. The data provided by experiments performed with MSC supernatants strongly suggests that soluble factors play a major role, at least in vitro. Moreover, the large sequestration of MSC in the lungs following intravenous administration (32) and the minimal time required—less than 20 min—for transplanted MSC to arrest DC migration to the draining lymph nodes, makes very unlikely that intravenously injected MSC could reach the subcutaneous tissue where DC were implanted. Indeed the potential role of soluble factors released in vivo by MSC at a distance from the target organ has been recently suggested by the demonstration that the interaction of intravenously injected MSC with the lung microenvironment leads to the release of the anti-inflammatory molecule TSG-6, resulting in the improvement of the myocardial function after global ischemia (33). It has been also recently demonstrated that MSC-derived metalloproteases could paracrinally cleave MSC-derived CCL2, leading to the generation of an antagonistic form of CCL2 with the ability of inhibiting encephalitogenic T cells (34). Moreover MSC-derived soluble factors have been shown to significantly modify the migration pattern of immune cells, resulting in the reversal of fulminant hepatic failure (35). Thus, injected MSC appear to quickly modify the recipient immune response upon interaction with the host tissues (36). Taken together, these observations suggest that both soluble factors, as well as cell-to-cell contact mechanism (11), could be involved in the modulation of DC effectors functions by MSC.
The results reported in this study are unique in providing a detailed analysis of MSC effects on DC functions in vitro as well as in vivo, and link this impairment to the inability of priming T cells and mounting an efficient antigen-specific immune response in the secondary lymphoid organs. Although the possible exploitation of MSC for the treatment of immune-mediated diseases is currently under scrutiny (37), these results confirm that MSC have a profound and immediate effect on immune cells in vivo.
Materials and Methods
A summary of experimental techniques is given here, with full methods and associated references presented in SI Materials and Methods.
BM-Derived MSC and DC.
MSC were isolated as previously described (20) and BM derived DC were generated as described elsewhere (38, 39).
In Vitro CD4 and CD8 Cell Cultures.
After LPS activation, mature ctr-DC and MSC-conditioned DC were pulsed with pOVA323-339 or with OVA antigen and cultured with pure naive CD4+ and CD8+ T cells labeled with 2.5 mM of 5-(and 6)-carboxyfluorescein diacetate, succinimidyl ester (CFSE, Molecular Probes). For detailed methods, see SI Materials and Methods.
DC Transplantation.
CFSE–labeled naive OVA-specific T cells (3 × 106) were injected into syngeneic Balb/c mice (iv). LPS-activated DC were pulsed with 2 μM of pOVA323-339 peptide and subcutaneously (sc) injected (2 × 106 per mouse) into the footpad 24 h after the adoptive transfer of DO11.10 cells. Three h later, Balb/c mice were iv injected with MSC (1.25 × 106/mouse). On day 3, priming of CFSE-labeled KJ1-26 positive DO11.10-specific T cells was evaluated. Lymph node T cells were stimulated with PMA and Ionomycin (Sigma-Aldrich) for 4 h and Brefeldin A for the last 2 h. Anti-TNF-α, IL-2, and IFN-γ (Pharmingen BD) were used for intracellular staining.
DC Migration Study.
For the in vivo analysis of CCR7+ CD49dβ1+ DC in the draining lymph nodes, we sc injected 2 × 106 CFSE-labeled DC in naive animals followed by the iv administration of 1.25 × 106 MSC or PBS (controls). On day 3 post DC injection, we isolated the draining lymph nodes from the two animal groups (three animals per group) and analyzed CCR7 and CD49dβ1 expression on CD11c high CFSE+ DC. DC radioactive labeling was performed with 99mTc-exametazime (HMPAO, Ceretec, GE Healthcare) according to a procedure described in SI Materials and Methods and elsewhere (40, 41).
Supplementary Material
Acknowledgments
We thank Prof. S. Ferrone for kindly supplying antibodies against the antigen processing machinery and Fabio Grassi for kindly providing us with DO.11.10 and OT.1 transgenic mice. This research was supported by grants from the Fondazione Italiana Sclerosi Multipla (to A.U. and E.T.), the Italian Ministry of Health (Ricerca Finalizzata) (to A.U.), the Italian Ministry of the University and Scientific Research (to A.U.), the “Progetto LIMONTE” (to A.U.), and the Fondazione CARIGE (to A.U.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1103650108/-/DCSupplemental.
References
- 1.Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276(5309):71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 2.Uccelli A, Pistoia V, Moretta L. Mesenchymal stem cells: A new strategy for immunosuppression? Trends Immunol. 2007;28:219–226. doi: 10.1016/j.it.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 3.Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8:726–736. doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- 4.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 5.De Smedt T, et al. Regulation of dendritic cell numbers and maturation by lipopolysaccharide in vivo. J Exp Med. 1996;184:1413–1424. doi: 10.1084/jem.184.4.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.MartIn-Fontecha A, et al. Regulation of dendritic cell migration to the draining lymph node: Impact on T lymphocyte traffic and priming. J Exp Med. 2003;198:615–621. doi: 10.1084/jem.20030448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pevsner-Fischer M, et al. Toll-like receptors and their ligands control mesenchymal stem cell functions. Blood. 2007;109:1422–1432. doi: 10.1182/blood-2006-06-028704. [DOI] [PubMed] [Google Scholar]
- 8.Tomchuck SL, et al. Toll-like receptors on human mesenchymal stem cells drive their migration and immunomodulating responses. Stem Cells. 2008;26(1):99–107. doi: 10.1634/stemcells.2007-0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krampera M, et al. Role for interferon-gamma in the immunomodulatory activity of human bone marrow mesenchymal stem cells. Stem Cells. 2006;24:386–398. doi: 10.1634/stemcells.2005-0008. [DOI] [PubMed] [Google Scholar]
- 10.Ren G, et al. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. 2008;2(1):141–150. doi: 10.1016/j.stem.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 11.Glennie S, Soeiro I, Dyson PJ, Lam EW, Dazzi F. Bone marrow mesenchymal stem cells induce division arrest anergy of activated T cells. Blood. 2005;105:2821–2827. doi: 10.1182/blood-2004-09-3696. [DOI] [PubMed] [Google Scholar]
- 12.Jiang XX, et al. Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood. 2005;105:4120–4126. doi: 10.1182/blood-2004-02-0586. [DOI] [PubMed] [Google Scholar]
- 13.Beyth S, et al. Human mesenchymal stem cells alter antigen-presenting cell maturation and induce T-cell unresponsiveness. Blood. 2005;105:2214–2219. doi: 10.1182/blood-2004-07-2921. [DOI] [PubMed] [Google Scholar]
- 14.Nauta AJ, Kruisselbrink AB, Lurvink E, Willemze R, Fibbe WE. Mesenchymal stem cells inhibit generation and function of both CD34+-derived and monocyte-derived dendritic cells. J Immunol. 2006;177:2080–2087. doi: 10.4049/jimmunol.177.4.2080. [DOI] [PubMed] [Google Scholar]
- 15.Aldinucci A, et al. Inhibition of immune synapse by altered dendritic cell actin distribution: A new pathway of mesenchymal stem cell immune regulation. J Immunol. 2010;185:5102–5110. doi: 10.4049/jimmunol.1001332. [DOI] [PubMed] [Google Scholar]
- 16.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 17.Spaggiari GM, Abdelrazik H, Becchetti F, Moretta L. MSCs inhibit monocyte-derived DC maturation and function by selectively interfering with the generation of immature DCs: Central role of MSC-derived prostaglandin E2. Blood. 2009;113:6576–6583. doi: 10.1182/blood-2009-02-203943. [DOI] [PubMed] [Google Scholar]
- 18.Zhang B, et al. Mesenchymal stem cells induce mature dendritic cells into a novel Jagged-2-dependent regulatory dendritic cell population. Blood. 2009;113(1):46–57. doi: 10.1182/blood-2008-04-154138. [DOI] [PubMed] [Google Scholar]
- 19.Bartholomew A, et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002;30(1):42–48. doi: 10.1016/s0301-472x(01)00769-x. [DOI] [PubMed] [Google Scholar]
- 20.Zappia E, et al. Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood. 2005;106:1755–1761. doi: 10.1182/blood-2005-04-1496. [DOI] [PubMed] [Google Scholar]
- 21.Sudres M, et al. Bone marrow mesenchymal stem cells suppress lymphocyte proliferation in vitro but fail to prevent graft-versus-host disease in mice. J Immunol. 2006;176:7761–7767. doi: 10.4049/jimmunol.176.12.7761. [DOI] [PubMed] [Google Scholar]
- 22.Augello A, Tasso R, Negrini SM, Cancedda R, Pennesi G. Cell therapy using allogeneic bone marrow mesenchymal stem cells prevents tissue damage in collagen-induced arthritis. Arthritis Rheum. 2007;56:1175–1186. doi: 10.1002/art.22511. [DOI] [PubMed] [Google Scholar]
- 23.Hill M, et al. IDO expands human CD4+CD25high regulatory T cells by promoting maturation of LPS-treated dendritic cells. Eur J Immunol. 2007;37:3054–3062. doi: 10.1002/eji.200636704. [DOI] [PubMed] [Google Scholar]
- 24.Manetti R, et al. Interleukin 12 induces stable priming for interferon gamma (IFN-gamma) production during differentiation of human T helper (Th) cells and transient IFN-gamma production in established Th2 cell clones. J Exp Med. 1994;179:1273–1283. doi: 10.1084/jem.179.4.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neves BM, et al. Activation of phosphatidylinositol 3-kinase/Akt and impairment of nuclear factor-kappaB: Molecular mechanisms behind the arrested maturation/activation state of Leishmania infantum-infected dendritic cells. Am J Pathol. 2010;177:2898–2911. doi: 10.2353/ajpath.2010.100367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bevan MJ. Cross-priming. Nat Immunol. 2006;7:363–365. doi: 10.1038/ni0406-363. [DOI] [PubMed] [Google Scholar]
- 27.Whiteside TL, Stanson J, Shurin MR, Ferrone S. Antigen-processing machinery in human dendritic cells: Up-regulation by maturation and down-regulation by tumor cells. J Immunol. 2004;173:1526–1534. doi: 10.4049/jimmunol.173.3.1526. [DOI] [PubMed] [Google Scholar]
- 28.Racanelli V, et al. Alterations in the antigen processing-presenting machinery of transformed plasma cells are associated with reduced recognition by CD8+ T cells and characterize the progression of MGUS to multiple myeloma. Blood. 2010;115:1185–1193. doi: 10.1182/blood-2009-06-228676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Groettrup M, Soza A, Kuckelkorn U, Kloetzel PM. Peptide antigen production by the proteasome: Complexity provides efficiency. Immunol Today. 1996;17:429–435. doi: 10.1016/0167-5699(96)10051-7. [DOI] [PubMed] [Google Scholar]
- 30.Nandi D, Jiang H, Monaco JJ. Identification of MECL-1 (LMP-10) as the third IFN-gamma-inducible proteasome subunit. J Immunol. 1996;156:2361–2364. [PubMed] [Google Scholar]
- 31.Martín-Fontecha A, et al. Induced recruitment of NK cells to lymph nodes provides IFN-gamma for T(H)1 priming. Nat Immunol. 2004;5:1260–1265. doi: 10.1038/ni1138. [DOI] [PubMed] [Google Scholar]
- 32.Barbash IM, et al. Systemic delivery of bone marrow-derived mesenchymal stem cells to the infarcted myocardium: Feasibility, cell migration, and body distribution. Circulation. 2003;108:863–868. doi: 10.1161/01.CIR.0000084828.50310.6A. [DOI] [PubMed] [Google Scholar]
- 33.Lee RH, et al. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009;5(1):54–63. doi: 10.1016/j.stem.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rafei M, et al. Mesenchymal stromal cells ameliorate experimental autoimmune encephalomyelitis by inhibiting CD4 Th17 T cells in a CC chemokine ligand 2-dependent manner. J Immunol. 2009;182:5994–6002. doi: 10.4049/jimmunol.0803962. [DOI] [PubMed] [Google Scholar]
- 35.Parekkadan B, et al. Mesenchymal stem cell-derived molecules reverse fulminant hepatic failure. PLoS ONE. 2007;2:e941. doi: 10.1371/journal.pone.0000941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Uccelli A, Prockop DJ. Why should mesenchymal stem cells (MSCs) cure autoimmune diseases? Curr Opin Immunol. 2010;22:768–774. doi: 10.1016/j.coi.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 37.Uccelli A, Laroni A, Freedman MS. Mesenchymal stem cells for the treatment of multiple sclerosis and other neurological diseases. Lancet Neurol. 2011;10:649–656. doi: 10.1016/S1474-4422(11)70121-1. [DOI] [PubMed] [Google Scholar]
- 38.Inaba K, et al. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176:1693–1702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lutz MB, et al. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223:77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
- 40.Botti C, et al. Comparison of three different methods for radiolabelling human activated T lymphocytes. Eur J Nucl Med. 1997;24:497–504. doi: 10.1007/BF01267680. [DOI] [PubMed] [Google Scholar]
- 41.Blocklet D, et al. 111In-oxine and 99mTc-HMPAO labelling of antigen-loaded dendritic cells: In vivo imaging and influence on motility and actin content. Eur J Nucl Med Mol Imaging. 2003;30:440–447. doi: 10.1007/s00259-002-1001-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.