Abstract
Macrophage migration inhibitory factor (MIF) is a pleiotropic inflammatory cytokine that has been implicated in various inflammatory diseases. Chronic inflammation is a mainstay of liver fibrosis, a leading cause of morbidity worldwide, but the role of MIF in liver scarring has not yet been elucidated. Here we have uncovered an unexpected antifibrotic role for MIF. Mice genetically deleted in Mif (Mif−/−) showed strongly increased fibrosis in two models of chronic liver injury. Pronounced liver fibrosis in Mif−/− mice was associated with alterations in fibrosis-relevant genes, but not by a changed intrahepatic immune cell infiltration. Next, a direct impact of MIF on hepatic stellate cells (HSC) was assessed in vitro. Although MIF alone had only marginal effects on HSCs, it markedly inhibited PDGF-induced migration and proliferation of these cells. The inhibitory effects of MIF were mediated by CD74, which we detected as the most abundant known MIF receptor on HSCs. MIF promoted the phosphorylation of AMP-activated protein kinase (AMPK) in a CD74-dependent manner and, in turn, inhibition of AMPK reversed the inhibition of PDGF-induced HSC activation by MIF. The pivotal role of CD74 in MIF-mediated antifibrotic properties was further supported by augmented liver scarring of Cd74−/− mice. Moreover, mice treated with recombinant MIF displayed a reduced fibrogenic response in vivo. In conclusion, we describe a previously unexplored antifibrotic function of MIF that is mediated by the CD74/AMPK signaling pathway in HSCs. The results imply MIF and CD74 as targets for treatment of liver diseases.
Chronic liver diseases are a major health burden worldwide and among the leading causes of death in the United States and Europe (1). Increased mortality in liver diseases is mainly attributed to progressive fibrogenesis, which ultimately leads to liver cirrhosis and hepatocellular carcinoma in many patients (2, 3). Hence, the process of liver scarring is considered a valuable target to reduce the clinical consequences of chronic liver diseases.
Fibrogenesis is believed to be a chronic wound-healing response, which is characterized by a tight interplay between inflammatory and matrix-producing cellular pathways (4). Within the liver, different cell populations are thought to contribute directly to matrix production in response to inflammatory stimuli. These cells include hepatic stellate cells (HSCs), periportal myofibroblasts, and bone marrow-derived fibrocytes (4). However, HSCs seem to be the quantitatively predominant cell type for fibrosis progression in most human liver diseases and their respective animal models (5). During chronic hepatic inflammation, HSCs are a target of numerous immune mediators, including cytokines and chemokines, which directly modulate their profibrogenic phenotype (6, 7). Notably, there are also immune cell-derived mediators that inhibit HSC functions (8). Thus, the action of one mediator might only be viewed in the context of other cytokines and growth factors, which might operate synergistically or antagonistically in vivo.
Macrophage migration inhibitory factor (MIF) is a key proinflammatory cytokine and chemokine-like function cytokine that is rapidly released from various immune cells but also from endothelial cells, tissue macrophages, and certain parenchymal cells upon inflammatory and stress stimulation (9, 10). MIF plays a nonredundant role in several inflammatory diseases, including sepsis (11, 12), rheumatoid arthritis (13, 14), obesity (15), and atherosclerosis (16, 17). Because most of these diseases were ameliorated by genetic Mif deletion or MIF neutralization, anti-MIF therapies have been considered to be of potential clinical value (18).
MIF is also expressed within the liver and is up-regulated during thioacetamide (TAA)-induced liver fibrosis in rats (19). In Con A-induced hepatic damage, a model of T-cell mediated hepatitis (20), Mif-deficient mice were protected from severe acute liver injury (21), suggesting that MIF can display detrimental effects in the liver. However, no functional studies on the role of MIF in chronic liver-disease models have yet been performed.
In contrast to these data and the general conception of a proinflammatory spectrum of action of MIF, recent findings demonstrate that MIF might also exert beneficial effects under defined pathological conditions. These pivotal studies identified cardioprotective effects of MIF in the ischemic heart and during ischemia/reperfusion injury (22, 23). Interestingly, the cardioprotective effect of MIF during ischemia/reperfusion injury is age-dependent and strongly correlates with reduced MIF expression in the senescent heart (24). At the molecular level, MIF target-cell effects are mediated by interactions with three distinct receptor proteins. Activation of inflammatory and atherogenic leukocyte recruitment by MIF is mediated by noncognate interaction between MIF and the chemokine receptors CXCR2 and CXCR4 (16), but proproliferative effects and regulation of B-cell and tumor cell survival by MIF depends on its interaction with CD74 (25). CD74 is the plasma membrane form of the MHC class II chaperone invariant chain Ii and has recently been demonstrated to function as a cell-surface receptor for MIF (26). Of note, the beneficial effects of MIF in ischemia/reperfusion injury were found to be mediated through CD74 and by activation of AMP-activated protein kinase (AMPK) in cardiomyocytes (22). The AMPK pathway is activated under cellular stress conditions and affects diverse molecular cascades, which together increase cellular ATP production and limit energy consumption (27). However, although consistent data have been generated in heart injury, the role of MIF as a potential up-stream regulator of AMPK signaling in other organs and under chronic inflammatory conditions (as it may occur in liver fibrogenesis) is unknown. Here, we have investigated the role of MIF in liver fibrosis and describe a previously unexplored and unexpected antifibrogenic effect of MIF in different models of experimental liver injury in vitro and in vivo.
Results
Mif-Deficient Mice Display Exacerbated Liver Fibrosis After Experimental Liver Injury.
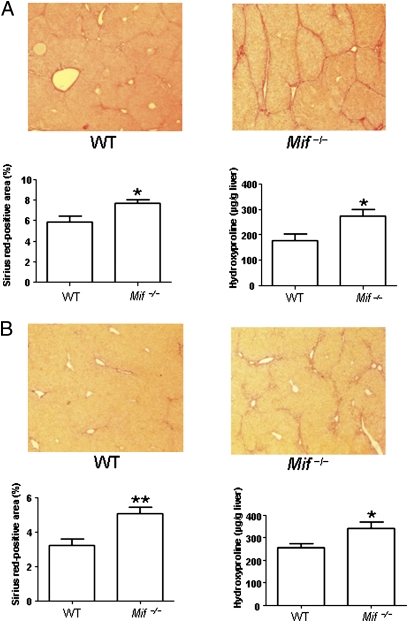
To assess the functional relevance of MIF in liver fibrosis, we treated Mif-deficient (Mif−/−) mice and WT C57BL/6 mice with carbontetrachloride (CCl4), which is an established model of chronic liver injury (7). Mice were killed and liver fibrosis was assessed histologically and by determination of the collagen-specific amino acid hydroxyproline following 6 wk of treatment. As shown in Fig. 1A, Mif−/− mice showed significantly increased liver scarring compared with WT mice in this model. The enhanced fibrogenic response in the Mif−/− mice was evident both after quantification of the Sirius red-positive area (P < 0.05) and by increased hepatic levels of hydroxyproline (P < 0.05). As MIF has generally been considered a pivotal proinflammatory cytokine and Mif−/− mice are protected from damage in most inflammation-related disease models (18), the increased liver damage in the Mif−/− mice was a surprising result. We therefore subjected Mif−/− and WT mice to another model of chronic liver injury (i.e., administration of TAA) to confirm the findings in the CCl4 model. As depicted in Fig. 1B, an increased propensity of Mif−/− mice to develop more severe liver fibrosis was also clearly evident in this independent model. The Sirius red-positive area and the hydroxyproline levels were again significantly increased in Mif−/− compared with WT mice (P < 0.01 and P < 0.05, respectively), suggesting model-independent antifibrotic properties of MIF in mice.
Fig. 1.
Enhanced liver fibrosis in Mif−/− mice in two independent experimental models. (A) CCl4 model: representative Sirius red stainings (Upper) of WT and Mif−/− mice after challenge with CCl4 for 6 wk. Sirius red stainings from 12 mice per group were quantitated (Lower Left). Increased fibrosis in Mif−/− mice compared with WT (n = 12 per group) was further validated by significantly increased concentrations of hydroxyproline (Lower Right). (B) TAA model: challenge with TAA for 6 wk of WT vs. Mif−/− mice. Exaggerated fibrosis in Mif−/− mice compared with WT (n = 12 per group) is evident by representative Sirius red staining (Upper) and quantification of Sirius red-positive area (Lower Left) and increased concentrations of hydroxyproline (Lower Right). Asterisks indicate statistical significance: *P < 0.05, **P < 0.01. (Magnification: A and B, 200×.)
Liver Damage in Mif−/− Mice Is Associated with Altered Expression of Fibrosis-Related Genes and HSC Activation.
In the experimental models used in this study, liver fibrosis is considered to be the result of an interplay between the infiltration of certain immune-cell subsets and the subsequent activation of HSCs (28). As MIF has been shown to modulate the recruitment of immune cells through interaction with its receptors CXCR2 and CXCR4 (16), we next evaluated potential differences in hepatic immune cell infiltration between the Mif−/− and WT mice.
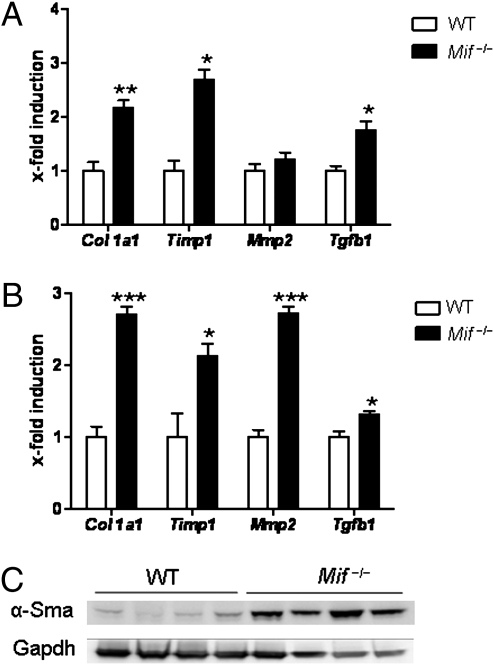
However, FACS analysis revealed no major differences in immune-cell subset infiltration between the mouse strains in both models of liver injury (Fig. S1). As other cells might then be responsible for the profibrotic phenotype observed in the Mif−/− mice, we next assessed the expression of critical HSC-associated genes after fibrosis induction in Mif−/− vs. WT mice. In accordance with the overall enhanced liver fibrosis, the mRNA expression of Col1a1, Timp1, Mmp2, and Tgfb1 was increased in Mif−/− mice compared with their WT counterparts after chronic CCl4 treatment (Fig. 2A). The altered mRNA expression of these genes was confirmed in the mice treated with TAA (Fig. 2B), suggesting that HSC activation is a predominant pathological feature in Mif−/− mice during chronic liver injury. This hypothesis was confirmed by the strongly increased expression of α-smooth muscle actin (α-Sma) protein, a marker for HSC activation (5), in total liver lysates (Fig. 2C). Therefore, the exaggerated fibrogenic response in the Mif−/− mice appeared to be primarily because of effects on HSC biology rather than significant changes in the intrahepatic immune response.
Fig. 2.
Increased expression of HSC-related genes and α-Sma in Mif−/− mice. (A) CCl4 treatment of Mif−/− mice leads to significantly increased mRNA expression of Col1a1, Timp1, and Tgfb1 compared with WT mice. (B) Same as in A, except that fibrosis was induced by TAA treatment. Asterisks indicate statistical significance: *P < 0.05, **P < 0.01, ***P < 0.001. (C) Augmented HSC activation after CCl4 challenge in Mif−/− mice (lanes 5–8) compared with WT (lanes 1–4) is further evidenced by increased expression of α-Sma protein expression (Western blot from total liver lysates; loading control: Gapdh). Each lane represents a cell lysate from an independent mouse.
Inhibitory Effects of MIF on HSCs Are Mediated Through CD74.
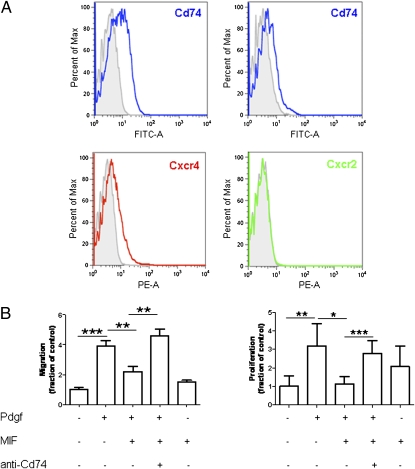
Overall, the receptors CD74, CXCR4, and CXCR2 have been shown to mediate the predominant effects of MIF in vitro and in vivo (10). We therefore determined the expression of each of these receptors on murine HSCs. As determined by FACS analysis, CD74 is the most abundant MIF receptor on HSCs, and CXCR4 was present at lower expression levels (Fig. 3A). In contrast, CXCR2 was not detected.
Fig. 3.
Surface expression and role of CD74 in regulation of HSCs by MIF. (A) Expression of MIF receptors on immortalized and primary HSCs. FACS analysis reveals marked expression of CD74 (Upper Left: immortalized murine HSCs, Upper Right: primary murine HSCs) and low-to-moderate expression CXCR4 on stellate cells. CXCR2 is not expressed on HSCs. (B) Chemotaxis (Left) and BrdU incorporation-based proliferation experiments (Right) experiments reveal that MIF inhibits PDGF-induced HSC behavior, but MIF alone does not have substantial effects on these cells. Note, that the inhibitory effects of MIF on PDGF-induced HSC activation can be completely blocked by pretreatment of the cells with neutralizing CD74 antibody (anti-CD74). Each type of incubation was performed at least twice in quadruplicate each. Asterisks indicate statistical significance: *P < 0.05, **P < 0.01, ***P < 0.001.
Thus, we next assessed the functional importance of MIF and CD74 for fibrogenic HSC responses in vitro. The potential modulation of HSC migration and proliferation by MIF was tested as these properties are among key features of HSCs during fibrogenesis. Recombinant MIF alone did not affect the migration of HSCs in vitro and only had a slight effect on proliferation (Fig. 3B). In contrast, MIF strongly inhibited the PDGF-induced migration and proliferation of HSCs (P < 0.001) (Fig. 3B). Notably, inhibition of PDGF-induced HSC activation by MIF was completely blocked by pretreatment of the cells with an inhibitory antibody to CD74 (P < 0.001) (Fig. 3B), suggesting that this receptor is responsible for the observed inhibition of PDGF-triggered migration and proliferation of HSCs by MIF in vitro.
Inhibition of PDGF-Induced HSC Activation by MIF Is Mediated by Increased Phosphorylation of AMPK.
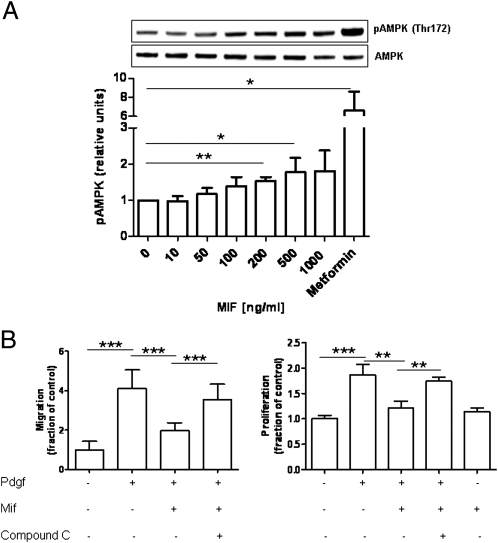
MIF has been shown to critically regulate cardiomyocyte responses to stress by CD74-dependent phosphorylation of AMPK (22). Thus, we evaluated whether MIF also affected the phosphorylation of AMPK in HSCs. In fact, short-term incubation of primary HSCs isolated from Mif−/− mice resulted in a dose-dependent phosphorylation of AMPK in these cells (Fig. 4A). The effect of MIF was less strong than that of Metformin, a well-known AMPK activator, but led to significantly increased pAMPK/AMPK ratios at MIF doses of 200 to 500 ng/mL that were previously shown to induce AMPK in cardiomyocytes. The functional significance of this finding was underscored by measuring MIF effects on HSC behavior after pretreatment of the cells with the AMPK inhibitor Compound C (29). In accordance with a functional role of AMPK phosphorylation in HSCs, the MIF-induced inhibition of PDGF-induced HSC activation was significantly blocked by pretreatment of the cells with the AMPK inhibitor (P < 0.001) (Fig. 4B).
Fig. 4.
Regulation of hepatic stellate cell activity by MIF is mediated by AMPK activation. (A) Recombinant MIF induces a dose-dependent phosphorylation of AMPK at position Thr-172. A representative Western blot is shown. The bar diagram represents mean values of three independent experiments. (B) Inhibition of PDGF-induced HSC migration and proliferation by MIF is mediated by AMPK activation. HSCs were incubated with PDGF and MIF in the presence or absence of the AMPK inhibitor Compound C, which significantly reverts the inhibitory effects of MIF. All types of incubation were performed at least twice in quadruplicate each. Asterisks indicate statistical significance: *P < 0.05, ***P < 0.001.
Mice Deficient in Cd74 Have Augmented Liver Fibrosis After CCl4 Treatment and Recombinant MIF Administration Ameliorates Fibrogenesis in Vivo.
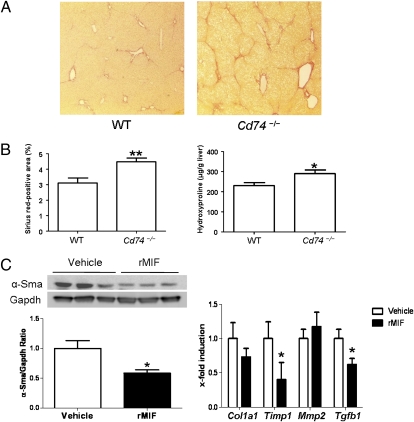
As CD74 seems to mediate the main antifibrotic effects of MIF in vitro, we next subjected mice with a targeted deletion of the Cd74 gene (Cd74−/−) to the CCl4 fibrosis model. In line with our in vitro results, Cd74−/− mice displayed increased liver scarring compared with WT mice in vivo (Fig. 5A). This difference was evident both after quantification of liver fibrosis by assessment of the Sirius red-positive area (P < 0.01) and by determination of hepatic hydroxyproline content (P < 0.05) (Fig. 5B). Furthermore, Cd74−/− mice showed significantly increased Col1a1 mRNA expression compared with their WT counterparts (P < 0.05) (Fig. 5C). As for the Mif−/− mice, Cd74−/− mice showed no relevant differences in immune cell infiltration rates, except for a higher number of NKT (NK1.1+ CD3+) cells within the liver after CCl4 challenge (Fig. S2).
Fig. 5.
Enhanced liver fibrosis in Cd74−/− mice in CCl4 fibrosis model. (A) Representative Sirius red stainings of WT and Cd74−/− mice after challenge with CCl4. (Magnification: 200×.) (B) Increased fibrosis in Cd74−/− mice (n = 8 per group) as validated by quantification of Sirius red-positive areas (Left) and hydroxyproline content (Right). (C) Daily administration of recombinant MIF represses the CCl4 induced activation of stellate cells as assessed by total α-Sma protein expression. Three representative samples of each group and the overall ratio of α-Sma/Gapdh are displayed. (D) The reduced fibrogenic response in mice treated with rMIF is validated by repression of fibrosis related-genes compared with vehicle-treated mice. *P < 0.05, **P < 0.01.
As described above, CXCR4 is also expressed on murine HSCs and could thus potentially also be involved in mediating the observed antifibrotic effects of MIF. We thus analyzed mice with a heterozygous deletion of this receptor (Cxcr4+/−) for their fibrogenic response in vivo. In contrast to mice with a deletion for Cd74, the Cxcr4+/− mice did not show any appreciable differences in fibrosis parameters compared with WT controls after CCl4 challenge (Fig. S3).
In extension of these data, we next tested whether therapeutic application of recombinant (rMIF) to wild-type mice is able to inhibit the CCl4-induced fibrogenic response in vivo. Indeed, daily intraperitoneal injections of rMIF led to a reduced activation of HSCs, as demonstrated by biochemical and histological α-Sma expression (Fig. 5C and Fig. S4). Furthermore, MIF administration resulted in a strong repression of major fibrosis-relevant genes compared with vehicle-treated mice (Fig. 5D).
Discussion
In this study, we have uncovered an unexpected antifibrotic role of the proinflammatory cytokine MIF in hepatic fibrogenesis in vitro and in vivo. As liver fibrosis in the murine models used in our study is considered to have an inflammatory phenotype with secondary HSC activation (7), we had anticipated that Mif−/− mice would exhibit a reduced severity of liver fibrosis. However, the contrary was observed in both models of liver fibrosis in vivo.
Immune-cell infiltration is considered to be an integral part of the fibrogenic response in the models used in our study. However, the observed differences in infiltrated immune-cell subsets in the damaged liver were marginal or not present at all, fostering the conclusion that an altered leukocyte recruitment profile was probably not responsible for the exacerbated fibrosis phenotype seen in the Mif−/− mice. Thus, unlike in atherogenic, arthritic, and other murine models of inflammation, where MIF/CXCR2- or MIF/CXCR4-mediated leukocyte recruitment processes play an important pathogenic role (16, 30–33), MIF-driven hepatic leukocyte infiltration does not appear to contribute to liver fibrogenesis. Because CXCR4 has been shown to mediate important profibrotic effects in human HSCs in vitro (34), we also assessed the hypothesis that CXCR4 might be a contributing MIF receptor during liver fibrogenesis in vivo. These experiments were performed in mice with a heterozygous deletion of Cxcr4 (Cxcr4+/−) (35), which show reduced Cxcr4 expression levels and an ameliorated phenotype in atherosclerosis models (36). However, when Cxcr4+/− mice were subjected to the CCl4 fibrosis model, no major differences in liver scarring, HSC activation, or immune-cell infiltration were detected compared with litter-matched WT mice. Thus, although we cannot fully exclude important effects of the CXCR4 ligands MIF and CXCL12 during liver fibrosis, a major contribution of this receptor pathway to liver fibrosis seems unlikely in the mouse models studied herein.
In contrast to the lack of an immune cell-driven phenotype in the Mif−/− mice, a prominent feature of the mice was a strong dysregulation of HSC-associated genes in both models. Furthermore, α-Sma protein content was clearly increased in the livers of Mif−/− mice compared with WT mice. As HSCs are considered as key players in liver fibrogenesis (5), we assessed the direct effects of MIF on these cells in vitro. We first determined the abundance of the MIF receptors expressed on the surface of these cells. Both immortalized and primary murine HSCs expressed high levels Cd74, and Cxcr4 was found expressed at low, yet measurable levels. CXCR4 has been described on human HSCs before (34), but Cd74/invariant chain expression so far has only been detected on rat HSCs in the context of class II-associated antigen presentation after stimulation with IFN-γ (37). Here we have extended this observation by demonstrating marked Cd74 expression on unstimulated mouse HSCs.
Recent reports have clearly suggested that CD74 is involved in mediating proliferative effects of MIF on various target cells (26, 38–41). Furthermore, this receptor has been implicated in protective effects of MIF on stress-induced cardiac dysfunction (22, 23). In these latter studies, the MIF/CD74 axis was found to activate the stress- and starvation-activated kinase AMPK, thereby modulating intracellular energy-saving pathways (22). The importance of the AMPK pathway has also been demonstrated in human HSC cells in vitro. In these studies, different AMPK activators, such as adiponectin, were found to inhibit PDGF-induced HSC proliferation (42, 43). Notably, adiponectin has also been shown to ameliorate chronic liver injury after pharmacological application in vivo (44, 45), suggesting that the AMPK pathway might be a pharmacological target within the liver. In light of these observations, we systematically assessed whether the antifibrotic effects of MIF in the liver could be mediated by an AMPK-mediated pathway. In fact, MIF was able to inhibit the PDGF-induced proliferation and migration of isolated HSCs, and these effects were found to be dependent on CD74 binding and consecutive AMPK phosphorylation. Thus, HSCs, similar to cardiomyocytes, seem to be an important target of cell-protective MIF action upon stress. We speculate that these MIF effects only occur under energy-consuming conditions, as MIF alone did not modify the proliferation or migration of HSCs. Of note, HSCs are certainly exposed to PDGF during liver fibrogenesis in vivo, PDGF representing one of the strongest mitogens for these cells (46).
An essential role for CD74 in liver fibrosis also became apparent in our in vivo models. Mice with a targeted deletion in the Cd74 gene displayed an exaggerated fibrogenic response compared with WT animals, although we cannot exclude that CD74 might also mediate other cellular effects apart from AMPK activation (26). Nevertheless, together with our in vitro findings and the clear-cut evidence on cell-protective effects of MIF/CD74/AMPK in cardiomyocytes (22, 23), our in vivo comparison between the Cd74−/− and WT mice in the CCl4 model is strongly supportive of the importance of the MIF/CD74/AMPK pathway in chronic liver damage. Accordingly, we also observed that systemic application of rMIF repressed the CCl4-induced activation of HSCs and fibrosis-associated genes in vivo. These therapeutic effects of MIF appear to be similar to the known prominent AMPK activator adiponectin (44) and strongly suggest that this pathway plays a nonredundant role in liver fibrosis.
In summary, we describe herein an unexpected antifibrotic pathway of MIF through CD74/AMPK-mediated inhibition of PDGF-mediated HSC activation. These results underscore the importance of this pathway in the ischemic heart and imply unique therapeutic targets for the treatment of chronic liver diseases.
Materials and Methods
Proteins, Antibodies, and Other Reagents.
Recombinant murine MIF was prepared as described elsewhere (16) or purchased from R&D Systems. Recombinant PDGF-B was purchased from R&D Systems. Metformin (1,1-Dimethylbiguanide hydrochloride), AMPK inhibitor Compound C (6-[4-(2-Piperidin-1-ylethoxy)phenyl]-3-pyridin-4-ylpyrazolo[1,5-a]pyrimidine), and LPS from Escherichia coli 0111:B4 were from Sigma. The neutralizing anti-mouse CD74 antibody (clone ln-1) was bought from BD Pharmingen.
Murine in Vivo Experiments.
All animal experiments were approved by the animal welfare committee of the Bezirksregierung Cologne, Germany. Mice were subjected to two different fibrosis models. In the first model, C57BL/6 Mif−/− (23) and WT mice (n = 12 per group) received intraperitoneal injections of CCl4 for 6 wk (0.6 mg/kg, twice weekly). Mice were killed 3 d after the last injection. Subjection of Cd74−/− and Cxcr4+/− mice to the CCl4 was done identically except that n = 8 per group were used. In the second model, Mif−/− and WT mice (n = 12 per group) received intraperitoneal injections of TAA for 6 wk (100 mg/kg, three times weekly).
In a pharmacological approach, WT mice (n = 6 per group) received daily intraperitoneal injections of 10 μg of biologically active, endotoxin-free, recombinant MIF (16) or vehicle concomitantly to CCl4 for 10 d. Mice were killed 3 d after the last CCl4 injection and the fibrogenic response was assessed within the livers by α-Sma quantification and RT-PCRs of fibrosis-related genes.
In all animals, liver fibrosis was assessed histologically by quantification of the α-Sma+ cells and the Sirius red-positive area on 10 low-power fields (magnification: 200×) per slide through use of the National Institutes of Health software ImageJ, which is available from http://rsbweb.nih.gov. Collagen contents of the livers of treated mice were measured as described previously (7).
Expression Analysis of Murine Fibrosis-Related Genes.
Total RNA was isolated from livers of mice and reversely transcribed using Super-Script (Invitrogen). Quantitative RT-PCR was carried out for Col1a1, Timp1, Mmp2, α-Sma, and Tgfb1, with “Assays on Demand” from http://www.appliedbiosystems.com.
Hepatic Immune Cell Isolation and Flow Cytometric Analysis.
Single-cell suspensions were isolated from freshly harvested livers using mechanical and enzymatic digestion. Viable white blood cells were purified from the suspension by centrifugation for 20 min at 800 × g with a density gradient separation medium (Lympholyte-H; Cederlane Laboratories). Peripheral blood mononuclear cells were collected from the gradient/supernatant interface and then washed in HBSS supplemented with 1% BSA and 2 mM EDTA. For flow cytometry analysis, cells were stained with fluorochrome-conjugated antibodies for CD45, CD3, F4/80, and NK1.1 (eBioscience) and the relative numbers were quantified using the FACSCanto II (Becton Dickinson). Data were analyzed using FlowJo software (Tree Star).
Immortalized or primary mouse HSCs (for isolation protocol see below) were stained with fluorochrome-conjugated antibodies for CD74 (FITC-conjugated; BD Pharmingen), CXCR2 and CXCR4 (both PE-conjugated), or the appropriate isotype controls (R&D Systems). Cells were subjected to flow cytometry analysis using a FACS Canto (BD Bioscience). Data were analyzed using FlowJo software (Tree Star).
Cell Migration Assay.
The cell migration assays were performed using a modified Boyden chamber. Briefly, the HSCs (2 × 105 cells/well) were placed in the upper chamber in DMEM without FCS. The cells were exposed to PDGF-B (100 ng) and recombinant MIF (500 ng) in the lower chamber.
For blockade experiments, the HSCs were preincubated with 12 μg anti-CD74 antibody or 25 μmol Compound C for 60 min. After 4 h of incubation at 37 °C, cells migrated to the lower chamber were counted in six randomly chosen fields (magnification: 100×). All experiments were performed at least twice in quadruplicate each.
Cell Proliferation Assay.
Proliferation of immortalized HSCs was assessed by a colorimetric immunoassay based on the measurement of BrdU incorporation during DNA synthesis (Cell Proliferation Elisa; Roche Applied Science) following the manufacturer's instructions. Briefly, cells were starved for 16 h in DMEM (PAA Laboratories) without FCS and stimulated with PDGF-B (100 ng) and recombinant MIF (500 ng) for 24 h. Blockade of CD74 or AMPK was done as described for the migration assay. After preincubation, cells were labeled with BrdU for 2 h. BrdU incorporation was assessed after removal of labeling media, fixation of cells and DNA denaturation by adding an anti–BrdU-POD antibody and subsequent substrate reaction.
Isolation and Cultivation of Immortalized and Primary Mouse HSC.
Use and cultivation of the GRX HSC cell line has been described previously (47). Primary HSCs were isolated from Mif−/− [background: C57BL/6 (16)] at the age of 40 wk. Isolation was performed according to a protocol that is based on enzymatic collagenase and pronase digestion of the liver followed by centrifugation of the crude cell suspension through a Nycodenz gradient. After isolation, cells were resuspended in DMEM supplemented with 10% FCS and 4 mM l-glutamine and plated on plastic dishes. Cells were cultured at 37 °C in a humidified atmosphere with 5% CO2. After 24 h, cell debris and nonadherent cells were removed by replacing with fresh medium. Starting from day 2 after isolation, cells were stimulated with 100 ng/mL LPS daily for 5 d. Cells then showed the morphology of activated myofibroblasts and were used for the in vitro experiments. Scouting experiments with HSCs from WT mice, which gave similar HSC yields, had shown that rMIF-elicited pAMPK responses were less pronounced in those cells, likely because of desensitization effects by autocrine MIF. Thus, Mif−/− HSCs were used for the functional studies.
Analysis of AMPK Phosphorylation and α-Sma by Western Blotting.
Primary mouse HSCs were seeded into 48-well plates and allowed to adhere. After overnight serum starvation in DMEM containing 0.5% FCS, cells were stimulated with varying concentrations of recombinant mouse MIF or 20 μM Metformin for 30 min. Cell lysates were subjected to Western blotting as previously described (16). For protein detection, membranes were probed with primary phospho-AMPKα (Thr172), AMPKα, or α-Sma antibodies (Cell Signaling Technologies) and the appropriate peroxidase-conjugated secondary antibody. Bands were detected by chemoluminescence and intensity quantified using the AIDA image analyzer software (Fuji/Raytest Isotopenmessgerät GmbH).
Statistical Analysis.
Data are given as means ± SEM. Continuous variables were compared by two-sided t tests with Welch's correction in case of unequal variances. Variables in contingency tables were compared by Fisher's exact test, with which we also calculated odds ratios for the genetic analysis. P values lower than 0.05 were considered significant in all analyses. Statistical tests were performed by GraphPad Prism 4.0.
Supplementary Material
Acknowledgments
We thank all members of the Q3 platform of the SFB-TRR57 for their help in cell isolation procedures. This study was supported by the Deutsche Forschungsgemeinschaft (SFB-TRR57 P07) and the National Institutes of Health.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1107023108/-/DCSupplemental.
References
- 1.Schuppan D, Afdhal NH. Liver cirrhosis. Lancet. 2008;371:838–851. doi: 10.1016/S0140-6736(08)60383-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States N Engl. J Med. 1999;340:745–750. doi: 10.1056/NEJM199903113401001. [DOI] [PubMed] [Google Scholar]
- 3.Ginès P, Cárdenas A, Arroyo V, Rodés J. Management of cirrhosis and ascites. N Engl J Med. 2004;350:1646–1654. doi: 10.1056/NEJMra035021. [DOI] [PubMed] [Google Scholar]
- 4.Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134:1655–1669. doi: 10.1053/j.gastro.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Friedman SL. Hepatic stellate cells: Protean, multifunctional, and enigmatic cells of the liver. Physiol Rev. 2008;88(1):125–172. doi: 10.1152/physrev.00013.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209–218. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berres ML, et al. Antagonism of the chemokine Ccl5 ameliorates experimental liver fibrosis in mice. J Clin Invest. 2010;120:4129–4140. doi: 10.1172/JCI41732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wasmuth HE, et al. Antifibrotic effects of CXCL9 and its receptor CXCR3 in livers of mice and humans. Gastroenterology. 2009;137:309–319, 319.e1-3. doi: 10.1053/j.gastro.2009.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calandra T, Roger T. Macrophage migration inhibitory factor: A regulator of innate immunity. Nat Rev Immunol. 2003;3:791–800. doi: 10.1038/nri1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schober A, Bernhagen J, Weber C. Chemokine-like functions of MIF in atherosclerosis. J Mol Med (Berl) 2008;86:761–770. doi: 10.1007/s00109-008-0334-2. [DOI] [PubMed] [Google Scholar]
- 11.Bernhagen J, et al. MIF is a pituitary-derived cytokine that potentiates lethal endotoxaemia. Nature. 1993;365:756–759. doi: 10.1038/365756a0. [DOI] [PubMed] [Google Scholar]
- 12.Calandra T, et al. Protection from septic shock by neutralization of macrophage migration inhibitory factor. Nat Med. 2000;6(2):164–170. doi: 10.1038/72262. [DOI] [PubMed] [Google Scholar]
- 13.Baugh JA, et al. A functional promoter polymorphism in the macrophage migration inhibitory factor (MIF) gene associated with disease severity in rheumatoid arthritis. Genes Immun. 2002;3(3):170–176. doi: 10.1038/sj.gene.6363867. [DOI] [PubMed] [Google Scholar]
- 14.Morand EF, Leech M, Bernhagen J. MIF: A new cytokine link between rheumatoid arthritis and atherosclerosis. Nat Rev Drug Discov. 2006;5:399–410. doi: 10.1038/nrd2029. [DOI] [PubMed] [Google Scholar]
- 15.Verschuren L, et al. MIF deficiency reduces chronic inflammation in white adipose tissue and impairs the development of insulin resistance, glucose intolerance, and associated atherosclerotic disease. Circ Res. 2009;105(1):99–107. doi: 10.1161/CIRCRESAHA.109.199166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bernhagen J, et al. MIF is a noncognate ligand of CXC chemokine receptors in inflammatory and atherogenic cell recruitment. Nat Med. 2007;13:587–596. doi: 10.1038/nm1567. [DOI] [PubMed] [Google Scholar]
- 17.Zernecke A, Bernhagen J, Weber C. Macrophage migration inhibitory factor in cardiovascular disease. Circulation. 2008;117:1594–1602. doi: 10.1161/CIRCULATIONAHA.107.729125. [DOI] [PubMed] [Google Scholar]
- 18.Hoi AY, Iskander MN, Morand EF. Macrophage migration inhibitory factor: a therapeutic target across inflammatory diseases. Inflamm Allergy Drug Targets. 2007;6(3):183–190. doi: 10.2174/187152807781696455. [DOI] [PubMed] [Google Scholar]
- 19.Hori Y, et al. Immunohistochemical study of macrophage migration inhibitory factor in rat liver fibrosis induced by thioacetamide. Eur J Histochem. 2003;47:317–324. [PubMed] [Google Scholar]
- 20.Tiegs G, Hentschel J, Wendel A. A T cell-dependent experimental liver injury in mice inducible by concanavalin A. J Clin Invest. 1992;90:196–203. doi: 10.1172/JCI115836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakajima H, et al. Lack of macrophage migration inhibitory factor protects mice against concanavalin A-induced liver injury. Liver Int. 2006;26:346–351. doi: 10.1111/j.1478-3231.2005.01216.x. [DOI] [PubMed] [Google Scholar]
- 22.Miller EJ, et al. Macrophage migration inhibitory factor stimulates AMP-activated protein kinase in the ischaemic heart. Nature. 2008;451:578–582. doi: 10.1038/nature06504. [DOI] [PubMed] [Google Scholar]
- 23.Qi D, et al. Cardiac macrophage migration inhibitory factor inhibits JNK pathway activation and injury during ischemia/reperfusion. J Clin Invest. 2009;119:3807–3816. doi: 10.1172/JCI39738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma H, et al. Impaired macrophage migration inhibitory factor-AMP-activated protein kinase activation and ischemic recovery in the senescent heart. Circulation. 2010;122:282–292. doi: 10.1161/CIRCULATIONAHA.110.953208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Starlets D, et al. Cell-surface CD74 initiates a signaling cascade leading to cell proliferation and survival. Blood. 2006;107:4807–4816. doi: 10.1182/blood-2005-11-4334. [DOI] [PubMed] [Google Scholar]
- 26.Leng L, et al. MIF signal transduction initiated by binding to CD74. J Exp Med. 2003;197:1467–1476. doi: 10.1084/jem.20030286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hardie DG. Minireview: The AMP-activated protein kinase cascade: The key sensor of cellular energy status. Endocrinology. 2003;144:5179–5183. doi: 10.1210/en.2003-0982. [DOI] [PubMed] [Google Scholar]
- 28.Sahin H, Trautwein C, Wasmuth HE. Functional role of chemokines in liver disease models. Nat Rev Gastroenterol Hepatol. 2010;7:682–690. doi: 10.1038/nrgastro.2010.168. [DOI] [PubMed] [Google Scholar]
- 29.Jin J, et al. AMPK inhibitor Compound C stimulates ceramide production and promotes Bax redistribution and apoptosis in MCF7 breast carcinoma cells. J Lipid Res. 2009;50:2389–2397. doi: 10.1194/jlr.M900119-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gregory JL, et al. Reduced leukocyte-endothelial cell interactions in the inflamed microcirculation of macrophage migration inhibitory factor-deficient mice. Arthritis Rheum. 2004;50:3023–3034. doi: 10.1002/art.20470. [DOI] [PubMed] [Google Scholar]
- 31.Gregory JL, et al. Macrophage migration inhibitory factor induces macrophage recruitment via CC chemokine ligand 2. J Immunol. 2006;177:8072–8079. doi: 10.4049/jimmunol.177.11.8072. [DOI] [PubMed] [Google Scholar]
- 32.Vera PL, Iczkowski KA, Wang X, Meyer-Siegler KL. Cyclophosphamide-induced cystitis increases bladder CXCR4 expression and CXCR4-macrophage migration inhibitory factor association. PLoS ONE. 2008;3:e3898. doi: 10.1371/journal.pone.0003898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weber C, et al. Structural determinants of MIF functions in CXCR2-mediated inflammatory and atherogenic leukocyte recruitment. Proc Natl Acad Sci USA. 2008;105:16278–16283. doi: 10.1073/pnas.0804017105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hong F, et al. Hepatic stellate cells express functional CXCR4: Role in stromal cell-derived factor-1alpha-mediated stellate cell activation. Hepatology. 2009;49:2055–2067. doi: 10.1002/hep.22890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tachibana K, et al. The chemokine receptor CXCR4 is essential for vascularization of the gastrointestinal tract. Nature. 1998;393:591–594. doi: 10.1038/31261. [DOI] [PubMed] [Google Scholar]
- 36.Zernecke A, et al. Protective role of CXC receptor 4/CXC ligand 12 unveils the importance of neutrophils in atherosclerosis. Circ Res. 2008;102:209–217. doi: 10.1161/CIRCRESAHA.107.160697. [DOI] [PubMed] [Google Scholar]
- 37.Maubach G, Lim MC, Kumar S, Zhuo L. Expression and upregulation of cathepsin S and other early molecules required for antigen presentation in activated hepatic stellate cells upon IFN-gamma treatment. Biochim Biophys Acta. 2007;1773:219–231. doi: 10.1016/j.bbamcr.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 38.Lue H, et al. Macrophage migration inhibitory factor (MIF) promotes cell survival by activation of the Akt pathway and role for CSN5/JAB1 in the control of autocrine MIF activity. Oncogene. 2007;26:5046–5059. doi: 10.1038/sj.onc.1210318. [DOI] [PubMed] [Google Scholar]
- 39.Meyer-Siegler KL, Iczkowski KA, Leng L, Bucala R, Vera PL. Inhibition of macrophage migration inhibitory factor or its receptor (CD74) attenuates growth and invasion of DU-145 prostate cancer cells. J Immunol. 2006;177:8730–8739. doi: 10.4049/jimmunol.177.12.8730. [DOI] [PubMed] [Google Scholar]
- 40.Meyer-Siegler KL, Leifheit EC, Vera PL. Inhibition of macrophage migration inhibitory factor decreases proliferation and cytokine expression in bladder cancer cells. BMC Cancer. 2004;4:34. doi: 10.1186/1471-2407-4-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meyer-Siegler KL, et al. Macrophage migration inhibitory factor (MIF) gene polymorphisms are associated with increased prostate cancer incidence. Genes Immun. 2007;8:646–652. doi: 10.1038/sj.gene.6364427. [DOI] [PubMed] [Google Scholar]
- 42.Adachi M, Brenner DA. High molecular weight adiponectin inhibits proliferation of hepatic stellate cells via activation of adenosine monophosphate-activated protein kinase. Hepatology. 2008;47:677–685. doi: 10.1002/hep.21991. [DOI] [PubMed] [Google Scholar]
- 43.Caligiuri A, et al. Adenosine monophosphate-activated protein kinase modulates the activated phenotype of hepatic stellate cells. Hepatology. 2008;47:668–676. doi: 10.1002/hep.21995. [DOI] [PubMed] [Google Scholar]
- 44.Kamada Y, et al. Enhanced carbon tetrachloride-induced liver fibrosis in mice lacking adiponectin. Gastroenterology. 2003;125:1796–1807. doi: 10.1053/j.gastro.2003.08.029. [DOI] [PubMed] [Google Scholar]
- 45.Xu A, et al. The fat-derived hormone adiponectin alleviates alcoholic and nonalcoholic fatty liver diseases in mice. J Clin Invest. 2003;112(1):91–100. doi: 10.1172/JCI17797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wasmuth HE, et al. The Marburg I variant (G534E) of the factor VII-activating protease determines liver fibrosis in hepatitis C infection by reduced proteolysis of platelet-derived growth factor BB. Hepatology. 2009;49:775–780. doi: 10.1002/hep.22707. [DOI] [PubMed] [Google Scholar]
- 47.Zaldivar MM, et al. CXC chemokine ligand 4 (Cxcl4) is a platelet-derived mediator of experimental liver fibrosis. Hepatology. 2010;51:1345–1353. doi: 10.1002/hep.23435. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.