Abstract
Invariant natural killer T cell (NKT) cells (iNKT cells) produce both T-helper 1 (Th1) and T-helper 2 cytokines in response to α-Galactosylceramide (α-GalCer) stimulation and are thought to be the important effectors in the regulation of both innate and adaptive immunity involved in autoimmune disorders, microbial infections, and cancers. However, the anticancer effects of α-GalCer were limited in early clinical trial. In this study, several analogs of α-GalCer, containing phenyl groups in the lipid tails were found to stimulate murine and human iNKT cells to secrete Th1-skewed cytokines and exhibit greater anticancer efficacy in mice than α-GalCer. We explored the possibility of different Vβ usages of murine Vα14 iNKT or human Vα24 iNKT cells, accounting for differential cytokine responses. However, T-cell receptor Vβ analysis revealed no significant differences in Vβ usages by α-GalCer and these phenyl glycolipid analogs. On the other hand, these phenyl glycolipids showed greater binding avidity and stability for iNKT T-cell receptor when complexed with CD1d. These findings suggest that CD1d–phenyl glycolipid complexes may interact with the same population of iNKT cells but with higher avidity and stability to drive Th1 polarization. Thus, this study provides a key to the rational design of Th1 biased CD1d reactive glycolipids in the future.
Keywords: immune-modulating activity, structure, interaction, cancer immunotherapy
Invariant natural killer T cell (NKT) cells (iNKT cells) are characterized by the utilization of the invariant T-cell receptor (TCR)-α chain (Vα14 in mice and Vα24 in humans) and the coexpression of CD161 antigen (NK cell marker NK1.1 in mice and NKR-P1A in humans) (1–4). The glycolipid antigen α-Galactosylceramide (α-GalCer) presented by CD1d molecule could stimulate iNKT cells to rapidly produce abundant T-helper 1 (Th1) (e.g., IFN-γ) and T-helper 2 (Th2) cytokines (e.g., IL-4, IL-10) along with chemokines (5–9). As for tumor immunity, Th1 cytokines are associated with protective responses whereas Th2 cytokines are associated with suppression of tumor immunity (10). Thus, it was not surprising that the phase I clinical trial of α-GalCer showed meager antitumor responses, perhaps as a consequence of counteraction of Th1 cytokines by the Th2 cytokines (11–13). It has been reported that α-GalCer analogs with higher binding affinity for CD1d induced more Th1-polarized immune response (14) and analogs with the phenyl ring on the acyl chain displayed better anticancer activity than α-GalCer (15). To account for the Th1 biased immune modulatory activities of phenyl analogs, we explored the structure-activity relationships between iNKT TCR and CD1d-glycolipid complex to examine the following two possibilities: The first possibility was that each glycolipid might activate a particular repertoire of iNKT cells bearing specific beta chain. It has been shown that differential Vβ usages could contribute to the recognition of different lipid antigens in mice. For example, iNKT cells bearing Vβ7 preferentially recognized iGb3 (16, 17), whereas those bearing Vβ8.1/8.2 recognized α-GalCer (18). In humans, Vα24 iNKT cells could pair with other Vβ chains besides Vβ11 to generate CD1d-reactive TCRs (19). The second possibility was that glycolipids loaded onto CD1d would bind to iNKT cells having identical alpha and beta chains but differ in their binding affinity/avidity or stability. It was shown that the iNKT TCR did not have direct contact with the lipid tails of α-GalCer based on the crystal structure of CD1d–α-GalCer–iNKT TCR in mice and in humans (20, 21). Nevertheless, a series of analogs with the same glycan head as α-GalCer but with different chain length or presence of double bond on either of the two lipid tails would differ in the binding affinity and dissociation rate for human iNKT TCR when complexed with human CD1d (hCD1d) molecule (22).
In this study, we demonstrated that our phenyl analogs were recognized mostly by Vβ8.1/8.2 + Vα14iNKT cells in mice and by Vβ11 + Vα24iNKT cells in humans as was α-GalCer. However, these CD1d–phenyl glycolipid complexes have better binding avidity and stability for iNKT cells than α-GalCer. Understanding the mechanisms involved in differential immune-modulating activities should facilitate the design of glycolipids with a desired Th1/ Th2 polarity in the future.
Results
Cytokines Induced by Phenyl Glycolipids in Vitro in Murine Vα14 iNKT Cells and in Vivo.
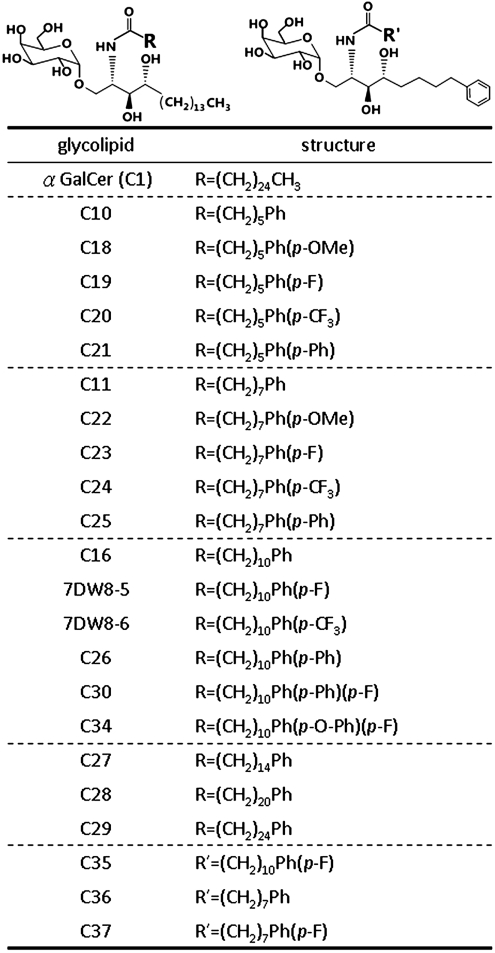
Previously, 16 α-GalCer analogs with various modifications on the acyl or sphingosine chain were synthesized. Among them, three analogs with phenyl group on the acyl chain (C10, C11, and C16) displayed greater induction of Th1-biased cytokines and anticancer effects than α-GalCer (15, 23). Based on these results, glycolipids with further modifications on phenyl group and varying carbon chain lengths were synthesized (Fig. 1). Among them, 17 glycolipids studied by Li et al. suggested that the 7DW8-5 had the greatest capacity for induction of IFN-γ production in human NKT cells and binding to CD1d (24). In this study, five glycolipids (C34, C30, C35, C36, and C37) were also evaluated and compared to 17 previously reported phenyl glycolipids. At first, the immune-modulating activities of phenyl glycolipids were screened by in vitro assay of IL-2 secretion from Vα14 iNKT hybridoma upon incubation with 100 ng/mL of various phenyl glycolipids. As shown in Fig. S1A, 13 of 18 compounds tested induced greater IL-2 secretion by three- to ninefold than aGerCer. Among those glycolipids with single phenyl group on the acyl chain, the longer the acyl chain, the lower the secretion of IL-2 was noted (C27 ∼ C29 < C16 < C11 < C10). The addition of -OMe (C18 and C22), -F (C19, C23, and 7DW8-5), and -CF3 (C20, C24, and 7DW8-6) on phenyl groups of glycolipids with six or eight carbon chain lengths did not affect cytokine response when compared to the phenyl group only compounds (C10, C11). On the other hand, especially with -F addition, similar modification on those with 11 carbon chain length significantly enhanced IL-2 secretion as compared to the parent compound, C16. In contrast, modification with two phenyl rings on the acyl chain (C21, C25, and C26) dampened the IL-2 secretion as compared to those with single phenyl ring (C10, C11, and C16, respectively). Surprisingly, the compound with Ph-O-Ph-F group (C34), induced significantly more IL-2 secretion than C26 with Ph-Ph group only. To differentiate the contribution of oxygen or fluoride addition to C34 activity, C30 (Ph-Ph-F) was synthesized and evaluated for cytokine induction in vivo. Mouse serum was harvested at 2 and 18 h after i.v. injection of glycolipids and the level of cytokines was examined by Luminex system. The production of IL-4 and IFN-γ was significantly higher in C34-treated mice than C30-treated mice (Fig. S1B). Notably, C34 was more Th1-skewed with far less IL-4 secretion but similar extent of IFN-γ induction as compared to C1. In addition, fluorescence activated cell sorter (FACS) analysis showed that C34 induced greater expansion of NKT cells (2.7 ± 0.3-fold) than C30 (1.8 ± 0.4-fold, relative to control) on day three after i.v injection at 0.1 μg/mouse. These data suggested that the oxygen linkage between two phenyl groups is important for the activity of C34. We next evaluated the immune-modulating activities of those glycolipids with single phenyl group added to both the acyl chain and sphigosine chain (C35, C36, and C37) in mice. Neither IFN-γ nor IL-4 secretion was induced in the mouse serum (Fig. S1C), and iNKT cells were neither activated nor expanded as revealed by the FACS analysis on day three after i.v injection. After these initial screening, six phenyl analogs were chosen for more detailed studies.
Fig. 1.
The structure of α-GalCer analogs. The analogs of α-GalCer (C1) are separated into two categories: those with one or two phenyl ring(s) on the acyl chain only and those with one phenyl ring on each lipid tail (C35, C36 and C37). The glycolipids with one acyl phenyl ring without further modifications were arranged in the order of their chain length: C10, C11, C16 and then C27 ∼ C29.
Phenyl Glycolipid Induced Cytokines/Chemokines Production Was CD1d-Dependent.
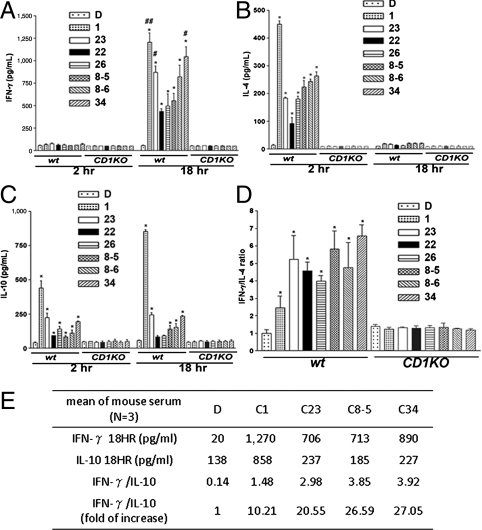
To evaluate the potency of glycolipids in inducing Th1/Th2 cytokines, BALB/c mice were i.v injected with 0.1 μg of C22, C23, C26, 7DW8-5 (8-5), 7DW8-6 (8-6), and C34, and serum was harvested at 2 and 18 h later. The secretion of IFN-γ, IL-10, and IL-12p70 rose at 18 h, whereas IL-4, MCP-1, KC peaked at 2 h (Fig. 2 and Fig. S2). C1, C23, and C34 induced significantly more Th1 cytokines (IFN-γ and IL-12p70) secretion than 7DW8-5 (Fig. 2A and Fig. S2A). Notwithstanding, C1 induced the highest amounts of Th2 cytokines (IL-4 and IL-10) (Fig. 2 B and C). When expressed as the ratio of IFN-γ/IL-4 or IFN-γ/IL-10, C34 was most Th1-driven, followed by 7DW8-5 and C23 (Fig. 2 D and E). Moreover, C34, C23 and 7DW8-5 also induced higher levels of chemokines (MCP-1 and KC) than other phenyl glycolipids (Fig. S2 B and C). No secretions of cytokines/chemokines were observed in CD1d-knockout mice after injection of these glycolipids, indicating that NKT cells activation by phenyl glycolipids was CD1d-dependent.
Fig. 2.
Phenyl glycolipids induced cytokines secretion was CD1d dependent in mice. Wild-type and CD1d knockout BALB/c mice were i.v. injected with indicated glycolipids (0.1 μg/mouse) or vehicle. Sera were collected at 2 and 18 h postinjection for measurement of IFN-γ (A), IL-4 (B), and IL-10 (C) as described in Materials and Methods. The Ratios of IFN-γ over IL-4 (D) and IFN-γ over IL-10 (E), normalized to DMSO control, were calculated. Assays were performed in triplicates and data were presented as mean ± SD. The symbol * represents, p < 0.05, compared with DMSO; #, p < 0.05; and ##, p < 0.01, compared with 7DW8-5 using a two-tailed Student t test.
Anticancer Efficacy of Phenyl Glycolipids.
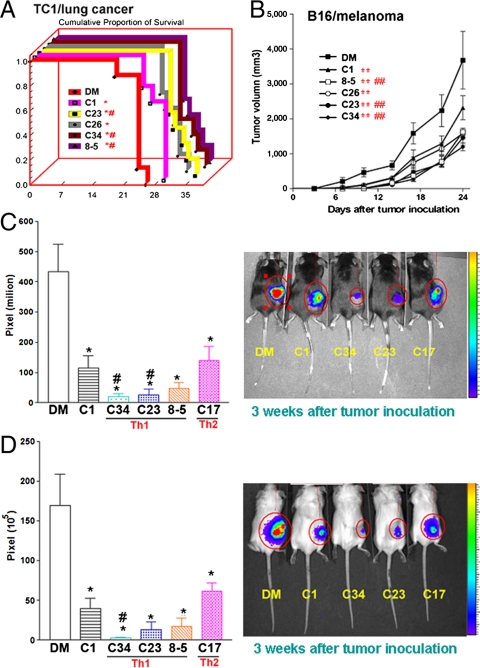
To examine the anticancer efficacy of phenyl glycolipids, mice were inoculated with lung cancer (TC1), breast cancer (4T1), and melanoma (B16), and i.v. injected with glycolipids (0.1 μg/mouse) weekly for 4 wk. The survival of lung tumor bearing mice was significantly prolonged after treatment with C1, C23, C26, C34, and 7DW8-5, as compared to the control group. Moreover, three phenyl glycolipids (C23, C34, and 7DW8-5) displayed better anticancer effects than C1 (Fig. 3A). In addition, these glycolipids showed similar ranking in their anticancer effects on suppressing tumor growth in melanoma-bearing mice (Fig. 3B). To further monitor the tumor growth in vivo, luciferase-containing TC1 and 4T1 cell lines were established. As shown in Fig. 3 C and D, the intensity of bioluminescence was significantly decreased in mice treated with C1, C34, C23, 7DW8-5, and C17 as compared to vehicle control mice, indicating the inhibition of tumor growth by these glycolipids. Among these phenyl glycolipids, C34 exhibited significantly greater anticancer efficacy than C1 in both cancer models (p < 0.05).
Fig. 3.
Anticancer efficacy of phenyl glycolipids. Three syngenic murine cancer models, lung cancer, melanoma, and breast cancer were used to evaluate the anticancer efficacy of phenyl glycolipids. (A) C57BL/6 mice (n = 5) were injected intravenously with lung cancer cells (TC1) and the indicated glycolipids (0.1 μg/mouse) or vehicle (0.1% DMSO) once a week for 4 wk. Mice survival was monitored and Kaplan–Meier survival curve was shown. The symbol * represents p < 0.05, compared with DMSO; and #, p < 0.05, compared with C1, were analyzed by the log-rank test. (B–D) C57BL/6 mice (n = 5) were s.c. inoculated with B16 melanoma cells (B) or TC1-GFP-Luciferase lung cancer cells (C), and BALB/c mice (n = 5) were s.c. inoculated with 4T1-GFP-Luciferase breast cancer cells (D). Three days later, mice were i.v. injected with indicated glycolipids (0.1 μg/mouse) or vehicle once a week for 4 wk. (B) The melanoma tumor growth was measured by caliper every 3 d for 24 d. The statistical significance among different groups was calculated using the generalized linear model by the SPSS software. The symbol ** represents p < 0.01, compared with DMSO; and ##, p < 0.01, compared with C1. The in vivo tumor growth of lung cancer (C) or breast cancer (D) was detected by IVIS system and the tumor growth was presented as pixel of bioluminescence (C and D, Left) and image (C and D, Right). The symbol * represents p < 0.05, compared with DMSO; and #, p < 0.05, compared with C1 (one-tailed Student t test).
TCR β Chain Usage of Vα14 iNKT Cells upon Phenyl Glycolipids Stimulation.
It has been reported that Vβ8.2 + iNKT cells bear higher binding avidity for mouse CD1d (mCD1d)-C1 or mCD1d-OCH complex than Vβ7 + iNKT cells (18, 25). To account for differential cytokine response induced by phenyl glycolipids, we explored the possibility of different Vβ usages of Vα14 iNKT cells. The mouse splenocytes were stimulated with phenyl glycolipids in vitro for 3 d, and the percentage of various Vβ chains in NK1.1 + iNKT cells was analyzed by FACS (Fig. S3A). Majority (> 60%) of the iNKT cells expressed Vβ8.1/8.2, and the percentage increased slightly upon stimulation with C1, C23 and C34, but not 7DW8-5, which was comparable to vehicle control. Vβ7 + iNKT cells that represented a minor population (< 10%) also increased after stimulation with all four glycolipids. These results demonstrated that the Vβ8.1/8.2 was the major beta chain used by iNKT cells for the recognition of phenyl glycolipids and C1.
Binding Avidity and Stability of mCD1d-Phenyl Glycolipids Complex with Vβ8.2 + Vα14iNKT Cells.
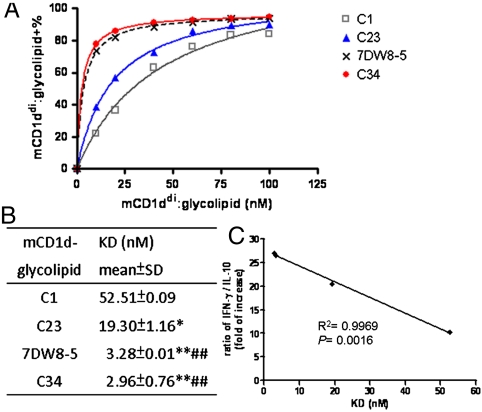
The basis for differential capacity of phenyl glycolipids in regulating secretion of cytokines/chemokines was investigated further by measuring the binding strengths between mCD1ddi–glycolipid complexes and iNKT cells. Various concentrations of mCD1ddi–glycolipid complexes were incubated with fixed amount of Vβ8.2 + Vα14iNKT hybridoma and the level of bound complexes at indicated concentration was analyzed by flow cytometry (Fig. 4A). The equilibrium dissociation constant (KD) of all mCD1ddi-glycolipid complexes with iNKT cells was determined by Scatchard transformation of the plot using Graphpad Prism software. As summarized in Fig. 4B, 7DW8-5 (3.28 ± 0.01 nM), and C34 (2.96 ± 0.76 nM) displayed lower KD values and hence higher binding strengths, for Vα14 iNKT cells than C1 (52.51 ± 0.09 nM) and C23 (19.30 ± 1.16 nM). Furthermore, an inverse correlation was noted between the KD values of these glycolipids and their ratios of IFN-γ/IL-10 induction, with R2 = 0.9969, p = 0.0016, (Fig. 4C), suggesting that the stronger binding strength of mCD1d–glycolipid complex with Vα14 iNKT cells might contribute to the greater secretion of Th1-skewed cytokines. Moreover, the rate of dissociation of CD1d–glycolipid complex from iNKT TCR could affect the duration of its interaction with TCR. To determine the half-life of ternary interaction, the decay of the percentage staining for dimer on iNKT cells was measured so as to assess the binding stability of the interaction between the dimer complexes and iNKT TCRs, as described previously (26). The mCD1d–glycolipid complexes were incubated with Vβ8.2 + Vα14iNKT hybridoma cells, the dissociated complexes were washed away at the indicated time points, and the remaining bound complexes were analyzed by FACS (Fig. S4). The half lives (in minutes) of CD1d dimer loaded with 7DW8-5 (215.8 ± 1.6), C23 (215.3 ± 10.1), or C34 (247.2 ± 10) were significantly longer than mCD1d-C1 (179.4 ± 94), p < 0.05. Taken together, the mCD1d dimer loaded with C23, 7DW8-5 or C34 exerted better binding avidity and stability for TCR on iNKT cells than mCD1d-C1. These results suggested that both the strength and the duration of interaction between CD1d–glycolipid complex and NKT cells might play a key role in modulating the production of cytokines/chemokines and the anticancer potency.
Fig. 4.
Binding avidity of murine CD1d-phenyl glycolipid complex with murine iNKT hybridoma cells. DN3A4-1.2 Vα14 iNKT hybridoma cells were incubated with various concentrations of indicated dimeric mCD1d-glycolipid complexes for 1 h at 4 °C, followed by FACS analysis. (A) The relationship between the percentage of binding and the concentration of CD1ddi-glycolipids complex was plotted. Data was representative of two independent experiments. (B) KD values were calculated from Scatchard transformation of the plot above. The symbol * represents p < 0.05; **, p < 0.01, compared with C1; and ##, p < 0.01, compared with C23, analyzed by a Student t test. (C) The correlation between cytokine secretion and binding avidity of mCD1d–glycolipid complexes for iNKT cells. Linear regression model was performed to analyze the ratio of IFN-γ over IL-10 (Fig. 2E) and the KD values of indicated glycolipids.
Cytokine Productions by Human Naïve iNKT Cells in Response to Phenyl Glycolipids.
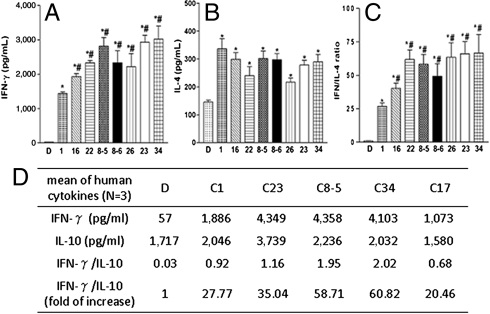
Next, we analyzed the effects of phenyl glycolipids on cytokine production by human NKT cells. Human naïve Vα24 iNKT cells were cultured with autologous immature CD14+ dendritic cells pulsed with 100 ng/mL of C1 or phenyl glycolipids (C16, C22, 7DW8-5, 7DW8-6, C26, C23, or C34) for 18 h. Supernatants were collected for determination of IFN-γ, IL-4 and IL-10. All phenyl glycolipids were able to induce significantly higher amounts of IFN-γ than C1 (Fig. 5A), but the production of IL-4 and IL-10 was comparable to C1, except for a greater IL-10 induction by C23 (Fig. 5 B and D). When expressed as the ratio of IFN-γ/IL-4 (Fig. 5C) or IFN-γ/IL-10 (Fig. 5D), all phenyl glycolipids triggered more Th1-skewed responses than C1.
Fig. 5.
The ability of phenyl glycolipids to stimulate cytokine productions of human naïve Vα24 iNKT cells. Human naïve Vα24 iNKT cells (1 × 105) were cultured with autologous immature CD14+ dendritic cells (5 × 104) pulsed with the indicated glycolipids at 100 ng/mL for 18 h. The production of IFN-γ (A), IL-4 (B) and IL-10 (D) were detected by Beadlyte Human Cytokine Kit and read by a Luminex100 system. The ratio of IFN-γ over IL-4 (C) and IFN-γ over IL-10 (D), normalized to DMSO, were calculated. Assays were performed in triplicates and data were presented as mean ± SD. The symbol * represents p < 0.05, compared with DMSO; and #, p < 0.05, compared with C1.
TCR β Chain Usage of Phenyl Glycolipids Expanded Human Vα24 iNKT Cells.
To determine the Vβ chain usages of human iNKT cells after phenyl glycolipids stimulation, purified Vα24 iNKT cells were stimulated by indicated glycolipids twice, followed by the addition of IL-2 after the removal of glycolipids. After the second stimulation and expansion, Vα24 iNKT cells were purified by magnetic cell sorting (MACS) (purity > 95%) and the usage of TCR beta chain was examined by FACS and spectratyping. From both analyses, Vβ11 was found to be the major beta chain used by Vα24 iNKT cells stimulated with C1, C34 and C17 or DMSO control (Fig. S5). Although there were variations in the Vβ11 percentages among individuals, in general, the percentage of Vβ11 + iNKT cells from C1- (90.94–97.31) and C34-expanded (72.27–95.54) NKT cells were higher than mock (11.34–90.7) and C17-expanded (58.13–92.05) Vα24 iNKT cells within each donor tested (Fig. S5 and Tables S1–S4). In addition, low usages of other β chains (Vβ3, Vβ6A, Vβ6B, Vβ13A, or Vβ13B) were observed, but their expression varied without regular pattern among these individuals (Tables S1–S4). Taken together, Vβ11 was the major β chain usage of C1, C34, or C17 activated Vα24 iNKT cells and its greater usage by C1 and C34-expanded iNKT cells may contribute to differential binding avidity of hCD1d–glcolipid complexes with Vβ11 + Vα24iNKT TCR.
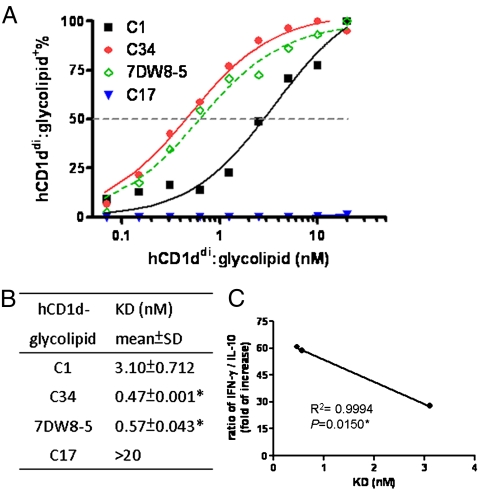
Binding Avidity of hCD1d–Phenyl Glycolipids Complex with Vβ11 + Vα24iNKT Cells.
As mentioned above, C1 and phenyl glycolipids were similar in their Vβ chain usage of Vα24 iNKT cells. It is possible that the variation in the binding avidity of CD1d–glycolipid complex with human iNKT cells might account for differential cytokines secretions, as found in mouse system. Various concentrations of hCD1ddi–glycolipid complexes were incubated with fixed amount of Vβ11 + Vα24iNKT cells and the level of bound complexes at indicated concentration was analyzed by flow cytometry (Fig. 6A). Fig. 6B showed that the binding strength of hCD1d loaded with C34 (0.47 ± 0.001 nM) and 7DW8-5 (0.57 ± 0.04 nM) was significantly greater than hCD1d-C1 (3.10 ± 0.71 nM) toward iNKT cells. In contrast, the KD value of hCD1d–C17 complex for Vα24 + /Vβ11 + iNKT cells was > 20 nM, suggesting that the lower binding avidity might be associated with the more Th2-skewed cytokine secretion (Fig. 6 A and B). Moreover, the KD values of hCD1d–glycolipid complex for Vα24 + /Vβ11 + iNKT cells correlated inversely with the IFN-γ/IL-10 ratio, with R2 = 0.999, p = 0.015 (Fig. 6C). These results suggested that the stronger interaction between hCD1d–glycolipid complexes and Vα24 + /Vβ11 + iNKT cells might drive the more Th1-polarized cytokine responses in humans.
Fig. 6.
The interaction between human CD1d–phenyl glycolipids complex with human Vα24+/Vβ11+iNKT cells. C1-expanded iNKT cells were incubated with various concentrations of hCD1d–glycolipid complexes and anti-mIgG1-PE antibody for 1 h at 4 °C, followed by staining with Vα24 and Vβ11 antibodies. The percentage of hCD1d–glycolipid in Vα24+/Vβ11+ population was determined by FACS. (A) The relationship between the binding percentage and the concentration of CD1ddi–glycolipids complex was plotted. (B) KD values of glycolipids were calculated from Scatchard transformation of the plot above. Assay was performed in duplicates and data was representative of two independent experiments. Student t test was used for statistical analysis. The symbol * represents p < 0.05, compared with C1. (C) The correlation between cytokine secretion and binding avidity of hCD1d–glycolipids for iNKT cells. Linear regression model was performed to analyze the ratio of IFN-γ over IL-10 (Fig. 5D) and the KD values of indicated glycolipids.
Discussion
In this study, most glycolipids with single phenyl ring were found to be more potent for iNKT cell activation than those with two phenyl rings on the acyl chain. Yet there were some exceptions to this phenomenon. First, C27, C28 or C29 having longer acyl chain triggered very little IL-2 production. This is consistent with the report that the acyl chain length exceeding fifteen carbons with single phenyl ring may not fit the A′ pocket of mCD1d binding grooves very well (27). Second, C34 with two phenyl rings on the acyl chain, unlike C26 and C30, induced high levels of IL-2 and IFN-γ secretions. In contrast, C35, C36 and C37 with one phenyl ring on each acyl and sphingosine chain could not activate iNKT cells as assessed by flow cytometry and serum IFN-γ or IL-4 after i.v injection of these compounds. Analogs of C35, C36, and C37 could not stimulate cytokine productions from human iNKT cells either (28). FACS staining revealed no detectable complexes of CD1d dimer loaded with these 3 glycolipids bound to mouse iNKT cells. This is in line with the report that C13, which contained the phenyl ring only on the sphingosine chain, exhibited very low affinity for mCD1d, possibly due to the difficulty for the sphingosine chain with the phenyl ring to fit into the limited space of the mCD1d F′ pocket (24).
Judging from the ratios of IFN-γ to IL-4 or IL-10 in mice and human systems, C23, 7DW8-5, and C34 elicited more Th1-biased responses as compared to C1 and other phenyl glycolipids. These three compounds, especially C34, were more efficacious than C1 for the treatment of lung, melanoma, and breast cancers in mice. This is in line with our report that C34 also exerted better antimicrobial responses in mice (29). The Th1-polarized cytokine production and greater antimicrobial or anticancer activities elicited by the three phenyl glycolipids likely reflected the immune responses downstream of early activation of iNKT cells. The early events may involve either preferential stimulation of iNKT cells bearing specific beta chain and/or differential binding avidity or stability of CD1d-phenyl glycolipids with NKT cells.
As measured by FACS staining of CD1d dimer-glycolipid complex bound to iNKT cells, phenyl glycolipids loaded onto mCD1d or hCD1d were found to have much stronger binding avidity than C1 toward Vα14 iNKT cells or Vα24 iNKT cells, respectively. The binding strength of the ternary interaction correlated well with the ratio of IFN-γ to IL-10 secreted in mice sera and human iNKT cells, with C34 showing the greatest Th1-driven potency and the strongest binding avidity. Besides binding avidity, the binding stability might have an impact on NKT cell activation because the duration of the interaction with iNKT cells could affect the downstream TCR signaling within the immunological synapse. It was reported that the shorter duration of NKT cell stimulation could trigger the release of preformed IL-4, whereas the longer NKT TCR stimulation could induce IFN-γ via de novo protein synthesis (30, 31). This is consistent with our observation that the association of mCD1d-C23, -7DW8-5 or -C34 with TCR of Vα14 iNKT cells was significantly longer than that of mCD1d-C1, with mCD1d-C34 complex displaying the longest interaction. In comparison, the Th1-skewed α-C-GalCer displayed weaker binding avidity than α-GalCer to iNKT TCR when complexed with CD1d. However, α-C-GalCer was more resistant to O-glycosidase degradation in vivo. Thus, CD1d-α-C-GalCer displayed longer half-life in vivo and stimulated iNKT cells longer (26). This may explain why the secretion of IFN-γ peaked at 24 h after α-C-GalCer injection while it peaked around 12 to approximately 18 h after α-GalCer stimulation (32). Thus, both the binding avidity and stability of the CD1d–glycolipid complex toward iNKT cells were important for the polarization of the Th1/Th2 cytokine secretion. These in vitro binding properties may serve as good indicators for predicting biological responses in vivo.
We also examined if phenyl glycolipids preferentially stimulated iNKT cells bearing certain specific beta chain. Using FACS analysis, C23, 7DW8-5 and C34 were found to predominantly activate the Vα14 iNKT cells with Vβ8.1/8.2, as C1. Similarly, the Th2-favored C20∶2 N-acyl variants of α-GalCer and sphigosine-truncated version OCH were also recognized mainly by Vβ8.1/8.2 + Vα14iNKT cells (25, 33). These findings suggested that the polarized Th1/Th2 induction by glycolipids could not be the consequence of preferred stimulation of Vα14 iNKT cells bearing different beta chains. However, we could not exclude the possibility that phenyl glycolipids may activate other types of NKT cells bearing different α chains like Vα10 as reported recently (34) and will pursue this in the future. On the other hand, Vα14 iNKT cells with diverse beta chains were reported to differ in their binding affinity/avidity for the same mCD1d-glycolipid complex (18, 21, 25, 35). For instance, Vα14 iNKT cells expressing Vβ8.2 interacted with mCD1d-C1 or mCD1d-OCH complex stronger than those expressing Vβ7 (25). Thus, we focused on the ternary interaction between CD1d-glycolipid complexes and iNKT cells with Vβ8.2, as described above.
In humans, FACS analysis using Vα24- and Vβ11-specific antibodies showed that some of the mitogen activated Vα24 iNKT cells did not use Vβ11 (2). Our detailed spectratyping examination revealed that Vβ11 was expressed at a high degree in C1- and C34-expanded cells, as compared to lower Vβ11 usage in C17-expanded Vα24 iNKT cells in most donors. Although Vβ11- Vα24 iNKT cells could also recognize CD1d, they were shown to produce less IFN-γ than Vβ11 + iNKT cells (19).Yet Vβ11 was still the major usage by Vα24 iNKT cells to recognize both Th1- and Th2-biased glycolipids in each individual. These lines of evidence suggested that the distinct cytokine patterns induced by these glycolipids should most likely come from the differential binding avidity of hCD1d-glycolipid with Vα24 iNKT cells using Vβ11 predominantly. Nonetheless, the difference in the expression level of Vβ11 on the cell surface may amplify the differential binding capacity of hCD1d-glcolipid complex to Vα24/Vβ11 iNKT TCR.
In conclusion, it was discovered that acyl modifications on the phenyl ring could promote Th1- biased polarization. C34, C23, and 7DW8-5 that contained additional modifications induced higher ratio of IFN-γ to IL-4 or IFN-γ to IL-10 in mice and in humans, exhibiting greater anticancer effects on breast, lung and melanoma tumors in mice. These biological responses induced by C23, 7DW8-5 and C34 could be attributed to their stronger interaction with NKT TCR than C1. In addition, C34 was superior to C23 or 7DW8-5 in several aspects including its binding strength and stability with NKT TCR, and its induction of Th1-biased immunity in mice and in humans. Thus, further development of C34 as a drug candidate for cancer therapy is warranted.
Materials and Methods
All glycolipids structures, mouse models, isolation and generation of cell lines and dendritic cells, FACS analysis, determination of cytokines, and binding avidity were described in SI Text.
DN3A4-1.2 Hybridoma Cytokine Assay.
CD1d-reactive T cell hybridoma with a Vα14 T cell antigen receptor, DN3A4-1.2, was kindly provided by Mitchell Kronenberg (36). T cell hybridoma cells were cultured in mCD1d-coated 96 wells and stimulated with indicated glycolipids at 100 ng/mL according to the published protocol (37). After incubation for 18 h, IL-2 released into the medium as the readout of the iNKT cells activation was measured by an ELISA assay.
Determination of Murine Cytokines/Chemokines Secretion.
BALB/c mice were intravenously injected with vehicle or glycolipids. Serum was collected at 2 and 18 h after injection for measurement of cytokines/chemokines by Beadlyte® Mouse Cytokine kit and read by a Luminex® 100™ system (Luminex).
Binding Avidity of Various CD1d-Loaded Glycolipids to Vα14 INKT Cells.
Binding avidity of CD1d-glycolipid complex to iNKT cells was determined as described previously (18). Briefly, murine CD1d:Ig dimer (BD Biosciences PharMingen) was loaded with glycolipids at a molar ratio of 1∶10 or vehicle for overnight at 37 °C. Murine 1.2 Vα14 iNKT cells were incubated with various doses of dimer-glycolipid complex in buffer containing azide (0.05%) for 1 h at 4 °C. After washing, these cells were stained with anti-mouse IgG1-PE mAb (A85-1) for 30 min at 4 °C, followed by washing, fixation with 4% paraformaldehyde, and the bound mCD1d dimer complexes were detected by flow cytometry. The binding curve and linear fit of the Scatchard transformation were plotted by Graphpad Prism software.
Spetratyping of Various Glycolipid-Expanded Vα24 iNKT Cell.
Human Vα24 iNKT cells were isolated from peripheral blood mononuclear cell (PBMC), cultured in the presence of C1, C34, and C17, and expanded as described in SI Text. Total RNA was extracted from treated cells using RNeasy Mini kit (Qiagen) and cDNA was generated using cDNA reverse transcription kit (Applied Biosystems by Life Technologies). Spetratyping of TCR β chain was performed by quantitative real time PCR with Taqman probe Vβ-specific forward primers (Applied Biosystems by Life Technologies) and universal Cβ reverse primer as described (38).
Supplementary Material
Acknowledgments.
We thank Academia Sinica in Taiwan for funding support and the excellent support provided by cell imaging/flow cytometry facility at the Division of Medical Biology, Genomics Research Center. We also thank Dr. Mitchell Kronenberg (La Jolla Institute for Allergy and Immunology) for kindly providing DN3A4-1.2 Vα14+ T hybridoma.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1114255108/-/DCSupplemental.
References
- 1.Lantz O, Bendelac A. An invariant T cell receptor alpha chain is used by a unique subset of major histocompatibility complex class I-specific CD4+ and CD4-8- T cells in mice and humans. J Exp Med. 1994;180:1097–1106. doi: 10.1084/jem.180.3.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dellabona P, Padovan E, Casorati G, Brockhaus M, Lanzavecchia A. An invariant V alpha 24-J alpha Q/V beta 11 T cell receptor is expressed in all individuals by clonally expanded CD4-8- T cells. J Exp Med. 1994;180:1171–1176. doi: 10.1084/jem.180.3.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Makino Y, Kanno R, Ito T, Higashino K, Taniguchi M. Predominant expression of invariant V alpha 14+ TCR alpha chain in NK1.1+ T cell populations. Int Immunol. 1995;7:1157–1161. doi: 10.1093/intimm/7.7.1157. [DOI] [PubMed] [Google Scholar]
- 4.Davodeau F, et al. Close phenotypic and functional similarities between human and murine alphabeta T cells expressing invariant TCR alpha-chains. J Immunol. 1997;158:5603–5611. [PubMed] [Google Scholar]
- 5.Kawano T, et al. CD1d-restricted and TCR-mediated activation of valpha14 NKT cells by glycosylceramides. Science. 1997;278:1626–1629. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- 6.Yoshimoto T, Paul WE. CD4pos, NK1.1pos T cells promptly produce interleukin 4 in response to in vivo challenge with anti-CD3. J Exp Med. 1994;179:1285–1295. doi: 10.1084/jem.179.4.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arase H, Arase N, Nakagawa K, Good RA, Onoe K. NK1.1+ CD4+ CD8- thymocytes with specific lymphokine secretion. Eur J Immunol. 1993;23:307–310. doi: 10.1002/eji.1830230151. [DOI] [PubMed] [Google Scholar]
- 8.Kawakami K, et al. Critical role of Valpha14+ natural killer T cells in the innate phase of host protection against Streptococcus pneumoniae infection. Eur J Immunol. 2003;33:3322–3330. doi: 10.1002/eji.200324254. [DOI] [PubMed] [Google Scholar]
- 9.Nieuwenhuis EE, et al. CD1d-dependent macrophage-mediated clearance of Pseudomonas aeruginosa from lung. Nat Med. 2002;8:588–593. doi: 10.1038/nm0602-588. [DOI] [PubMed] [Google Scholar]
- 10.van der Vliet HJ, et al. The immunoregulatory role of CD1d-restricted natural killer T cells in disease. Clin Immunol. 2004;112:8–23. doi: 10.1016/j.clim.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 11.Tahir SM, et al. Loss of IFN-gamma production by invariant NK T cells in advanced cancer. J Immunol. 2001;167:4046–4050. doi: 10.4049/jimmunol.167.7.4046. [DOI] [PubMed] [Google Scholar]
- 12.Dhodapkar MV, et al. A reversible defect in natural killer T cell function characterizes the progression of premalignant to malignant multiple myeloma. J Exp Med. 2003;197:1667–1676. doi: 10.1084/jem.20021650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giaccone G, et al. A phase I study of the natural killer T-cell ligand alpha-galactosylceramide (KRN7000) in patients with solid tumors. Clin Cancer Res. 2002;8:3702–3709. [PubMed] [Google Scholar]
- 14.Liang PH, et al. Quantitative microarray analysis of intact glycolipid-CD1d interaction and correlation with cell-based cytokine production. J Am Chem Soc. 2008;130:12348–12354. doi: 10.1021/ja8012787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang YJ, et al. Potent immune-modulating and anticancer effects of NKT cell stimulatory glycolipids. Proc Natl Acad Sci USA. 2007;104:10299–10304. doi: 10.1073/pnas.0703824104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schumann J, Mycko MP, Dellabona P, Casorati G, MacDonald HR. Cutting edge: Influence of the TCR Vbeta domain on the selection of semi-invariant NKT cells by endogenous ligands. J Immunol. 2006;176:2064–2068. doi: 10.4049/jimmunol.176.4.2064. [DOI] [PubMed] [Google Scholar]
- 17.Wei DG, Curran SA, Savage PB, Teyton L, Bendelac A. Mechanisms imposing the Vbeta bias of Valpha14 natural killer T cells and consequences for microbial glycolipid recognition. J Exp Med. 2006;203:1197–1207. doi: 10.1084/jem.20060418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schumann J, Voyle RB, Wei BY, MacDonald HR. Cutting edge: Influence of the TCR V beta domain on the avidity of CD1d:alpha-galactosylceramide binding by invariant V alpha 14 NKT cells. J Immunol. 2003;170:5815–5819. doi: 10.4049/jimmunol.170.12.5815. [DOI] [PubMed] [Google Scholar]
- 19.Exley M, Garcia J, Balk SP, Porcelli S. Requirements for CD1d recognition by human invariant Valpha24+ CD4-CD8- T cells. J Exp Med. 1997;186:109–120. doi: 10.1084/jem.186.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Borg NA, et al. CD1d-lipid-antigen recognition by the semi-invariant NKT T-cell receptor. Nature. 2007;448:44–49. doi: 10.1038/nature05907. [DOI] [PubMed] [Google Scholar]
- 21.Pellicci DG, et al. Differential recognition of CD1d-alpha-galactosyl ceramide by the V beta 8.2 and V beta 7 semi-invariant NKT T cell receptors. Immunity. 2009;31:47–59. doi: 10.1016/j.immuni.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCarthy C, et al. The length of lipids bound to human CD1d molecules modulates the affinity of NKT cell TCR and the threshold of NKT cell activation. J Exp Med. 2007;204:1131–1144. doi: 10.1084/jem.20062342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fujio M, et al. Structure-based discovery of glycolipids for CD1d-mediated NKT cell activation: Tuning the adjuvant versus immunosuppression activity. J Am Chem Soc. 2006;128:9022–9023. doi: 10.1021/ja062740z. [DOI] [PubMed] [Google Scholar]
- 24.Li X, et al. Design of a potent CD1d-binding NKT cell ligand as a vaccine adjuvant. Proc Natl Acad Sci USA. 2010;107:13010–13015. doi: 10.1073/pnas.1006662107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stanic AK, et al. Another view of T cell antigen recognition: cooperative engagement of glycolipid antigens by Va14Ja18 natural T(iNKT) cell receptor. J Immunol. 2003;171:4539–4551. doi: 10.4049/jimmunol.171.9.4539. [DOI] [PubMed] [Google Scholar]
- 26.Sullivan BA, et al. Mechanisms for glycolipid antigen-driven cytokine polarization by Valpha14i NKT cells. J Immunol. 2010;184:141–153. doi: 10.4049/jimmunol.0902880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schiefner A, Fujio M, Wu D, Wong CH, Wilson IA. Structural evaluation of potent NKT cell agonists: Implications for design of novel stimulatory ligands. J Mol Biol. 2009;394:71–82. doi: 10.1016/j.jmb.2009.08.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park JJ, et al. Syntheses and biological activities of KRN7000 analogues having aromatic residues in the acyl and backbone chains with varying stereochemistry. Bioorg Med Chem Lett. 2010;20:814–818. doi: 10.1016/j.bmcl.2009.12.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin KH, et al. In vivo protection provided by a synthetic new alpha-galactosyl ceramide analog against bacterial and viral infections in murine models. Antimicrob Agents Chemother. 2010;54:4129–4136. doi: 10.1128/AAC.00368-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berkers CR, Ovaa H. Immunotherapeutic potential for ceramide-based activators of iNKT cells. Trends Pharmacol Sci. 2005;26:252–257. doi: 10.1016/j.tips.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 31.Oki S, Chiba A, Yamamura T, Miyake S. The clinical implication and molecular mechanism of preferential IL-4 production by modified glycolipid-stimulated NKT cells. J Clin Invest. 2004;113:1631–1640. doi: 10.1172/JCI20862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fujii S, et al. Glycolipid alpha-C-galactosylceramide is a distinct inducer of dendritic cell function during innate and adaptive immune responses of mice. Proc Natl Acad Sci USA. 2006;103:11252–11257. doi: 10.1073/pnas.0604812103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu KO, et al. Modulation of CD1d-restricted NKT cell responses by using N-acyl variants of alpha-galactosylceramides. Proc Natl Acad Sci USA. 2005;102:3383–3388. doi: 10.1073/pnas.0407488102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Uldrich AP, et al. A semi-invariant V(alpha)10(+) T cell antigen receptor defines a population of natural killer T cells with distinct glycolipid antigen-recognition properties. Nat Immunol. 2011;12:616–623. doi: 10.1038/ni.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mallevaey T, et al. T cell receptor CDR2 beta and CDR3 beta loops collaborate functionally to shape the iNKT cell repertoire. Immunity. 2009;31:60–71. doi: 10.1016/j.immuni.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burdin N, et al. Selective ability of mouse CD1 to present glycolipids: alpha-galactosylceramide specifically stimulates V alpha 14+ NK T lymphocytes. J Immunol. 1998;161:3271–3281. [PubMed] [Google Scholar]
- 37.Wu D, et al. Bacterial glycolipids and analogs as antigens for CD1d-restricted NKT cells. Proc Natl Acad Sci USA. 2005;102:1351–1356. doi: 10.1073/pnas.0408696102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ochsenreither S, et al. Relative quantification of TCR Vbeta-chain families by real time PCR for identification of clonal T-cell populations. J Transl Med. 2008;6:1–8. doi: 10.1186/1479-5876-6-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.