Abstract
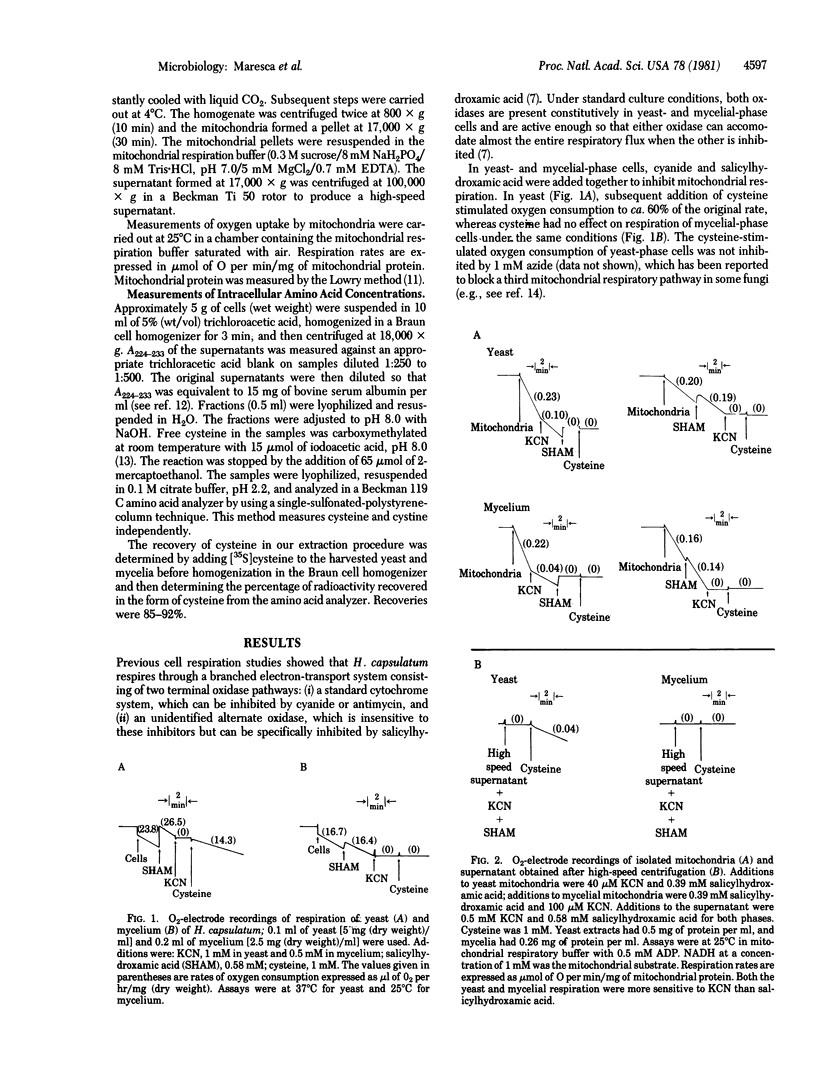
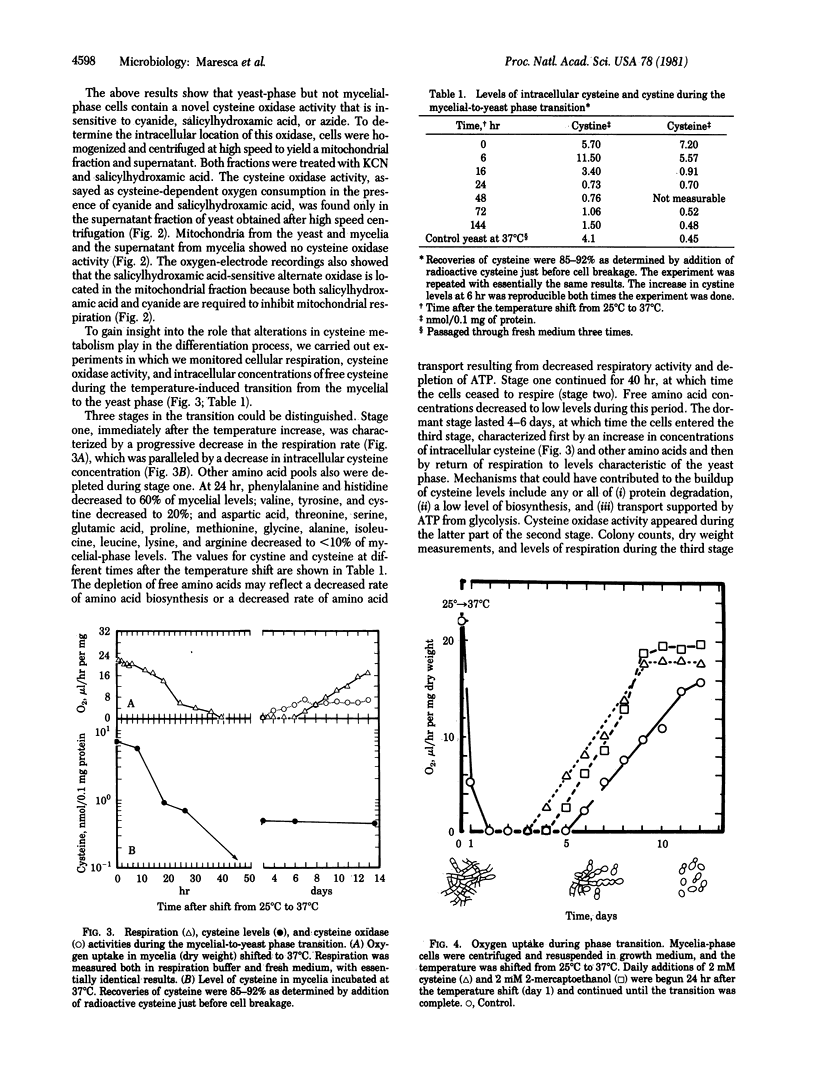
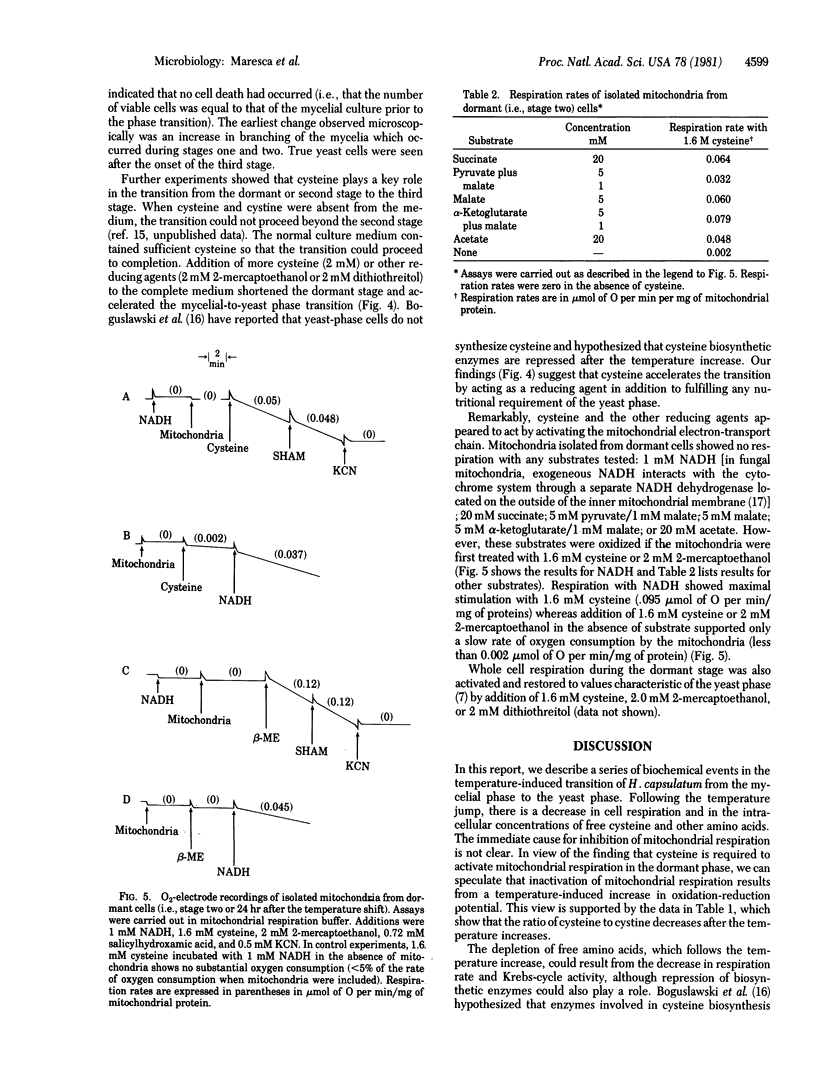
Three stages can be distinguished in the temperature-induced mycelial-to-yeast phase transition of Histoplasma capsulatum. Stage one is characterized by a progressive decrease in the respiration rate and in the intracellular concentrations of cysteine and other amino acids. By stage two, respiration has ceased completely and free cysteine has fallen to low levels. Exogenous cysteine is required during the second stage for activation of mitochondrial respiration (stage three) and completion of the morphological transition. Mitochondria isolated from cells in the second stage show no respiration with NADH, succinate, or other substrates unless they are first incubated with cysteine. In addition, a novel, cytosolic cysteine oxidase appears during the latter part of the second stage. In stage three, the respiration rate rises, intracellular concentrations of free cysteine and other amino acids increase to levels characteristic of yeast, and the morphological transition is completed. The results support the idea that alterations in cysteine metabolism play a key role in this differentiation process.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angeletti R. H., Bradshaw R. A., Wade R. D. Subunit structure and amino acid composition of mouse submaxillary gland nerve growth factor. Biochemistry. 1971 Feb 2;10(3):463–469. doi: 10.1021/bi00779a018. [DOI] [PubMed] [Google Scholar]
- Boguslawski G., Akagi J. M., Ward L. G. Possible role for cysteine biosynthesis in conversion from mycelial to yeast form of Histoplasma capsulatum. Nature. 1976 May 27;261(5558):336–338. doi: 10.1038/261336a0. [DOI] [PubMed] [Google Scholar]
- Edwards D. L., Unger B. W. Cyanide- and hydroxamate-resistant respiration in Neurospora crassa. J Bacteriol. 1978 Mar;133(3):1130–1134. doi: 10.1128/jb.133.3.1130-1134.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrison R. G., Dodd H. T., Hamilton J. W. The uptake of low molecular weight sulfur-containing compounds by Histoplasma capsulatum and related dimorphic fungi. Mycopathol Mycol Appl. 1970;40(2):171–180. doi: 10.1007/BF02051995. [DOI] [PubMed] [Google Scholar]
- Groves W. E., Davis F. C., Jr, Sells B. H. Spectrophotometric determination of microgram quantities of protein without nucleic acid interference. Anal Biochem. 1968 Feb;22(2):195–210. doi: 10.1016/0003-2697(68)90307-2. [DOI] [PubMed] [Google Scholar]
- Knight R. H., Body B. A., Kobayashi G. S., Medoff G. Balanced growth and morphogenesis of Histoplasma capsulatum in a defined synthetic medium. Sabouraudia. 1980 Mar;18(1):39–50. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lambowitz A. M., Slayman C. W. Cyanide-resistant respiration in Neurospora crassa. J Bacteriol. 1971 Dec;108(3):1087–1096. doi: 10.1128/jb.108.3.1087-1096.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maresca B., Lambowitz A. M., Kobayashi G. S., Medoff G. Respiration in the yeast and mycelial phases of Histoplasma capsulatum. J Bacteriol. 1979 May;138(2):647–649. doi: 10.1128/jb.138.2.647-649.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maresca B., Medoff G., Schlessinger D., Kobayashi G. S. Regulation of dimorphism in the pathogenic fungus Histoplasma capsulatum. Nature. 1977 Mar 31;266(5601):447–448. doi: 10.1038/266447a0. [DOI] [PubMed] [Google Scholar]
- McVeigh I., Houston W. E. Factors affecting mycelial to yeast phase conversion and growth of the yeast phase of Histoplasma capsulatum. Mycopathol Mycol Appl. 1972 Jun 15;47(1):135–151. doi: 10.1007/BF02126161. [DOI] [PubMed] [Google Scholar]
- Rippon J. W. Monitored environment system to control cell growth, morphology, and metabolic rate in fungi by oxidation-reduction potentials. Appl Microbiol. 1968 Jan;16(1):114–121. doi: 10.1128/am.16.1.114-121.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHERR G. H. Studies on the dimorphism of Histoplasma capsulatum. I. The roles of -SH groups and incubation temperature. Exp Cell Res. 1957 Feb;12(1):92–107. doi: 10.1016/0014-4827(57)90296-3. [DOI] [PubMed] [Google Scholar]
- Slayman C. L. Adenine nucleotide levels in Neurospora, as influenced by conditions of growth and by metabolic inhibitors. J Bacteriol. 1973 May;114(2):752–766. doi: 10.1128/jb.114.2.752-766.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]



