Abstract
Synaptic terminals are known to expand and contract throughout an animal's life. The physiological constraints and demands that regulate appropriate synaptic growth and connectivity are currently poorly understood. In previous work, we identified a Drosophila model of lysosomal storage disease (LSD), spinster (spin), with larval neuromuscular synapse overgrowth. Here we identify a reactive oxygen species (ROS) burden in spin that may be attributable to previously identified lipofuscin deposition and lysosomal dysfunction, a cellular hallmark of LSD. Reducing ROS in spin mutants rescues synaptic overgrowth and electrophysiological deficits. Synapse overgrowth was also observed in mutants defective for protection from ROS and animals subjected to excessive ROS. ROS are known to stimulate JNK and fos signaling. Furthermore, JNK and fos in turn are known potent activators of synapse growth and function. Inhibiting JNK and fos activity in spin rescues synapse overgrowth and electrophysiological deficits. Similarly, inhibiting JNK, fos, and jun activity in animals with excessive oxidative stress rescues the overgrowth phenotype. These data suggest that ROS, via activation of the JNK signaling pathway, are a major regulator of synapse overgrowth. In LSD, increased autophagy contributes to lysosomal storage and, presumably, elevated levels of oxidative stress. In support of this suggestion, we report here that impaired autophagy function reverses synaptic overgrowth in spin. Our data describe a previously unexplored link between oxidative stress and synapse overgrowth via the JNK signaling pathway.
Lysosomal dysfunction leads to poor digestion of damaged macromolecules and organelles, resulting in the accumulation of biological waste. During aging, this “waste” manifests as lipofuscin, a nondegradable, autofluorescent intralysosomal polymeric agglomeration of lipids and proteins (1). Lipofuscin is viewed as a hallmark of aging cells and is notable in long-lived cells, such as neurons. Lysosomal storage disorders (LSD) are characterized in part by lipofuscin accumulation, reduced lysosomal function, and neurodegeneration. Ectopic synaptogenesis and dendritogenesis has also been demonstrated in models of LSDs (2, 3), although the mechanisms causing this growth are unclear. Lipofuscin deposition is proposed to hamper autophagic degradation in general and particularly organelle turnover, promoting the accumulation of senescent mitochondria and lysosomal iron, producing increasing amounts of reactive oxygen species (ROS) (4). A similar mechanism may occur in LSD, where lysosomal dysfunction can lead to an accumulation of autophagosomes, either because of a defect in autophagosomal clearance in the lysosome or an induction of autophagy (5, 6). Oxidative stress (OS), potentially activated by lysosomal accumulation, is an increasingly recognized feature of LSDs (see for example ref. 7) and may in turn induce a further increase in autophagy. The pathways by which OS/ROS go on to contribute to neuronal dysfunction in aging or diseased tissue currently remain obscure (8).
We have previously identified in Drosophila loss-of-function mutations in spinster (spin), an endosomal protein, that give rise to shortened lifespan, accumulation of lipofuscin, swollen lysosomes, neurodegeneration, and synaptic overgrowth (9–12), all hallmarks of LSD. Neuromuscular synaptic overgrowth in spin was shown to require a permissive TGF-β signal (9), an important developmental pathway regulating the growth of this synapse (9, 13–16). Synapse growth and function is also regulated by the jun N-terminal kinase (JNK)/activator protein-1 (AP-1) pathway (17–19). AP-1 is a transcriptional regulator composed of the DNA binding proteins fos and jun. In hiw mutants, loss of an E3-ubiquitin ligase and the consequent failure to degrade wallenda, a JNK kinase kinase (JNKKK) generates synaptic overgrowth by the activation of JNK/AP-1 (19, 20). In other cellular contexts the JNK/AP-1 pathway is well known to be activated by OS (21), particularly via the activation of apoptosis-signal–regulating kinase (ASK), a JNKKK stimulated by oxidation of thioredoxin (22) and the regulation of JNK itself by interaction with GST (23). OS has been shown to be a major activator of autophagy and antioxidative responses via JNK/AP-1 activity (24–26). Other than hiw and wallenda, factors signaling upstream of JNK regulating synaptic development are relatively unknown. The known activation and regulation of the JNK signaling pathway by ROS suggests that this stimulus may play a potent role in the activation of synapse growth and function.
We examined and found in our Drosophila LSD model spin evidence for an OS burden. We hypothesized and found that decreasing OS reduces synaptic overgrowth and alleviates physiological dysfunction. We further tested if larvae with OS generated by independent genetic and pharmacological manipulations have similarly overgrown synapses. Finally, we examined the role of JNK/AP-1 in regulating synaptic growth in response to OS. JNK/AP-1 are well known mediators of the cellular response to various stressors including OS. We demonstrate here that OS is a major activator of synapse growth via activation of the JNK/AP-1 pathway.
Results
Mutations in the Late Endosomal Protein spin Lead to an OS Burden.
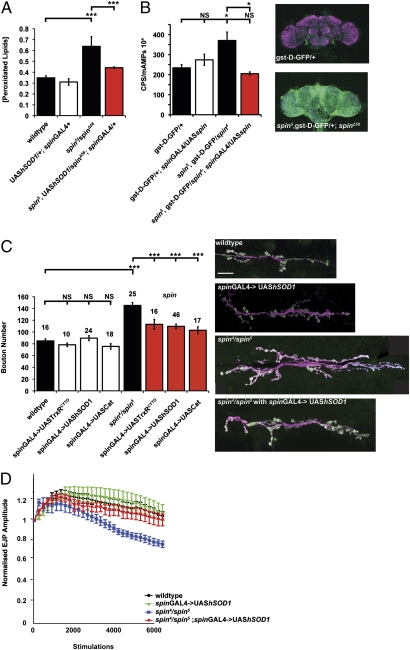
The presence of lipofuscin and swollen late endosome/lysosomes in the spin mutant has previously been documented (9–11). Lipofuscin consists of undegraded material known to generate OS through Fenton reactions in the disrupted lysosome (1, 27). To test if neuromuscular junction (NMJ) overgrowth is caused by OS, we verified the presence of OS in spin using a lipid peroxidation assay (Fig. 1A) and a reporter transgene for OS (Fig. 1B). Lipid peroxidation is the degradation of polyunsaturated lipids to malondialdehyde (MDA) and 4-hydroxyalkenals by the action of free radicals (28) and adducts generated by MDA may give rise to fluorescent lipofuscin (29). Elevated levels of aldehydes are commonly used as a marker of OS. We found that MDA levels in spin are increased by 80% in spin mutants, an increase rescued by the expression of a superoxide dismutase 1 (hSOD1) transgene, a well-characterized antioxidant enzyme (30) (Fig. 1A). This finding suggests pathological levels of ROS in spin. High levels of ROS in spin are also indicated using a GST (gst-D-GFP) reporter transgene where GFP expression is controlled by a gst-D promoter, an element recognized and activated by the NF-E2-related factor-2 (Nrf2) transcription factor under conditions of OS (31). Increased levels of GFP in spin are shown here both by fluorimetry and confocal microscopy (Fig. 1B), confirming activation of the cellular OS response pathway.
Fig. 1.
spin mutants bear a ROS burden: Relieving OS provides a partial rescue of overgrowth at the NMJ and alleviates electrophysiological deficits. (A) spin mutants have significantly increased levels of peroxidated lipids (***P < 0.001, Student t test), rescued by expression of hSOD1 under the control of spinGAL4. Error bars show SEM (n = 3). (B) spin mutants have increased levels of gst-D-GFP expression (*P < 0.05, ANOVA), indicating activation of the NRF2 OS response. Increased GFP is shown by fluorimetry, bars and show emission levels at the peak amplitude of GFP fluorescence; error bars show SEM (n = 3). Increased GFP can also be seen in z-stacks of adult heads (neuropil, in magenta). Expression of spin under the control of spinGAL4 rescues this phenotype. (C) Overexpression of antioxidant transgenes simultaneously expressed pre- and postsynaptically using spinGAL4 significantly reduces overgrowth at the NMJ. Furthermore, spin4/spin5 has a 96% increase in bouton number compared with wild-type, from 85 ± 3.3 (n = 16) to 151 ± 8.8 (n = 32) (***P < 0.001, ANOVA). Expression of UAS-trxR1CYTO, UAS-hSOD1 or UAS-cat under control of spinGAL4 does not change bouton number. UAS-trxR1CYTO;spinGAL4/+, UAShSod1/+;spinGAL/+, and UAS-cat/+;spinGAL4/+ have bouton numbers of 78 ± 2.7 (n = 10), 90 ± 3.3 (n = 24), and 76 ± 8.7 (n = 18), respectively. Expressing antioxidant transgenes in spin mutants significantly rescues bouton number to 113 ± 8.3 (n = 10), 110 ± 4.5 (n = 46), and 103 ± 4.5 (n = 17), respectively (***P < 0.001, ANOVA different from spin and *P > 0.05 compared with wild-type). Error bars show SEM; numbers above each bar are the n values. Images of the NMJ at muscle 6/7 in segment A3, with nerves shown in magenta (HRP) and boutons in green (syt). (Scale bar, 20 μm.) (D) EJP amplitudes decrease in spin with repeated stimulation. EJP amplitudes return to control levels when hSOD1 is expressed. Expressing this transgene in a wild-type background does not affect responses to repeated stimulation.
Reducing ROS in spin Mutants Rescues NMJ Overgrowth and Electrophysiological Deficits.
Mutations in spin cause significant overgrowth of the NMJ, with an 80% increase in bouton number (9). Bouton number is a common measure of synaptic development at the Drosophila larval NMJ and is the quantification of synaptic growth that we use throughout our study as an assay for synaptic growth (32). The spin mutant muscles do not significantly differ in size from wild-type controls (Table S1). We therefore do not need to normalize bouton number to muscle area to allow for the presumed coupling during development between muscle size and synaptic growth (9, 33, 34). We asked if synapse overgrowth in spin mutants is generated by increased levels of ROS. Through expression of antioxidant transgenes we reduced OS in spin. To rescue cells affected by the loss of spin, UAS-hSOD1, UAS-catalase (cat), and UAS-thioredoxin-reductase (TrxR1) were expressed pre- and postsynaptically under the control of a spin promoter (spinGAL4, expressed both in muscle and nerve). In a wild-type background, expression of hSOD1, cat, and TrxR1 does not affect bouton number. Overexpression of hSOD1, cat, or TrxR1 in spin mutant larvae significantly rescue synapse overgrowth by 60% (Fig. 1C). In another synaptic overgrowth mutant, hiw, a modest but significant reduction in overgrowth can be seen when we express hSOD1. This finding suggests that OS contributes to the overgrowth seen in this mutant (Fig. S1). These data suggest increased levels of ROS are present in spin and contribute to synaptic overgrowth in spin and other synaptic overgrowth mutants.
Electrophysiological stimulation of control and spin mutant larval NMJs at a high frequency (10 Hz) resulted in an initial increase in excitatory junction potential (EJP) amplitudes over approximately the first 1,000 stimulations followed by a rundown in EJP amplitude. Similar responses have been reported in this preparation previously (35). Mutant spin larvae showed an increased rate and extent of rundown of (EJP) amplitudes to about 80% of their initial value, significantly different (P < 0.05) from control larvae with EJP amplitudes of about 100% of initial values after 6,000 stimuli (Fig. 1D). This increased rate of rundown in spin mutants is similar to previous reports (12). Expression of hSOD1 in a wild-type background had no effect on EJP amplitudes during high-frequency stimulation. Expression of hSOD1 restored the ability of spin mutant synapses to maintain synaptic transmission during high-frequency stimulation. EJP amplitudes in rescued larvae were not statistically different from wild-type.
OS Induces Synaptic Growth.
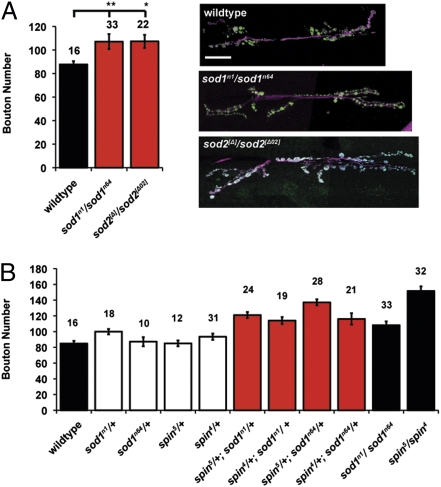
We examined further the role OS may have in generating synapse overgrowth independent of spin-induced lysosomal dysfunction. We determined synapse size in two different mutants known to have defective protection from ROS. The enzymes SOD1 and SOD2 are vital components of the cellular antioxidant defense system; SOD converts superoxide anions to hydrogen peroxide (30). SOD1 is predominantly localized to the cytosol and mitochondrial periplasm (36), and SOD2 is mitochondrially localized (37). Both sod1 and sod2 muscle fibers are undergrown; therefore, by not normalizing for synaptic growth coupling we underestimate the size of the synapse in these animals (Fig. S2 D–F and Table S1). On examination, larvae mutant for either sod1 or sod2 have a significant increase in bouton number, indicating a synaptic overgrowth (Fig. 2A). We observe a reduction in synaptic branching in sod2 mutants (Fig. S3 and Table S2) and reduced bouton size in sod1 mutants (Fig. S4 and Table S3); nonetheless, bouton number is increased compared with wild-type in all conditions.
Fig. 2.
OS induces growth of the NMJ. (A) Mutations in the OS protection system cause significantly overgrown NMJs. Wild-type larvae have a mean bouton number of 85 ± 3.3 (n = 16), which is increased by mutations in sod1 to 107 ± 4.9 (n = 21, **P < 0.01, ANOVA) and sod2 to 107 ± 5.9 (n = 22, *P < 0.05, ANOVA). Error bars show SEM; numbers above each bar are the n values. Images of the NMJ at muscle 6/7 in segment A3, with nerves shown in magenta (HRP) and boutons in green (syt). (Scale bar, 20 μm.) (B) Larvae carrying heterozygous mutations for both sod1 and spin are significantly overgrown compared with heterozygous mutations alone. Wild-type, sod1n1/+, sod1n64/+, spin4/+, and spin5/+ are not significantly different (P > 0.05). In all combinations, mutants of spin and sod1 are overgrown compared with wild-type or the constituent heterozygotes alone (P < 0.001, ANOVA).
Feeding paraquat to Drosophila has previously been used to induce OS. Paraquat is known to impair mitochondrial function by disturbing complex I and complex III of the mitochondrial respiratory chain (38), increasing generation of ROS (39). Drosophila larvae were reared on food containing 10-mM paraquat from the first-instar stage. Although paraquat feeding resulted in smaller sized animals, expression of UAS-hSOD1 in nerve and muscle reduced synapse growth by 30% compared with paraquat-fed controls (wild-type larvae with 10-mM paraquat bouton number, 80 ± 6.64; spinGAL4/UAS-hSOD1 with 10-mM paraquat, 57 ± 2.64) (Fig. S2A). Collectively, the observations that known mutations and a toxin, which increase OS, all induce synaptic growth suggest that exposure to OS during development can result in NMJ overgrowth.
OS and spin Overgrowth Share the Same Genetic Pathway; JNK/AP-1 Signaling Is Required for ROS-Induced Synapse Overgrowth.
Heterozygotes of two spin mutants, spin4/+ and spin5/+ have bouton numbers similar to wild-type. Similarly, sod1 mutant heterozygotes (sod1n1/+ and sod1n64/+) do not lead to significant overgrowth (P > 0.05, ANOVA). Combining these mutations in a single animal by removing one functional copy of spin and one copy of sod1 generates a significant increase in bouton number (P < 0.001, ANOVA). Animals carrying heterozygous mutations in both sod1 and spin have a synaptic overgrowth that resembles the spin or sod1 full-mutant phenotypes (Fig. 2B), suggesting a shared genetic pathway revealed by the synergy between the spin and sod1 heterozygous mutants.
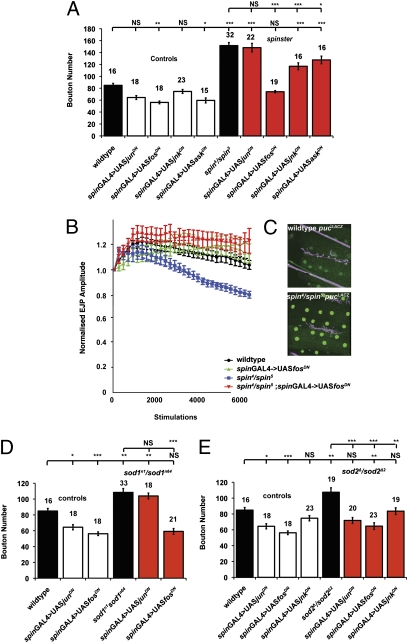
It is well documented that JNK and fos are activated in response to OS (21, 40, 41). Activation of the AP-1 pathway is known to directly transcriptionally activate puckered, a phosphatase inhibitor of JNK (42). We examined muscles in spin for the activation of the AP-1 pathway using the pucE69 enhancer trap, examining tissue for the induction of the β-gal reporter transgene. Increased expression of β-gal compared with wild-type was observed by immunofluorescence, indicating an activation of the AP-1 pathway in muscles in spin (Fig. 3C). We then asked if this pathway is essential to generate OS-induced synaptic overgrowth. Dominant-negative transgenes (and RNAi) (Fig. S5) for components of JNK/AP-1 signaling were expressed simultaneously pre- and postsynaptically (using spinGAL4). In spin, synaptic overgrowth is significantly reduced by depleting fos and JNK signaling (Fig. 3A), but reducing jun and ASK activity did not reduce overgrowth. Similar results were observed using RNAi transgenes targeted to fos and JNK in a spin mutant background (Fig. S5).
Fig. 3.
spin-, sod1-, and sod2-induced synaptic overgrowth is dependent on JNK/AP-1 signaling. (A) Expression of fos and JNK dominant-negative transgenes rescue synaptic overgrowth in spin. Depleting fos signaling rescues spin synaptic overgrowth, reducing bouton number from 166 ± 8.8 (n = 25) to 74 ± 1.7 (n = 19), generating a bouton number that is not significantly different from wild-type (P > 0.05, ANOVA) and significantly different from spin (***P < 0.001, ANOVA). Depleting jun did not significantly rescue synapse size, with a bouton number of 148 ± 3.6 (n = 22). Expression of a JNK dominant-negative transgene reduced bouton number to 117 ± 5.8 (n = 16), but expression of an ASK dominant-negative transgene did not rescue NMJ overgrowth with a bouton number of 142 ± 9.5 (n = 16). (B) EJP amplitudes decrease in spin with repeated stimulation. This rundown in EJP amplitudes is rescued to control levels when fos signaling is reduced by expression of fosDN. Expressing this transgene in a wild-type background does not affect synaptic responses to repeated stimulation. Expression of fosDN fully rescues the rundown of EJP amplitudes in spin mutants to the same level as wild-type EJPs. (C) Expression of pucLacZ, an enhancer trap-reporter of JNK/AP-1 activation, is increased in the muscle in spin compared with wild-type; nerves shown in magenta, LacZ in green. (D) In a wild-type background, spinGAL4 > UASjunDN and spinGAL4 > UASfosDN cause a significant reduction in bouton number, with mean bouton numbers of 64 ± 3.24 (n = 18) (*P < 0.05, ANOVA) and 56 ± 2.29 (n = 18) (***P < 0.001, ANOVA), respectively. Expression of junDN in a sod1 background does not rescue bouton number 104 ± 3.69 (n = 18), (P > 0.05 compared with sod1; **P < 0.01 compared with wild-type, ANOVA), whereas fosDN expression significantly reduces sod1 overgrowth to 59 ± 3.7 (n = 21), back to wild-type levels (P < 0.001 compared with sod1; P > 0.05 compared with wild-type). (E) In a wild-type background spinGAL4 > UAS-JNKDN causes no change in bouton number, 75 ± 3.31 (n = 23) (P > 0.05, ANOVA). Expression of transgenes, junDN, fosDN, or JNKDN in a sod2 mutant background significantly reduces overgrowth to 72 ± 3.77 (n = 20) (***P < 0.001, ANOVA), 65 ± 4.25 (n = 23) (***P < 0.001, ANOVA), and 84 ± 4.28 (n = 19), respectively (**P < 0.01, ANOVA).
As the fosDN transgene gave the greatest rescue of bouton number, we asked if depleting fos signaling could alleviate the electrophysiological deficit previously described in spin loss-of-function mutants. Expression of fosDN prevented the fatigue phenotype seen during repeated stimulation in spin (Fig. 3B). EJP amplitudes in the rescued larvae were not statistically different from controls, suggesting fos activation contributes to synapse dysfunction in spin.
In sod1 and sod2 mutant animals, expression of the fosDN transgene using spinGAL4 rescued synaptic overgrowth to wild-type levels (Fig. 3 D and E), but a junDN transgene rescued synaptic overgrowth in sod2 animals only (Fig. 3E). A similar reduction in OS-induced synaptic growth using fosDN and junDN transgenes is achieved in paraquat-fed animals (Fig. S6), although overall animal size is altered in paraquat-treated animals. These data suggest that fos is involved in all of the overgrowth phenotypes studied (spin, sod1, sod2, paraquat treatment), but we suggest that jun may be involved in synaptic growth where mitochondrial dysfunction may be the source for the generation of OS (sod2 and paraquat treatment).
Autophagy Genes Are Required for OS-Induced Overgrowth in spin.
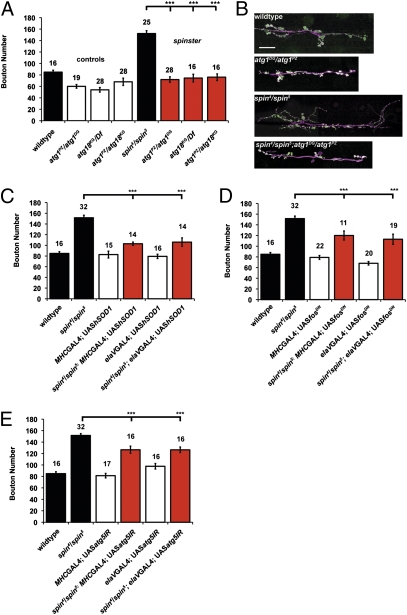
Autophagy is potently activated in response to OS and JNK activation (24), and acts to protect the cell by degradation of damaged proteins, lipids, and organelles (43, 44). Activation of autophagy produces an added burden to lysosomal function, particularly in the context of LSD, where lysosomal clearance is blocked, resulting in added lysosomal storage. We reasoned that activation of autophagy function would be critical to the process of synaptic overgrowth induced by OS in spin and that blocking autophagy function would reduce accumulation in the lysosome. When introduced into a spin mutant background, mutations in atg1 or atg18 rescue synapse overgrowth, resulting in a significant reduction in bouton number (Fig. 4 A and B). Heterozygous combinations of atg1 and atg18 similarly restrict synaptic growth in spin mutants. Although we observe a general decrease in branching in the atg and atg;spin mutant combinations, we suggest that the data demonstrate that an atg mutation can restrain spin synapse overgrowth by reducing the storage burden in the lysosomes.
Fig. 4.
Autophagy is required for spin induced overgrowth. (A) Autophagy is required for spin-induced synapse overgrowth. Mutations in the autophagy genes Atg1 (atg1PZ/atg1DG), Atg18 (atg18KG/Df), and hemizygotes (atg1PZ/atg18KG) cause a reduction in bouton number to 59 ± 5.8 (n = 19), 54 ± 4.0 (n = 28), and 68 ± 6.3 (n = 28), respectively. These mutations also reduce spin-induced overgrowth to 72 ± 4.9 (n = 28), 75 ± 6.7 (n = 16), and 76 ± 5.8 (n = 15), respectively (P > 0.05 compared with wild-type and atg1PZ/atg1DG ANOVA and ***P < 0.001 compared with spin). (B) Images of the NMJ at muscle 6/7 in segment A3, with nerves shown in magenta (HRP) and boutons in green (syt). (Scale bar, 20 μm.) (C) Expression of hSOD1 in the muscle or nerve partially rescues bouton number in a spin mutant to 103 ± 3.2 (n = 16) and 106 ± 8.0 (n = 16), respectively (***P < 0.001, ANOVA). (D) Expression of fosDN in the muscle or nerve partially rescues bouton number in a spin mutant to 120 ± 8.4 (n = 11) and 113 ± 9.5 (n = 9) (n = 16), respectively (***P < 0.001, ANOVA). (E) Expression of atg5IR in the muscle or nerve partially rescues bouton number in a spin mutant to 127 ± 5.57 (n = 16) and 126 ± 6.51 (n = 16), respectively (***P < 0.001, ANOVA).
Signaling from Pre- and Postsynaptic Compartments Contributes to Synaptic Overgrowth.
We expressed the hSOD1 transgene in either muscles only or nerves only in a spin mutant background. Under either condition, we partially rescued synaptic overgrowth (Fig. 4C). When we expressed the fosDN transgene in either nerves or muscles only in a spin mutant, we partially rescued synaptic overgrowth, each time by ∼50% (Fig. 4D). This finding suggests that both the pre- and postsynaptic compartment can contribute to synaptic overgrowth under conditions of OS via activation of the JNK/AP-1 pathway. In support of this observation, we note the elevated expression of the pucE69 β-gal reporter in muscles of spin mutants (Fig. 3C). Similarly, depletion of atg5 in either the nerve or muscle using transgenic RNAi significantly reduced synaptic overgrowth in spin (Fig. 4E). We propose that this result suggests that autophagy in muscle can contribute to synaptic overgrowth in spin by increasing the storage burden on lysosomes and potentiating the subsequent OS.
Discussion
OS Contributes to Synaptic Overgrowth and Electrophysiological Deficits in an LSD Model.
Our data show that OS causes growth of the Drosophila NMJ and is a contributory factor in the overgrowth seen in spin loss-of-function mutants, a model of a neurodegenerative LSD. OS, presence of lipofuscin, and dysregulated autophagy (5–7) are identified cellular hallmarks of LSD; excessive dendritogenesis and synaptogenesis are also observed (2, 3). The “pathogenic signaling cascade” driving synapse growth in LSD has yet to be identified. Lysosomes are rich in transition metals in a low pH and dysregulation in LSD produces an environment conducive for Fenton reactions generating ROS (1, 27). OS induces autophagy, contributing further to lysosomal build-up of undegraded material. A positively reinforcing cycle ensues: impaired lysosomal degradation increases OS and the induction of autophagy, causing a failure of autophagic clearance. In a model of LSD in Drosophila we focus on the effects of OS on synaptic development. We show that the LSD-like mutant spin has increased oxidative damage and activation of the OS response. We suggest spin lysosomal dysfunction generates OS, as seen in known LSDs (7). Our data support the proposal that OS and the activation of JNK/AP-1 in spin mutants promotes the generation of synaptic overgrowth (Fig. S7). We rescue the majority of the synaptic overgrowth in spin by reducing the OS burden. Our data are supported by the observation that OS generated independently of lysosomal dysfunction promotes synapse growth. OS also contributes to synaptic dysfunction in spin mutants; progressive reduction in EJP amplitudes with high-frequency stimulation is observed (12) (Fig. 1D). This reduction in EJP amplitude is similar in size to that observed in synapses defective in endocytosis, such as dap160, or deficient for ATP synthesis (45–47). It has previously been reported that the electrophysiological defect seen in spin mutants is a result of altered synaptic vesicle recycling and is inferred from both a reduction in FM1-43 dye uptake and the presence of multilamellar structures in the NMJ (12). The deficit in synaptic transmission observed in spin mutants can be fully rescued by expression of either hSOD1 or fosDN (Figs. 1D and 3B), suggesting that if OS and resultant fos signaling at the NMJ is reduced, normal synapse size and function can be restored.
OS Induces Growth of the Drosophila NMJ.
The synergistic effect on bouton number seen in heterozygotes of spin and sod1 are indicative of a threshold at which overgrowth is induced, indicating these mutations affect a shared pathway. An important suggestion that can be made as a result is that trafficking defects, previously suggested to cause overgrowth through continued expression/disinhibition of signaling pathways of growth signals (9, 48–50), may not be the only mechanism through which lysosomal dysfunction causes synapse overgrowth; OS may contribute to the activation of synaptic growth pathways.
Components of the JNK/AP-1 Signaling Pathway Are Differentially Required for OS-Induced Growth.
The requirement for JNK, fos, and jun activity for OS-induced synaptic growth is found to be different between spin, sod1, sod2 mutants, and paraquat-treated animals. This finding suggests differential activation of components of the JNK pathway in each condition. In Drosophila, fos can homodimerize and is known to act independently of jun in synapse overgrowth in hiw mutants and conditions of cytoskeletal disruption (19, 51). In sod2 mutants (and paraquat treatment), OS is generated predominantly in mitochondria, and under these conditions we observe a role for jun in synapse growth. This observation potentially suggests a context-dependent role for jun in the regulation of synaptic growth, which may indicate divergent responses to different cellular sources of OS.
Autophagy, OS, and Synaptic Growth.
OS is a potent activator of autophagy. Activation of autophagy has been shown to promote synapse growth in Drosophila via degradation of an upstream inhibitor of the JNK signaling pathway (20). Autophagic down-regulation of the hiw protein has been proposed as a mechanism to regulate synaptic growth (20). The hiw protein and its target wallenda (wnd) are both found presynaptically (19), but we observe JNK/AP-1 and autophagy activity contributing to synapse overgrowth in both the muscle and nerve. This observation suggests a previously unidentified muscle-derived JNK/AP-1 signal contributing to synapse growth that is likely to be independent of direct hiw/wnd regulation. The major identified transcriptional output of the JNK/AP-1 pathway is antioxidant and autophagic responses (24, 25). Autophagy and OS may directly contribute to the regulation of synapse growth mechanisms. The function of the cell-adhesion protein DE-cadherin is sensitive to OS (52), although the generality of this finding has yet to be defined. Autophagy has been observed to selectively phagocytose receptor proteins from an identified synapse (53, 54), suggesting a mechanism where autophagy can directly regulate the function and growth of the synapse independently of highwire degradation.
Taken together, our data suggest that the highly conserved JNK/AP-1 signaling pathway, a well-known mediator of synaptic growth and function, can be activated by OS to induce synaptic overgrowth. Many neurodegenerative disorders generate an OS burden in affected neurons. Investigating the effects of OS on synaptic development and function, as well as identifying signaling pathways, in such a disease context provides a potentially important insight into the pathology of a number of neurodegenerative diseases.
Materials and Methods
Wandering third-instar larvae were dissected and stained as described previously (9). Wandering third-instar larvae were dissected in modified HL3 (12). Intracellular recordings were made in muscle 6 from segments A3. Detailed methods can be found in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank John Roote, Fanis Missirlis, Manolis Fanto, Masayuki Miura, Aaron DiAntionio, Daisuke Yamamoto, Noreen Reist, Dirk Bohmann, the Developmental Studies Hybridoma Bank, Iowa and the Bloomington Drosophila Stock Center for stocks and reagents; and Matthias Landgraf, Sangeeta Chawla, and Chris Elliott for comments on the manuscript. This study was supported in part by a Quota studentship (to V.J.M.), a Biochemical Society Kreb's memorial scholarship (to S.C.), and Grant BB/I012273/1 (to S.T.S.) from the Biotechnology and Biological Sciences Research Council, and Medical Research Council Grants G0400580 (to S.T.S.) and G0802208 (to I.M.R.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1014511108/-/DCSupplemental.
References
- 1.Kurz T, Terman A, Brunk UT. Autophagy, ageing and apoptosis: The role of oxidative stress and lysosomal iron. Arch Biochem Biophys. 2007;462:220–230. doi: 10.1016/j.abb.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 2.Walkley SU, Wurzelmann S, Rattazzi MC, Baker HJ. Distribution of ectopic neurite growth and other geometrical distortions of CNS neurons in feline GM2 gangliosidosis. Brain Res. 1990;510:63–73. doi: 10.1016/0006-8993(90)90728-t. [DOI] [PubMed] [Google Scholar]
- 3.March PA, Wurzelmann S, Walkley SU. Morphological alterations in neocortical and cerebellar GABAergic neurons in a canine model of juvenile Batten disease. Am J Med Genet. 1995;57:204–212. doi: 10.1002/ajmg.1320570219. [DOI] [PubMed] [Google Scholar]
- 4.Terman A, Gustafsson B, Brunk UT. The lysosomal-mitochondrial axis theory of postmitotic aging and cell death. Chem Biol Interact. 2006;163:29–37. doi: 10.1016/j.cbi.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 5.Settembre C, Fraldi A, Rubinsztein DC, Ballabio A. Lysosomal storage diseases as disorders of autophagy. Autophagy. 2008;4:113–114. doi: 10.4161/auto.5227. [DOI] [PubMed] [Google Scholar]
- 6.Settembre C, et al. A block of autophagy in lysosomal storage disorders. Hum Mol Genet. 2008;17:119–129. doi: 10.1093/hmg/ddm289. [DOI] [PubMed] [Google Scholar]
- 7.Fu R, et al. Oxidative stress in Niemann-Pick disease, type C. Mol Genet Metab. 2010;101:214–218. doi: 10.1016/j.ymgme.2010.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keating DJ. Mitochondrial dysfunction, oxidative stress, regulation of exocytosis and their relevance to neurodegenerative diseases. J Neurochem. 2008;104:298–305. doi: 10.1111/j.1471-4159.2007.04997.x. [DOI] [PubMed] [Google Scholar]
- 9.Sweeney ST, Davis GW. Unrestricted synaptic growth in spinster—A late endosomal protein implicated in TGF-β-mediated synaptic growth regulation. Neuron. 2002;36:403–416. doi: 10.1016/s0896-6273(02)01014-0. [DOI] [PubMed] [Google Scholar]
- 10.Nakano Y, et al. Mutations in the novel membrane protein spinster interfere with programmed cell death and cause neural degeneration in Drosophila melanogaster. Mol Cell Biol. 2001;21:3775–3788. doi: 10.1128/MCB.21.11.3775-3788.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Usui-Aoki K, Nakano Y, Yamamoto D. Pathology of the adult central nervous system induced by genetic inhibition of programmed cell death in Drosophila pupae. Arch Insect Biochem Physiol. 2002;49:94–101. doi: 10.1002/arch.10011. [DOI] [PubMed] [Google Scholar]
- 12.Dermaut B, et al. Aberrant lysosomal carbohydrate storage accompanies endocytic defects and neurodegeneration in Drosophila benchwarmer. J Cell Biol. 2005;170:127–139. doi: 10.1083/jcb.200412001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aberle H, et al. wishful thinking encodes a BMP type II receptor that regulates synaptic growth in Drosophila. Neuron. 2002;33:545–558. doi: 10.1016/s0896-6273(02)00589-5. [DOI] [PubMed] [Google Scholar]
- 14.McCabe BD, et al. The BMP homolog Gbb provides a retrograde signal that regulates synaptic growth at the Drosophila neuromuscular junction. Neuron. 2003;39:241–254. doi: 10.1016/s0896-6273(03)00426-4. [DOI] [PubMed] [Google Scholar]
- 15.McCabe BD, et al. Highwire regulates presynaptic BMP signaling essential for synaptic growth. Neuron. 2004;41:891–905. doi: 10.1016/s0896-6273(04)00073-x. [DOI] [PubMed] [Google Scholar]
- 16.Rawson JM, Lee M, Kennedy EL, Selleck SB. Drosophila neuromuscular synapse assembly and function require the TGF-β type I receptor saxophone and the transcription factor Mad. J Neurobiol. 2003;55:134–150. doi: 10.1002/neu.10189. [DOI] [PubMed] [Google Scholar]
- 17.Sanyal S, Sandstrom DJ, Hoeffer CA, Ramaswami M. AP-1 functions upstream of CREB to control synaptic plasticity in Drosophila. Nature. 2002;416:870–874. doi: 10.1038/416870a. [DOI] [PubMed] [Google Scholar]
- 18.Sanyal S, Narayanan R, Consoulas C, Ramaswami M. Evidence for cell autonomous AP1 function in regulation of Drosophila motor-neuron plasticity. BMC Neurosci. 2003;4:20. doi: 10.1186/1471-2202-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Collins CA, Wairkar YP, Johnson SL, DiAntonio A. Highwire restrains synaptic growth by attenuating a MAP kinase signal. Neuron. 2006;51:57–69. doi: 10.1016/j.neuron.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 20.Shen W, Ganetzky B. Autophagy promotes synapse development in Drosophila. J Cell Biol. 2009;187:71–79. doi: 10.1083/jcb.200907109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang MC, Bohmann D, Jasper H. JNK signaling confers tolerance to oxidative stress and extends lifespan in Drosophila. Dev Cell. 2003;5:811–816. doi: 10.1016/s1534-5807(03)00323-x. [DOI] [PubMed] [Google Scholar]
- 22.Saitoh M, et al. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J. 1998;17:2596–2606. doi: 10.1093/emboj/17.9.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adler V, et al. Regulation of JNK signaling by GSTp. EMBO J. 1999;18:1321–1334. doi: 10.1093/emboj/18.5.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu H, Wang MC, Bohmann D. JNK protects Drosophila from oxidative stress by trancriptionally activating autophagy. Mech Dev. 2009;126:624–637. doi: 10.1016/j.mod.2009.06.1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jegga AG, Schneider L, Ouyang X, Zhang J. Systems biology of the autophagy-lysosomal pathway. Autophagy. 2011;7:477–489. doi: 10.4161/auto.7.5.14811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pattingre S, et al. Role of JNK1-dependent Bcl-2 phosphorylation in ceramide-induced macroautophagy. J Biol Chem. 2009;284:2719–2728. doi: 10.1074/jbc.M805920200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kurz T, Terman A, Gustafsson B, Brunk UT. Lysosomes and oxidative stress in aging and apoptosis. Biochim Biophys Acta. 2008;1780:1291–1303. doi: 10.1016/j.bbagen.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 28.Shimasaki H, Ueta N, Mowri HO, Inoue K. Formation of age pigment-like fluorescent substances during peroxidation of lipids in model membranes. Biochim Biophys Acta. 1984;792:123–129. [PubMed] [Google Scholar]
- 29.Uchida K. Lipofuscin-like fluorophores originated from malondialdehyde. Free Radic Res. 2006;40:1335–1338. doi: 10.1080/10715760600902302. [DOI] [PubMed] [Google Scholar]
- 30.McCord JM, Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein) J Biol Chem. 1969;244:6049–6055. [PubMed] [Google Scholar]
- 31.Sykiotis GP, Bohmann D. Keap1/Nrf2 signaling regulates oxidative stress tolerance and lifespan in Drosophila. Dev Cell. 2008;14:76–85. doi: 10.1016/j.devcel.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schuster CM, Davis GW, Fetter RD, Goodman CS. Genetic dissection of structural and functional components of synaptic plasticity. I. Fasciclin II controls synaptic stabilization and growth. Neuron. 1996;17:641–654. doi: 10.1016/s0896-6273(00)80197-x. [DOI] [PubMed] [Google Scholar]
- 33.Lnenicka GA, Keshishian H. Identified motor terminals in Drosophila larvae show distinct differences in morphology and physiology. J Neurobiol. 2000;43:186–197. [PubMed] [Google Scholar]
- 34.Wan HI, et al. Highwire regulates synaptic growth in Drosophila. Neuron. 2000;26:313–329. doi: 10.1016/s0896-6273(00)81166-6. [DOI] [PubMed] [Google Scholar]
- 35.Seabrooke S, Stewart BA. Synaptic transmission and plasticity are modulated by nonmuscle myosin II at the neuromuscular junction of Drosophila. J Neurophysiol. 2011;105:1966–1976. doi: 10.1152/jn.00718.2010. [DOI] [PubMed] [Google Scholar]
- 36.Crapo JD, Oury T, Rabouille C, Slot JW, Chang LY. Copper,zinc superoxide dismutase is primarily a cytosolic protein in human cells. Proc Natl Acad Sci USA. 1992;89:10405–10409. doi: 10.1073/pnas.89.21.10405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weisiger RA, Fridovich I. Mitochondrial superoxide dismutase. Site of synthesis and intramitochondrial localization. J Biol Chem. 1973;248:4793–4796. [PubMed] [Google Scholar]
- 38.Drechsel DA, Patel M. Chapter 21 Paraquat-induced production of reactive oxygen species in brain mitochondria. Methods Enzymol. 2009;456:381–393. doi: 10.1016/S0076-6879(08)04421-2. [DOI] [PubMed] [Google Scholar]
- 39.Bus JS, Aust SD, Gibson JE. Paraquat toxicity: Proposed mechanism of action involving lipid peroxidation. Environ Health Perspect. 1976;16:139–146. doi: 10.1289/ehp.7616139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103:239–252. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- 41.Weston CR, Davis RJ. The JNK signal transduction pathway. Curr Opin Cell Biol. 2007;19:142–149. doi: 10.1016/j.ceb.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 42.Martín-Blanco E, et al. puckered encodes a phosphatase that mediates a feedback loop regulating JNK activity during dorsal closure in Drosophila. Genes Dev. 1998;12:557–570. doi: 10.1101/gad.12.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arsham AM, Neufeld TP. A genetic screen in Drosophila reveals novel cytoprotective functions of the autophagy-lysosome pathway. PLoS One. 2009;4:e6068. doi: 10.1371/journal.pone.0006068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Simonsen A, et al. Promoting basal levels of autophagy in the nervous system enhances longevity and oxidant resistance in adult Drosophila. Autophagy. 2008;4:176–184. doi: 10.4161/auto.5269. [DOI] [PubMed] [Google Scholar]
- 45.Koh TW, Verstreken P, Bellen HJ. Dap160/intersectin acts as a stabilizing scaffold required for synaptic development and vesicle endocytosis. Neuron. 2004;43:193–205. doi: 10.1016/j.neuron.2004.06.029. [DOI] [PubMed] [Google Scholar]
- 46.Marie B, et al. Dap160/intersectin scaffolds the periactive zone to achieve high-fidelity endocytosis and normal synaptic growth. Neuron. 2004;43:207–219. doi: 10.1016/j.neuron.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 47.Verstreken P, et al. Synaptic mitochondria are critical for mobilization of reserve pool vesicles at Drosophila neuromuscular junctions. Neuron. 2005;47:365–378. doi: 10.1016/j.neuron.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 48.Kim S, Wairkar YP, Daniels RW, DiAntonio A. The novel endosomal membrane protein Ema interacts with the class C Vps-HOPS complex to promote endosomal maturation. J Cell Biol. 2010;188:717–734. doi: 10.1083/jcb.200911126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Korolchuk VI, et al. Drosophila Vps35 function is necessary for normal endocytic trafficking and actin cytoskeleton organisation. J Cell Sci. 2007;120:4367–4376. doi: 10.1242/jcs.012336. [DOI] [PubMed] [Google Scholar]
- 50.Wang X, Shaw WR, Tsang HT, Reid E, O'Kane CJ. Drosophila spichthyin inhibits BMP signaling and regulates synaptic growth and axonal microtubules. Nat Neurosci. 2007;10:177–185. doi: 10.1038/nn1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Massaro CM, Pielage J, Davis GW. Molecular mechanisms that enhance synapse stability despite persistent disruption of the spectrin/ankyrin/microtubule cytoskeleton. J Cell Biol. 2009;187:101–117. doi: 10.1083/jcb.200903166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.DeGennaro M, et al. Peroxiredoxin stabilization of DE-cadherin promotes primordial germ cell adhesion. Dev Cell. 2011;20:233–243. doi: 10.1016/j.devcel.2010.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rowland AM, Richmond JE, Olsen JG, Hall DH, Bamber BA. Presynaptic terminals independently regulate synaptic clustering and autophagy of GABAA receptors in Caenorhabditis elegans. J Neurosci. 2006;26:1711–1720. doi: 10.1523/JNEUROSCI.2279-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bamber BA, Rowland AM. Shaping cellular form and function by autophagy. Autophagy. 2006;2:247–249. doi: 10.4161/auto.2746. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.