Abstract
Bone marrow (BM) microenvironment (BMME) constitutes the sanctuary for leukemic cells. In this study, we investigated the molecular mechanisms for BMME-mediated drug resistance and BM lodgment in chronic myelogenous leukemia (CML). Gene-expression profile as well as signal pathway and protein analyses revealed that galectin-3 (Gal-3), a member of the β-gal–binding galectin family of proteins, was specifically induced by coculture with HS-5 cells, a BM stroma cell-derived cell line, in all five CML cell lines examined. It was also found that primary CML cells expressed high levels of Gal-3 in BM. Enforced expression of Gal-3 activated Akt and Erk, induced accumulation of Mcl-1, and promoted in vitro cell proliferation, multidrug resistance to tyrosine kinase inhibitors for Bcr-Abl and genotoxic agents as a result of impaired apoptosis induction, and chemotactic cell migration to HS-5–derived soluble factors in CML cell lines independently of Bcr-Abl tyrosine kinase. The conditioned medium from Gal-3–overexpressing CML cells promoted in vitro cell proliferation of CML cells and HS-5 cells more than did the conditioned medium from parental cells. Moreover, the in vivo study in a mice transplantation model showed that Gal-3 overexpression promoted the long-term BM lodgment of CML cells. These results demonstrate that leukemia microenvironment-specific Gal-3 expression supports molecular signaling pathways for disease maintenance in BM and resistance to therapy in CML. They also suggest that Gal-3 may be a candidate therapeutic target to help overcome BMME-mediated therapeutic resistance.
Keywords: Philadelphia-positive leukemia, bone marrow niche, chemoresistance, minimal residual disease
Chronic myelogenous leukemia (CML) is characterized by Bcr-Abl fusion tyrosine kinase (TK) as a result of the Philadelphia chromosome (Ph). Major advances in the treatment of CML have resulted from molecularly targeted therapeutic agents, such as imatinib mesylate (IM), which is the first-in-class Bcr-Abl TK inhibitor (TKI) and the more potent second-generation TKIs, such as nilotinib and dasatinib (Das) (1, 2). However, the complete elimination of CML clones has rarely been achieved by TKIs because of a variety of cell-intrinsic and cell-extrinsic protective mechanisms. The former include Bcr-Abl–related mechanisms, such as point mutations in the Abl kinase domain, and a variety of molecular abnormalities unrelated to Bcr-Abl (3–9). The latter include support of the bone marrow (BM) microenvironment (BMME), the so-called leukemia niche, which consists of soluble factors and supporting tissues, such as BM stromal cells (BMSCs), extracellular matrix (ECM), or hypoxia (10–19). Various new agents have been proposed for overcoming cell-intrinsic mechanisms for drug resistance (20, 21), and the precise molecular mechanisms for CML cell protection and maintenance by BMME sanctuary are not yet fully understood.
With the aim of developing new therapeutic strategies to overcome BMME-mediated protection of CML cells, we investigated the molecular mechanisms regulated by BMME, e.g., BMSCs and ECM, which enable leukemic cells to reside in the BM niche. Our study identified the involvement of galectin-3 (Gal-3), a member of the β-gal–binding galectin family of proteins, in BMME-mediated cell proliferation, protection, and BM lodgment. Gal-3 associates with cell proliferation, migration, adhesion, and apoptosis (22–26), and moreover is associated with disease progression, metastasis, and drug resistance in various cancers (27–31), but the role of Gal-3 in leukemia has remained largely unknown.
Results
Identification of Gal-3 as Candidate Mediator of Leukemia Proliferation and Drug Resistance Caused by BMME.
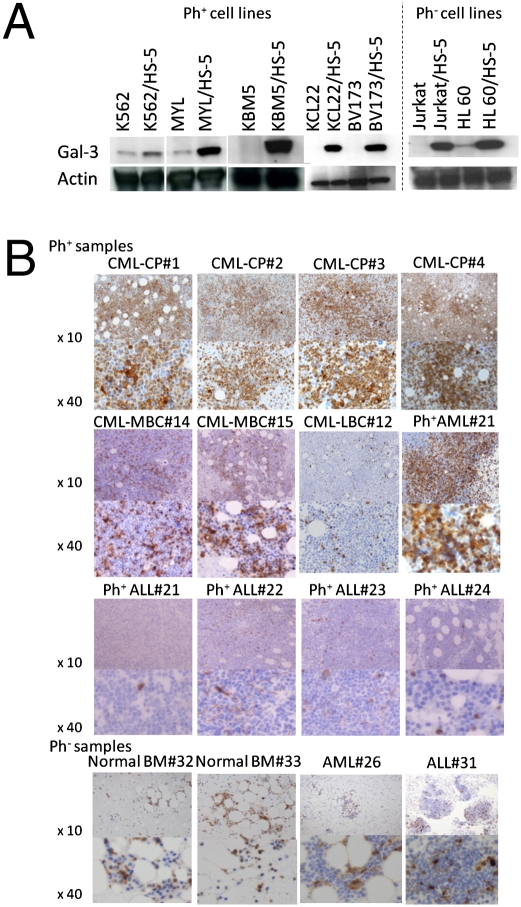
Leukemic cells are supported not only by BMSCs (and their secretion) but also by ECM (13, 32). We first used coculture with HS-5, a BMSC-derived cell line, to examine whether the acquisition of resistance to cell death by TKIs or by genotoxic agents is induced in Ph-positive (Ph+) CML cell lines (MYL, K562, BV173, and KCL22). HS-5, an immortalized human BMSC-derived cell line, potently secretes various hematopoietic growth factors (33). The coculture rendered both MYL cells and K562 cells partly resistant to TKIs, doxorubicin (DOX), cytarabine (CA), etoposide (i.e., VP16), and vincristine (VCR). BV173 cells, which are primarily resistant to induction of cell death by TKIs, did not become more resistant to IM and Das as a result of coculture with HS-5, but they acquired resistance to cell death induced by DOX, CA, VP16, and VCR. KCL22 also acquired resistance to cell death by DOX (Fig. S1A). Ph-negative (Ph−) cell lines and Jurkat T (i.e., Jurkat) and HL60 cells also acquired resistance to cell death by DOX by the coculture with HS-5 (Fig. S1B). We next used microarray-based assays to investigate the changes in gene expression profiles in MYL cells as a result of coculture with HS-5 and adhesion to fibronectin (FN). In MYL with HS-5 or MYL with FN, 902 and 910 genes were up-regulated more than 2.0-fold, respectively, whereas 563 and 550 genes were down-regulated by less than half of their expression levels, respectively, in comparison with levels of control (Fig. S1C and Table S1). Among the 284 genes commonly up-regulated in MYL with HS-5 and MYL with FN, we focused on Gal-3 as one of the candidate mediators of BMME-mediated leukemia proliferation/protection because of its pleiotropic cellular function, the interaction with cell signaling molecules downstream of Bcr-Abl TK (Fig. S1D), and the association with progression of various cancers (27–31). Because the levels of galectin-3 mRNA increased 3.84-fold as a result of coculture with HS-5, and 2.83-fold as a result of adhesion to FN in MYL cells, it was likely that Gal-3 was induced by cell adhesion and was further increased by HS-5–derived soluble factors. The induction of Gal-3 by the coculture with HS-5 was also confirmed at the protein level not only in MYL cells, but also in all leukemic cell lines examined regardless of their Ph status, whereas Gal-3 protein expression was absent or extremely low in normal liquid culture (Fig. 1A), suggesting that Gal-3 is especially inducible in the presence of BMME components in leukemic cells.
Fig. 1.
Gal-3 expression in CML cell lines and primary CML cells. (A) Induction by coculture with HS-5 in CML cell lines. (B) Immunohistochemical staining of Gal-3 in patient-derived BM samples. Data are representative of the results for all patients examined (Table S2). LBC, lymphoid blast crisis; MBC, myeloid blast crisis.
Gal-3 Is Highly Expressed in Primary Treatment-Naive CML Cells in BM.
We next investigated the expression of Gal-3 in BM-derived primary leukemic cells from 25 Ph+ leukemias (20 CMLs and five acute leukemias) and six Ph− patients with acute leukemia. Of the leukemic cells of 20 patients with CML, those of all but one Ph+ patient with blast crisis phase leukemia were positive for Gal-3. Ph+ cells from CML in chronic phase (CP) were especially highly positive for Gal-3 expression. In contrast, the frequency of Gal-3–positive cells from most patients with acute leukemia was as low as that of BM hematopoietic cells from healthy volunteers, regardless of Ph status (Table S2 and Fig. 1B). In normal BM, cells of myeloid/monocytic series, but not of lymphoid or erythroid series, were positive for Gal-3. These results suggest that Gal-3 expression in the BM milieu is more predominant in CML, especially in CML-CP.
Gal-3 Overexpression Promotes Cell Proliferation and Chemotactic Cell Migration and Confers Drug Resistance to Leukemic Cells in Vitro.
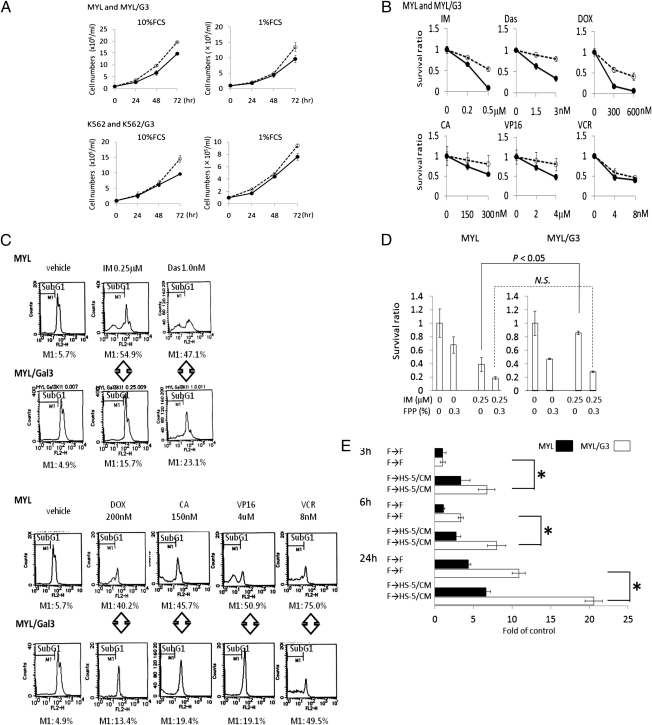
To characterize the role of Gal-3 in CML, we generated Gal-3 stably overexpressing MYL (MYL/G3) and K562 (K562/G3) subcell lines (Fig. S2). Gal-3 overexpression conferred moderately higher in vitro proliferation potency to both cell lines in medium containing 10% FCS as well as in low-nutrient 1% FCS-containing medium (Fig. 2A), whereas the growth of cells transfected with the mock plasmid was not significantly different from that of their respective parental cells. Both MYL/G3 and K562/G3 were less sensitive than their respective parental cells to cell death induced by TKIs as well as by conventional anticancer agents (Fig. 2B and Fig. S3A). Gal-3–overexpressing Ph− Jurkat/G3 cells were also less sensitive to conventional anticancer agents (Fig. S3B). This diminished sensitivity to cell death caused by chemotherapeutic agents was caused by a reduction in apoptosis (Fig. 2C). In contrast, the drug sensitivity of cells transfected with the mock plasmid was not significantly impaired. To confirm that MYL/G3 acquired a drug-resistant phenotype as result of Gal-3 overexpression, we examined the effect of an inhibitor for Gal-3, fractionated citrus pectin powder (FPP) (34), on MYL cells and MYL/G3 cells. MYL and MYL/G3 showed similar sensitivity to cell death induced by FPP, whereas the addition of FPP overcame resistance to IM-induced cell death in MYL/G3 cells (Fig. 2D). Furthermore, the addition of FPP overcame HS-5–induced resistance against IM in MYL cells (Fig. S3C). We also investigated the role of Gal-3 in cell migration of leukemic cells by using conditioned medium (CM) from HS-5 cells (CM/HS-5) as the source of BMSC-derived chemotactic stimuli. CM/HS-5–stimulated cell migration of MYL cells and Gal-3 overexpression further promoted chemotactic and nonchemotactic cell migration in MYL cells (Fig. 2E). These findings revealed that Gal-3 overexpression promotes cell proliferation, multidrug resistance, and cell migration in CML cells.
Fig. 2.
Effects of Gal-3 overexpression on leukemic cell lines. (A) Cell proliferation potency. Solid lines represent parental cells and dotted lines represent Gal-3–overexpressing cells. (B) Cell-killing effects of TKIs and genotoxic agents. The x axis shows the drug concentration and the y axis shows survival cell ratio relative to untreated cells after 48 h treatment. Solid lines represent parental cells and dotted lines represent Gal-3–overexpressing cells. (C) Apoptosis induction determined by DNA content analyses. MYL and MYL/G3 cells were treated for 48 h with the agents indicated. The proportions of subG1 fractions (M1) were assumed to be cells undergoing apoptosis. (D) Gal-3 inhibitor overcomes Gal-3–induced resistance to cell death by IM in MYL cells. Cells were treated with IM and/or FPP for 48 h. N.S., not statistically significant. (E) The effect of Gal-3 on chemotactic cell migration. The number of migrated cells in MYL after 3 h incubation was assumed to be 1.0. Asterisks indicate statistically significant differences (P < 0.05). F, serum-free medium; HS-5/CM, CM of HS-5 cells; F→HS-5/CM, upper chamber supplemented with serum-free medium and lower chamber filled with HS-5/CM. Bars indicate SD.
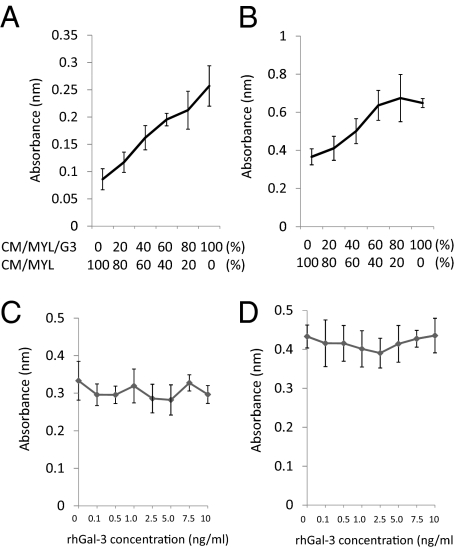
We also examined the involvement of extracellular Gal-3 in the resistance to cell death by chemotherapeutic agents and in the cell migration ability of leukemic cells. Gal-3 concentrations in CM from MYL (CM/MYL), CM from MYL/G3 (CM/MYL/G3), CM from K562 (CM/K562), and CM from K562/G3 (CM/K562/G3) were 0.25 ng/mL, 0.77ng/mL, 0.19 ng/mL, and 9.49 ng/mL, respectively. The addition of recombinant human Gal-3 protein (rhGal-3; ProSci) up to 10.0 ng/mL did not confer CML cell lines more resistance to IM or DOX (Fig. S4A), and did not promote cell migration of leukemic cell (Fig. S4B), indicating that intracellular Gal-3 expression is essential for the higher resistance to apoptosis and the higher cell migration ability of leukemic cells.
CM from Gal-3–Overexpressing CML Cells Contains More Proliferative Factors for Leukemic Cells and BMSCs.
Leukemic cells excrete growth factors, which stimulate the growth of adjacent leukemic cells as well as BM supporting cells via autocrine and paracrine loops, and thereby create a malignant niche (35). To investigate the involvement of Gal-3 in this scenario, MYL cells or HS-5 cells were cultured with media containing various ratios of CM/MYL and CM/MYL/G3 individually. MYL cells and HS-5 cells proliferated more at higher concentrations of CM/MYL/G3 (Fig. 3), indicating that MYL/G3 cells excrete more growth factors for both MYL cells themselves and BMSCs. These findings were the same for K562 and K562/G3 (Fig. S5). In contrast, the addition of rhGal-3 did not enhance the cell proliferation of MYL or HS-5 cells (Fig. 3 C and D), suggesting that an undefined soluble factor other than Gal-3 promotes the growth of leukemic cells and HS-5.
Fig. 3.
CM of Gal-3–overexpressing cells contains more growth-promoting soluble factors. MYL cells (A) or HS-5 cells (B) were grown with mixtures of various concentrations of CM/MYL and CM/MYL/G3. MYL cells (C) or HS-5 cells (D) were grown in complete medium containing various concentrations of rhGal-3 for 96 h. Cell proliferation was determined by means of methyl-thiazol-diphenyl-tetrazolium (MTT) assay. An increasing in concentration of CM/MYL/G3 promoted the cell proliferation of MYL cells and HS-5 cells, whereas the addition of rhGal-3 up to 10 ng/mL did not.
Molecular Sequelae Following Gal-3 Overexpression in Leukemic Cells.
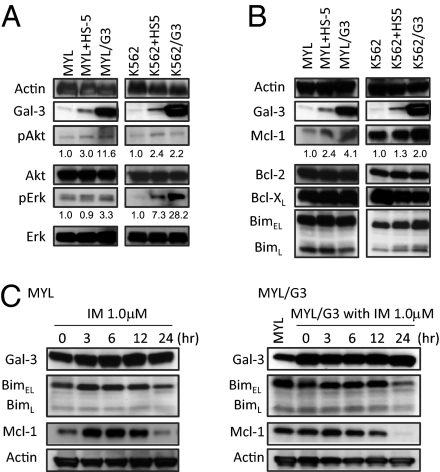
Coculture with HS-5 and enforced Gal-3 overexpression led to the activation of Akt and Erk in MYL and K562 (Fig. 4A). Moreover, coculture with HS-5 and Gal-3 overexpression resulted in the accumulation of Mcl-1, a member of the antiapoptotic Bcl-2 family of proteins, in MYL and K562, as well as a slight increase of BimEL, a proapoptotic BH3-only protein (Fig. 4B). This accumulation of BimEL may be the result of the accumulation of Mcl-1, which binds to BimEL in cytoplasm (36, 37). Although recent studies have identified Gal-3 as the substrate of c-Abl in solid cancers (38, 39), Gal-3 expression was not reduced by TKI in MYL and MYL/G3 (Fig. 4C), indicating that Gal-3 expression does not depend on Bcr-Abl TK activity in CML cells.
Fig. 4.
Western blot analyses. Coculture with HS-5 and enforced Gal-3 overexpression induce phosphorylation of Akt and Erk (A), and causes Mcl-1 (B) to accumulate in MYL and K562. The expression levels of pAkt, Akt, pErk, Erk, Mcl-1, and Actin were calculated by using ImageJ software. The relative ratios of expression levels of pAkt/Akt, pErk/Erk, and Mcl-1/Actin of parental cells in normal cell culture were considered to be 1.0. (C) IM treatment (1.0 μM) for the indicated periods did not reduce Gal-3 in MYL and MYL/G3, whereas it caused accumulation of dephosphorylated BimEL (faster migrated bands) and BimL. Mcl-1 accumulation was observed only when parental MYL cells were treated with IM.
In Vivo Role of Gal-3 in Leukemia.
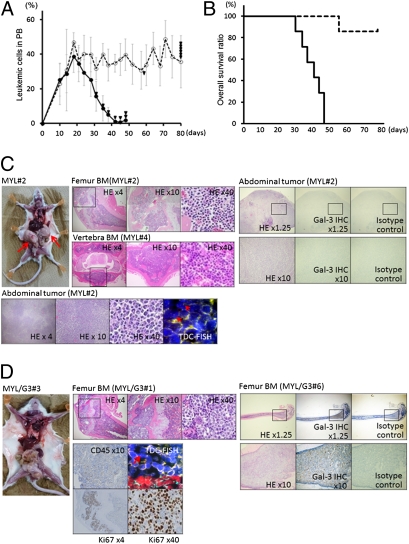
Finally, we examined the in vivo role of Gal-3 in leukemia by studying a mouse CML model transplanted with MYL/mock cells (group A) or MYL/G3 cells (group B). Although transplanted leukemic cells increased in a similar manner in the peripheral blood (PB) of both groups during the first 3 wk, the number of PB leukemic cells of group A mice then gradually decreased, whereas those of group B mice were preserved until death (Fig. 5A). The survival period of group A was significantly shorter than that of group B (P = 0.025; Fig. 5B); namely, all mice of group A died by day 48, whereas only one of seven mice in group B died during the observation period. Surprisingly, the sites of disease involvement at the mice's death showed major differences between the two groups. Most mice from group A showed extensive extramedullary involvement, such as intraabdominal, mediastinal, and/or s.c. tumors isolated from BM, whereas only one of seven mice showed BM involvement at their time of death. In contrast, all mice in group B showed BM involvement, and sometimes outgrew BM, but none exhibited tumors isolated from BM (Fig. 5 C and D, Table 1, and Fig. S6). These findings suggest that Gal-3 overexpression facilitates BM homing and lodgment of CML cells. We also speculate that the reason for the shorter survival of group A mice is that the tumors expanded much faster when leukemic cells had advanced outside the BM, and that this may have had a significantly more deleterious effect on mice in group A than on mice in group B.
Fig. 5.
In vivo role of Gal-3. (A) Percentages of transplanted leukemic cells in peripheral leukocytes of mice transplanted with MYL/mock cells (group A; solid line) and mice transplanted with MYL/G3 cells (group B; dotted line). The x axis shows days after transplantation and the y axis shows means ± SD of percentages of peripheral leukemic cells. Triangles indicate points of mouse deaths. (B) Overall survival periods of group A (solid line) and group B (dotted line). (C and D) Macroscopic and microscopic findings of mice transplanted with MYL/mock cells (C) or MYL/G3 cells (D). Data shown are representative of all mice examined (Table 1). In mice transplanted with MYL/mock cells, extensive extramedullary tumors (arrows) with Ph+ Gal-3–negative leukemic cells [detected by tissue double color (TDC)-FISH and immunohistochemistry (IHC)] were identified in all mice (C). In contrast, none of the mice transplanted with MYL/G3 showed extramedullary involvement, whereas BM was at least partly replaced by Ph+ leukemic cells expressing high level of Gal-3, which were also positive for human CD45 and Ki67 antigens (D). Direct invasion of leukemic cells outside BM was sometimes observed. HE, H&E staining.
Table 1.
Date of mice used in the present study
| Organ involvement at death |
||||||||
| Max. leukemic cells in PB |
BM invasion |
Extramedullary tumor |
||||||
| Mouse no. | Survival, d | % | Day | Femoral | Vertebral | Expansion from BM | Isolated from BM | Cause of death |
| MYL 1 | 31 | 36.8 | 14 | — | — | — | Mediastinum | D |
| MYL 2 | 35 | 40.0 | 18 | — | — | — | Abdominal cavity | D |
| MYL 3 | 38 | 53.6 | 18 | — | — | Lower jaw | s.c. Tissue abdominal cavity | D |
| MYL 4 | 42 | 52.9 | 21 | — | — | — | Abdominal cavity axillary LN | D |
| MYL 5 | 45 | 38.0 | 28 | — | — | — | Abdominal cavity s.c. tissue | D |
| MYL 6 | 48 | 42.6 | 18 | + | + | — | s.c. Tissue abdominal cavity | D |
| MYL 7 | 48 | 42.0 | 21 | — | — | — | s.c. Tissue abdominal cavity, soft tissue around the testis | D |
| MYL/G3 1 | 57 | 73.3 | 14 | + | + | Sacrum | — | B |
| MYL/G3 2 | 80 | 57.7 | 71 | + | + | Sacrum | — | E |
| MYL/G3 3 | 80 | 50.0 | 48 | + | + | — | — | E |
| MYL/G3 4 | 80 | 54.0 | 71 | + | + | Sacrum | — | E |
| MYL/G3 5 | 80 | 48.0 | 18 | + | + | Sacrum, right femur | — | E |
| MYL/G3 6 | 80 | 48.0 | 68 | + | + | — | — | E |
| MYL/G3 7 | 80 | 57.14 | 24 | + | + | — | — | E |
B, Loss of >10% body wight; D, deterioration caused by tumor; E, euthanasia; LN, lymph node; Max.: maximum.
Discussion
The present study demonstrates that Gal-3 was specifically induced when leukemic cells were cultured with BMSCs in vitro, and that Gal-3 is predominantly expressed in CML cells, but not in acute leukemias. These findings prompted us to further investigate BMME-specific roles of Gal-3 in CML. As the results, enforced Gal-3 overexpression caused at least partial resistance to apoptotic induction by TKIs and genotoxic agents. As the levels of drug resistance in Gal-3 gene transferred leukemic cells were similar to those in parental leukemic cell lines cocultured with HS-5, the inducibility of Gal-3 may at least partly explain the underlying molecular mechanisms of BMME-mediated drug resistance. As the molecular sequelae of Gal-3 overexpression, Erk and Akt, which are the essential downstream signaling molecules of Bcr-Abl (40), are activated in CML cells in a Bcr-Abl–independent manner. Simultaneously, Mcl-1 increased as the result of Gal-3 overexpression in CML cells. These results were consistent with those of previous studies showing that BMSC support activates Erk and Akt and increases Mcl-1 (41, 42), and the present study suggested Gal-3 as one of the positive mediators for these processes. Moreover, it has been reported that Gal-3 has an NWGR motif seen in the BH1 domain of Bcl-2 and may promote cell survival by interacting with Bcl-2 (27, 43). Bcl-2 family proteins have been shown to directly regulate cellular fate in the context of Bcr-Abl TK signaling, and Bim is essential for apoptosis by means of the blockade of Bcr-Abl TK signaling (36, 44–46). Because Mcl-1 protects mitochondrial integrity by binding to and keeping BimEL in check, and also inactivates other BH3-only proteins essential for genotoxic damage-induced apoptosis (47), Mcl-1 overexpression induced by Gal-3 may constitute one of the mechanisms for drug resistance of CML cells in BMME. Because Gal-3 expression induced by the BM milieu was not influenced by Bcr-Abl TK activity, Gal-3 induced by BM milieu stimuli may further augment the signaling for leukemia progression in combination with Bcr-Abl TK signaling, and also may maintain downstream pathways active even during treatment with TKIs.
In addition, the present study suggested the model that Gal-3 overexpression in CML cells exerts cell-extrinsic growth-promoting effects on CML cells as well as BMSCs, thereby accelerating the positive feedback mechanisms for leukemia proliferation and maintenance in the BM milieu in CML (Fig. S7), and promotes BM lodgment of CML cells in vivo. Although a number of studies have aimed to establish CML animal models by using xenograft models with human leukemic cells or transgene of bcr-abl into murine hematopoietic cells, most models have failed to recapitulate human CML-CP, which is clinically silent with persistent leukemic cell proliferation in BM and PB. Like the mice in group A in the present experiments, the survival periods of most previous CML models are frequently short as a result of progressive extramedullary involvements with or without BM leukemic lesion (48–51). The underlying molecular mechanism for this difference has remained unverified so far, but BMME-specific induction of Gal-3 expression in leukemic cells may be a clue to help solve this uncovered question. Also, soluble factors excreted by Gal-3–overexpressing CML cells, which promote this positive feedback machinery, are currently under investigation.
With respect to therapeutic applications, Gal-3 overexpression is expected to contribute to the generation of minimal residual disease as a result of the simultaneous promotion of BM lodgment and drug resistance, which makes the association between the expression levels of Gal-3 and the degree of response to TKIs a matter of considerable interest. However, because most patients with CML-CP with high levels of Gal-3 showed optimal response to TKIs (52), we underwrite the hypothesis that Gal-3 is a possible universal target in most patients with CML-CP, but is not a specific target in poor responders to TKIs. On the contrary, Gal-3 expression in leukemic cells in the advanced phase of CML and Ph+ acute lymphoblastic leukemia is less than that of CML-CP; in addition, its expression does not differ significantly at onset and at relapse (Table S3). It is therefore important to verify that the loss of Gal-3 expression is mechanistically involved in disease stage progression and systemic organ dissemination in CML.
In conclusion, the present study disclosed that BMME-induced Gal-3 in CML cells may play an important role in drug resistance and leukemia lodgment in the BM milieu. Molecular-targeted agents against Gal-3, such as GCS-100, actually cause a decrease in Mcl-1 (53). The combined use of such compounds and TKIs is expected to be valuable for overcoming BMME-mediated protection of CML cells.
Materials and Methods
Cell Lines and Generation of Gal-3–Overexpressing Leukemic Cell Sublines.
K562, BV173, KBM5 (American Type Culture Collection), MYL (54), and KCL22 (55) cell liens were established from Ph+ patients with CML. Jurkat T is a human T-cell lymphoblast-like cell line, and HL60 (American Type Culture Collection) was established from cases of acute myelogenous leukemia; both are Ph−. Gal-3–overexpressing subcell lines of MYL, K562, and Jurkat cells were generated by means of transfection of pEF1Galec3.neo plasmid (gift from Fu-Tong Liu, University of California, Davis, CA) (56). MYL and K562 cells were also transfected with a mock pEF1 plasmid empty vector as control, and were designated as MYL/mock and K562/mock, respectively. Following coculture assays, leukemic cells were positively isolated from HS-5 cells by using CD45 Microbeads and MiniMacs Separator (Miltenyi Biotec).
Microarray Analysis and Signal Pathway Analysis.
MYL cells were cultured in normal medium on a noncoated plate as control, on a FN-coated plate, or on a plate preseeded with HS-5 for 48 h. Total RNA was isolated, and gene expression was analyzed with Affymetrix Gene Chip arrays and GeneChip Scanner 3000 (Affymetrix). Array data analysis was carried out with Affymetrix GeneChip operating software, version 1.0., and genes showing at least a 2.0-fold difference in expression levels from control were considered to be positive. For signal pathway analysis, data were also analyzed with the Ingenuity pathway analysis software (Ingenuity Systems).
Mouse Xenografte Model for CML.
Approval was obtained from the institutional review board at Kyoto University Hospital for a study using mice. Fourteen male NOD/SCID mice at 6 wk of age were sublethally irradiated (2 Gy), and 1.0 × 106 MYL/mock cells (group A) or 1.0 × 106 MYL/G3 cells (group B) were transplanted i.v. via their tail veins into seven mice each. Body weight and the percentage of leukemic cells in PB were monitored at least twice per week until day 80. For survival analysis, death was determined by spontaneous death or elective killing as a result of pain, the loss of more than 10% of maximum body weight of the individual mouse, or suffering or dying according to established criteria. All survived mice were subjected to euthanasia on day 80. We performed a macroscopic as well as microscopic analysis of BM of femoral bone and vertebra, and also of the tumors in each mouse at death. Tissue dual-color FISH was performed as previously described (57). The data shown are representative of three independent experiments.
Supplementary Material
Acknowledgments
We appreciate the scientific support of Drs. Y. Kamitsuji and E. Kawata and Mss. K. Mizushima, N. Sakamoto, and A. Kazami. This work was partly supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (to J.K. and M.T.) and by grants from the Sagawa Foundation for Promotion of Cancer Research and the Kanae Foundation for the Promotion of Medical Science (to J.K.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1111138108/-/DCSupplemental.
References
- 1.Jabbour E, Fava C, Kantarjian H. Advances in the biology and therapy of patients with chronic myeloid leukaemia. Best Pract Res Clin Haematol. 2009;22:395–407. doi: 10.1016/j.beha.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 2.Santos FP, Ravandi F. Advances in treatment of chronic myelogenous leukemia—new treatment options with tyrosine kinase inhibitors. Leuk Lymphoma. 2009;50(suppl 2):16–26. doi: 10.3109/10428190903383427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Donato NJ, et al. Imatinib mesylate resistance through BCR-ABL independence in chronic myelogenous leukemia. Cancer Res. 2004;64:672–677. doi: 10.1158/0008-5472.can-03-1484. [DOI] [PubMed] [Google Scholar]
- 4.Gorre ME, et al. Clinical resistance to STI-571 cancer therapy caused by BCR-ABL gene mutation or amplification. Science. 2001;293:876–880. doi: 10.1126/science.1062538. [DOI] [PubMed] [Google Scholar]
- 5.Hu Y, et al. Requirement of Src kinases Lyn, Hck and Fgr for BCR-ABL1-induced B-lymphoblastic leukemia but not chronic myeloid leukemia. Nat Genet. 2004;36:453–461. doi: 10.1038/ng1343. [DOI] [PubMed] [Google Scholar]
- 6.La Rosée P, Deininger MW. Resistance to imatinib: Mutations and beyond. Semin Hematol. 2010;47:335–343. doi: 10.1053/j.seminhematol.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 7.Milojkovic D, Apperley J. Mechanisms of resistance to imatinib and second-generation tyrosine inhibitors in chronic myeloid leukemia. Clin Cancer Res. 2009;15:7519–7527. doi: 10.1158/1078-0432.CCR-09-1068. [DOI] [PubMed] [Google Scholar]
- 8.Perrotti D, Jamieson C, Goldman J, Skorski T. Chronic myeloid leukemia: Mechanisms of blastic transformation. J Clin Invest. 2010;120:2254–2264. doi: 10.1172/JCI41246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Soverini S, et al. ABL mutations in late chronic phase chronic myeloid leukemia patients with up-front cytogenetic resistance to imatinib are associated with a greater likelihood of progression to blast crisis and shorter survival: A study by the GIMEMA Working Party on Chronic Myeloid Leukemia. J Clin Oncol. 2005;23:4100–4109. doi: 10.1200/JCO.2005.05.531. [DOI] [PubMed] [Google Scholar]
- 10.Damiano JS, Hazlehurst LA, Dalton WS. Cell adhesion-mediated drug resistance (CAM-DR) protects the K562 chronic myelogenous leukemia cell line from apoptosis induced by BCR/ABL inhibition, cytotoxic drugs, and gamma-irradiation. Leukemia. 2001;15:1232–1239. doi: 10.1038/sj.leu.2402179. [DOI] [PubMed] [Google Scholar]
- 11.Gregory MA, et al. Wnt/Ca2+/NFAT signaling maintains survival of Ph+ leukemia cells upon inhibition of Bcr-Abl. Cancer Cell. 2010;18:74–87. doi: 10.1016/j.ccr.2010.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krämer A, et al. Adhesion to fibronectin stimulates proliferation of wild-type and bcr/abl-transfected murine hematopoietic cells. Proc Natl Acad Sci USA. 1999;96:2087–2092. doi: 10.1073/pnas.96.5.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meads MB, Gatenby RA, Dalton WS. Environment-mediated drug resistance: A major contributor to minimal residual disease. Nat Rev Cancer. 2009;9:665–674. doi: 10.1038/nrc2714. [DOI] [PubMed] [Google Scholar]
- 14.Takeuchi M, et al. Glyoxalase-I is a novel target against Bcr-Abl+ leukemic cells acquiring stem-like characteristics in a hypoxic environment. Cell Death Differ. 2010;17:1211–1220. doi: 10.1038/cdd.2010.6. [DOI] [PubMed] [Google Scholar]
- 15.Vianello F, et al. Bone marrow mesenchymal stromal cells non-selectively protect chronic myeloid leukemia cells from imatinib-induced apoptosis via the CXCR4/CXCL12 axis. Haematologica. 2010;95:1081–1089. doi: 10.3324/haematol.2009.017178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Y, et al. Adaptive secretion of granulocyte-macrophage colony-stimulating factor (GM-CSF) mediates imatinib and nilotinib resistance in BCR/ABL+ progenitors via JAK-2/STAT-5 pathway activation. Blood. 2007;109:2147–2155. doi: 10.1182/blood-2006-08-040022. [DOI] [PubMed] [Google Scholar]
- 17.Weisberg E, et al. Stromal-mediated protection of tyrosine kinase inhibitor-treated BCR-ABL-expressing leukemia cells. Mol Cancer Ther. 2008;7:1121–1129. doi: 10.1158/1535-7163.MCT-07-2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williams RT, den Besten W, Sherr CJ. Cytokine-dependent imatinib resistance in mouse BCR-ABL+, Arf-null lymphoblastic leukemia. Genes Dev. 2007;21:2283–2287. doi: 10.1101/gad.1588607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yokota A, et al. Osteoclasts are involved in the maintenance of dormant leukemic cells. Leuk Res. 2010;34:793–799. doi: 10.1016/j.leukres.2009.08.034. [DOI] [PubMed] [Google Scholar]
- 20.Bixby D, Talpaz M. Mechanisms of resistance to tyrosine kinase inhibitors in chronic myeloid leukemia and recent therapeutic strategies to overcome resistance. Hematology (Am Soc Hematol Educ Program) 2009;2009:461–476. doi: 10.1182/asheducation-2009.1.461. [DOI] [PubMed] [Google Scholar]
- 21.Cilloni D, Messa E, Rotolo A, Saglio G. Emerging drugs for chronic myeloid leukemia. Expert Opin Emerg Drugs. 2010;15:175–184. doi: 10.1517/14728211003621220. [DOI] [PubMed] [Google Scholar]
- 22.Krześlak A, Lipińska A. Galectin-3 as a multifunctional protein. Cell Mol Biol Lett. 2004;9:305–328. [PubMed] [Google Scholar]
- 23.Matarrese P, et al. Galectin-3 overexpression protects from apoptosis by improving cell adhesion properties. Int J Cancer. 2000;85:545–554. [PubMed] [Google Scholar]
- 24.Matarrese P, et al. Galectin-3 overexpression protects from cell damage and death by influencing mitochondrial homeostasis. FEBS Lett. 2000;473:311–315. doi: 10.1016/s0014-5793(00)01547-7. [DOI] [PubMed] [Google Scholar]
- 25.Nangia-Makker P, Nakahara S, Hogan V, Raz A. Galectin-3 in apoptosis, a novel therapeutic target. J Bioenerg Biomembr. 2007;39:79–84. doi: 10.1007/s10863-006-9063-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thijssen VL, Poirier F, Baum LG, Griffioen AW. Galectins in the tumor endothelium: opportunities for combined cancer therapy. Blood. 2007;110:2819–2827. doi: 10.1182/blood-2007-03-077792. [DOI] [PubMed] [Google Scholar]
- 27.Fukumori T, Kanayama HO, Raz A. The role of galectin-3 in cancer drug resistance. Drug Resist Updat. 2007;10:101–108. doi: 10.1016/j.drup.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim SJ, et al. Increased serum 90K and Galectin-3 expression are associated with advanced stage and a worse prognosis in diffuse large B-cell lymphomas. Acta Haematol. 2008;120:211–216. doi: 10.1159/000193223. [DOI] [PubMed] [Google Scholar]
- 29.Saussez S, Camby I, Toubeau G, Kiss R. Galectins as modulators of tumor progression in head and neck squamous cell carcinomas. Head Neck. 2007;29:874–884. doi: 10.1002/hed.20559. [DOI] [PubMed] [Google Scholar]
- 30.Shekhar MP, Nangia-Makker P, Tait L, Miller F, Raz A. Alterations in galectin-3 expression and distribution correlate with breast cancer progression: Functional analysis of galectin-3 in breast epithelial-endothelial interactions. Am J Pathol. 2004;165:1931–1941. doi: 10.1016/S0002-9440(10)63245-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Y, et al. Regulation of prostate cancer progression by galectin-3. Am J Pathol. 2009;174:1515–1523. doi: 10.2353/ajpath.2009.080816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nair RR, Tolentino J, Hazlehurst LA. The bone marrow microenvironment as a sanctuary for minimal residual disease in CML. Biochem Pharmacol. 2010;80:602–612. doi: 10.1016/j.bcp.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roecklein BA, Torok-Storb B. Functionally distinct human marrow stromal cell lines immortalized by transduction with the human papilloma virus E6/E7 genes. Blood. 1995;85:997–1005. [PubMed] [Google Scholar]
- 34.Jackson CL, et al. Pectin induces apoptosis in human prostate cancer cells: correlation of apoptotic function with pectin structure. Glycobiology. 2007;17:805–819. doi: 10.1093/glycob/cwm054. [DOI] [PubMed] [Google Scholar]
- 35.Colmone A, et al. Leukemic cells create bone marrow niches that disrupt the behavior of normal hematopoietic progenitor cells. Science. 2008;322:1861–1865. doi: 10.1126/science.1164390. [DOI] [PubMed] [Google Scholar]
- 36.Kuroda J, Taniwaki M. Involvement of BH3-only proteins in hematologic malignancies. Crit Rev Oncol Hematol. 2009;71:89–101. doi: 10.1016/j.critrevonc.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 37.Wuillème-Toumi S, et al. Reciprocal protection of Mcl-1 and Bim from ubiquitin-proteasome degradation. Biochem Biophys Res Commun. 2007;361:865–869. doi: 10.1016/j.bbrc.2007.07.070. [DOI] [PubMed] [Google Scholar]
- 38.Balan V, Nangia-Makker P, Jung YS, Wang Y, Raz A. Galectin-3: A novel substrate for c-Abl kinase. Biochim Biophys Acta. 2010;1803:1198–1205. doi: 10.1016/j.bbamcr.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li X, et al. c-Abl and Arg tyrosine kinases regulate lysosomal degradation of the oncoprotein Galectin-3. Cell Death Differ. 2010;17:1277–1287. doi: 10.1038/cdd.2010.8. [DOI] [PubMed] [Google Scholar]
- 40.McCubrey JA, et al. Targeting survival cascades induced by activation of Ras/Raf/MEK/ERK, PI3K/PTEN/Akt/mTOR and Jak/STAT pathways for effective leukemia therapy. Leukemia. 2008;22:708–722. doi: 10.1038/leu.2008.27. [DOI] [PubMed] [Google Scholar]
- 41.McMillin DW, et al. Tumor cell-specific bioluminescence platform to identify stroma-induced changes to anticancer drug activity. Nat Med. 2010;16:483–489. doi: 10.1038/nm.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Balakrishnan K, Burger JA, Wierda WG, Gandhi V. AT-101 induces apoptosis in CLL B cells and overcomes stromal cell-mediated Mcl-1 induction and drug resistance. Blood. 2009;113:149–153. doi: 10.1182/blood-2008-02-138560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Akahani S, Nangia-Makker P, Inohara H, Kim HR, Raz A. Galectin-3: a novel antiapoptotic molecule with a functional BH1 (NWGR) domain of Bcl-2 family. Cancer Res. 1997;57:5272–5276. [PubMed] [Google Scholar]
- 44.Kuroda J, et al. Bim and Bad mediate imatinib-induced killing of Bcr/Abl+ leukemic cells, and resistance due to their loss is overcome by a BH3 mimetic. Proc Natl Acad Sci USA. 2006;103:14907–14912. doi: 10.1073/pnas.0606176103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kuroda J, et al. Apoptosis-based dual molecular targeting by INNO-406, a second-generation Bcr-Abl inhibitor, and ABT-737, an inhibitor of antiapoptotic Bcl-2 proteins, against Bcr-Abl-positive leukemia. Cell Death Differ. 2007;14:1667–1677. doi: 10.1038/sj.cdd.4402168. [DOI] [PubMed] [Google Scholar]
- 46.Shah NP, et al. Transient potent BCR-ABL inhibition is sufficient to commit chronic myeloid leukemia cells irreversibly to apoptosis. Cancer Cell. 2008;14:485–493. doi: 10.1016/j.ccr.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 47.Villunger A, et al. p53- and drug-induced apoptotic responses mediated by BH3-only proteins puma and noxa. Science. 2003;302:1036–1038. doi: 10.1126/science.1090072. [DOI] [PubMed] [Google Scholar]
- 48.Ilaria RL., Jr Animal models of chronic myelogenous leukemia. Hematol Oncol Clin North Am. 2004;18:525–543, vii. doi: 10.1016/j.hoc.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 49.Kuroda J, et al. The third-generation bisphosphonate zoledronate synergistically augments the anti-Ph+ leukemia activity of imatinib mesylate. Blood. 2003;102:2229–2235. doi: 10.1182/blood-2003-01-0305. [DOI] [PubMed] [Google Scholar]
- 50.Ren R. The molecular mechanism of chronic myelogenous leukemia and its therapeutic implications: studies in a murine model. Oncogene. 2002;21:8629–8642. doi: 10.1038/sj.onc.1206090. [DOI] [PubMed] [Google Scholar]
- 51.Van Etten RA. Models of chronic myeloid leukemia. Curr Oncol Rep. 2001;3:228–237. doi: 10.1007/s11912-001-0055-y. [DOI] [PubMed] [Google Scholar]
- 52.Baccarani M, et al. European LeukemiaNet Evolving concepts in the management of chronic myeloid leukemia: Recommendations from an expert panel on behalf of the European LeukemiaNet. Blood. 2006;108:1809–1820. doi: 10.1182/blood-2006-02-005686. [DOI] [PubMed] [Google Scholar]
- 53.Streetly MJ, et al. GCS-100, a novel galectin-3 antagonist, modulates MCL-1, NOXA, and cell cycle to induce myeloma cell death. Blood. 2010;115:3939–3948. doi: 10.1182/blood-2009-10-251660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ito T, Tanaka H, Kimura A. Establishment and characterization of a novel imatinib-sensitive chronic myeloid leukemia cell line MYL, and an imatinib-resistant subline MYL-R showing overexpression of Lyn. Eur J Haematol. 2007;78:417–431. doi: 10.1111/j.1600-0609.2007.00835.x. [DOI] [PubMed] [Google Scholar]
- 55.Kubonishi I, Miyoshi I. Establishment of a Ph1 chromosome-positive cell line from chronic myelogenous leukemia in blast crisis. Int J Cell Cloning. 1983;1:105–117. doi: 10.1002/stem.5530010205. [DOI] [PubMed] [Google Scholar]
- 56.Yang RY, Hsu DK, Liu FT. Expression of galectin-3 modulates T-cell growth and apoptosis. Proc Natl Acad Sci USA. 1996;93:6737–6742. doi: 10.1073/pnas.93.13.6737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Matsumoto Y, et al. Detection of t(14;18) in follicular lymphoma by dual-color fluorescence in situ hybridization on paraffin-embedded tissue sections. Cancer Genet Cytogenet. 2004;150:22–26. doi: 10.1016/j.cancergencyto.2003.08.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.