Abstract
Background
To evaluate the analgesic efficacy of bromfenac sodium ophthalmic solution 0.09% compared with ketorolac tromethamine ophthalmic solution 0.5% in laser epithelial keratomileusis (LASEK) or epithelial keratomileusis (epi-LASEK), sometimes referred to as epi-LASIK.
Methods
Eighty eyes (from 40 patients, 18 men and 22 women) undergoing bilateral simultaneous LASEK or epi-LASEK were randomized to receive ketorolac in one eye and bromfenac in the other. Mean age was 33.13 ± 9.34 years. One drop of bromfenac or ketorolac was instilled in each eye 15 minutes and one minute prior to surgery, and two and four hours following surgery. Patients were instructed to instill the medications on-label each day through postoperative day 4. The subjects completed pain and visual blurriness assessments from day of surgery to postoperative day 4. Uncorrected visual acuity was tested on postoperative days 1 and 6.
Results
For each of the five days, pain scores for bromfenac-treated eyes were significantly less than that for ketorolac-treated eyes (P < 0.01). Of the 40 patients, 32 (80%) said bromfenac provided better postoperative analgesia than ketorolac. There was no statistically significant difference in visual blurriness scores between the two groups (P > 0.1). Uncorrected visual acuity did not vary significantly between the treatment groups (P > 0.1). No serious adverse events were noted.
Conclusion
Bromfenac is subjectively superior to ketorolac in reducing postoperative pain following LASEK or epi-LASEK. The subjects tolerated the drugs well with no serious adverse outcomes and no difference in uncorrected visual acuity.
Keywords: LASEK, epi-LASEK, epi-LASIK, ketorolac, bromfenac, postoperative pain, non-steroidal anti-inflammatory drugs
Introduction
Laser refractive surgery has gone through several phases, beginning in the 1990s with photorefractive keratectomy. In photorefractive keratectomy, the cornea is reshaped to correct ametropia by removing corneal epithelial cells via a brush, which then leaves an irregular surface. Unfortunately, this technique often resulted in discomfort and pain that persisted beyond 48 hours.1
Subsequently, the procedure of laser-assisted in situ keratomileusis (LASIK) was developed. In LASIK a flap in the corneal stroma is created in lieu of removing epithelial cells. In 1999, laser epithelial keratomileusis (LASEK) was introduced to eliminate the flap-related complications of LASIK.
In LASEK, the epithelium is first loosened by ethanol, then rolled to the periphery before laser treatment on the stroma. The epithelium is subsequently placed back on the stroma. Patients undergoing LASEK have been reported to have a lower risk of postoperative ectasia, indicating that the surface treatment may be safer.2
Epithelial LASEK, or epi-LASEK, sometimes referred to as LASIK, is the latest surface ablation treatment, combining LASIK, LASEK, and photorefractive keratectomy. Epi-LASEK uses an epikeratome for planar separation of the epithelium, thereby keeping Bowman’s membrane intact. The goal of this planar separation is to reduce healing time, which may decrease patient discomfort.3 In epi-LASEK, the epithelium is rolled to the periphery and replaced after laser treatment (similarly to LASEK).
Regardless of which refractive surgical technique is used, they will all cause injury to the corneal sensory nociceptive fibers, which can then lead to various degrees of pain.1 Chemical mediators, such as prostaglandins, are released during ocular surgery and also contribute to postoperative pain. The pain created from the corneal wound and chemical mediators can be mitigated by bandage contact lenses and oral and topical analgesia. In ophthalmology, the primary topical analgesia used for the control of pain is nonsteroidal anti-inflammatory drugs (NSAIDs).4–6
Nonsteroidal anti-inflammatory drugs
Ocular surgery creates inflammation caused by the conversion of arachidonic acid to prostaglandin by cyclooxygenase (COX). As a class, NSAIDs are accepted as having the ability to inhibit COX, thereby reducing inflammation.7,8 Rowen reported that the preoperative administration of an NSAID will have its maximum analgesic effect in the early postoperative phase, when the pain is most intense.9
Several clinical studies have shown that topical ophthalmic NSAIDs are effective in controlling pain associated with refractive laser vision correction, cataract surgery, and corneal abrasions.7,10–19 There are several topical ophthalmic NSAIDs in branded and generic formulations currently available in the US. Two of the most commonly used NSAIDs are bromfenac and ketorolac. Bromfenac sodium ophthalmic solution 0.09% (Xibrom®, ISTA Pharmaceuticals Inc, Irvine, CA) has been shown to be safe and effective in controlling pain following photorefractive keratectomy,16,20 cataract surgery,11,21,22 and corneal abrasions.23 Ketorolac tromethamine ophthalmic solution 0.5% (Acular®, Allergan Inc, Irvine, CA) has been shown to be safe and effective in controlling pain following photorefractive keratectomy,12,17 radial keratotomy,19 corneal abrasion,13 and cataract surgery.14 In addition, ketorolac 0.5% has been shown to reduce corneal sensitivity significantly.15 This study compared the safety and efficacy of bromfenac sodium ophthalmic solution 0.09% and ketorolac tromethamine ophthalmic solution 0.5% in managing ocular pain following LASEK or epi-LASEK.
Materials and methods
Patients
A total of 40 patients were enrolled in the study. Subjects had to be at least 18 years old and scheduled to undergo bilateral simultaneous LASEK (n = 32) or epi-LASEK (n = 8). Other inclusion criteria included: a difference in prescription in both eyes not exceeding 3 diopters (D); best corrected visual acuity of at least 20/25; completion of a self-assessment pain report; anticipated return for all required study visits; and predicted compliance with all instructions. Patients were excluded if they were taking either topical or systemic NSAIDs, were taking and/or had taken either of the investigational drugs within 45 days of surgery, and/or were taking any kind of blood thinner. Pregnant women, nursing mothers, or mothers of infants younger than six months of age were excluded from the study.
In addition, patients were excluded if they had any systemic or ocular disease that might interfere with proper wound healing, such as a history of ocular trauma, uveitis, ocular infection and/or inflammation, anemia, diabetes mellitus, rheumatoid arthritis, and/or pulmonary disease other than reactive airways disease. Patients who had a history of alcohol abuse, tobacco use (smoking more than one pack of cigarettes per day), or drug abuse, as well as those with a history of sensitivity to sulfites or NSAIDs, were excluded.
Study design
This study was a prospective, randomized, single-center study. All surgeries were performed by one surgeon (EWC). An independent investigational review board (IRB Inc, Plantation, FL) approved the project, and informed consent was obtained from the subjects after the nature of procedures had been explained. ISTA Pharmaceuticals Inc provided ketorolac tromethamine ophthalmic solution 0.5% and bromfenac sodium ophthalmic solution 0.09% free of charge for this study and monetary compensation to the subjects to encourage participation.
Test articles and dose regimen
Patients were screened 7–30 days prior to surgery. Each right eye was randomized to receive either ketorolac tromethamine ophthalmic solution 0.5% or bromfenac sodium ophthalmic solution 0.09%. Each left eye was assigned to receive the NSAID alternative to the NSAID to which the right eye was randomized. For example, if the right eye was assigned to receive ketolorac, the left eye was assigned to receive bromfenac, and vice versa. On the day of surgery, one drop of bromfenac or ketorolac was instilled in each eye 15 minutes and one minute prior to surgery and two and four hours following surgery.
Patients were given vials marked by right and left eye indicators and by number 1 through number 4. For postoperative days 1 and 2, patients were instructed to instill the drops in the designated eye in the correct vial order (from number 1 through number 4) upon waking, at noon, at dinner time, and before bed, as shown in Table 1. To mask patients to the medications, each eye was instilled four times a day. For the ketorolac-treated eye, all vials contained ketorolac and were dosed on-label (one drop four times daily). For the bromfenac-treated eye, vials 1 and 3 contained bromfenac, and vials 2 and 4 contained a placebo (Refresh Plus® artificial tears, Allergan Inc), because on-label bromfenac use is one drop two times daily. For postoperative day 3 and day 4, the subjects were asked to only use vials 1 and 2 upon waking and at dinner time in order to taper the NSAIDs to half their dosages. The subjects were instructed to use labeled preservative-free artificial tears every hour in between instilling drops from the numbered vials to maintain lubrication of both eyes and facilitate epithelial healing.
Table 1.
Topical NSAID dose regimen on postoperative days 1 and 2
| Waking | Noon | Dinner time | Before bed | ||||
|---|---|---|---|---|---|---|---|
| Bromfenac-treated eye | Vial 1: Bromfenac | Tears every hour | Vial 2: Placebo | Tears every hour | Vial 3: Bromfenac | Tears every hour | Vial 4: Placebo |
| Ketorolac-treated eye | Vial 1: Ketorolac | Tears every hour | Vial 2: Ketorolac | Tears every hour | Vial 3: Ketorolac | Tears every hour | Vial 4: Ketorolac |
All other postoperative drug regimens were identical for both eyes. These included: one drop twice daily of antibiotic; one drop twice daily of prednisolone acetate ophthalmic suspension (Pred Forte®, Allergan Inc); oral methylprednisolone (Medrol Dosepak®, Pfizer Inc, New York, NY) according to tapering directions on the back of the blister pack (subjects with high prescription, for example, ≥−6 D or ≥−3 D cylinder, took two packs on alternating days); 400 mg of gabapentin (Neurontin®, Pfizer Inc) daily through postoperative day 3; and vitamin C 500 mg twice daily for three months. Topical antibiotics were prescribed as part of the surgeon’s regular postoperative care, which were levofloxacin ophthalmic solution 0.5% (Quixin®, Vistakon Pharmaceuticals Inc, Jacksonville, FL), gatifloxacin ophthalmic solution 0.3% (Zymar®, Allergan Inc), or moxifloxacin HCl ophthalmic solution 0.5% (Vigamox®, Alcon Laboratories Inc, Fort Worth, TX). Subjects applied the same antibiotic to each eye.
Surgical technique
Bilateral LASEK or epi-LASEK was performed by a single surgeon (EWC) using the VISX Star S4 IR Excimer Laser System (Abbott Laboratories Inc, Abbott Park, IL). Before surgery, topical tetracaine 0.5%, prednisolone acetate ophthalmic suspension 1% (EconoPred Plus®, Alcon), and levofloxacin ophthalmic solution 1.5% were applied once to each eye. Alcohol-assisted separation for LASEK was performed by application of 50% ethanol in a trephine well for 10 seconds, followed by rinsing of the eye with balanced salt solution and epithelial separation using a hockey spatula. Mechanical separation of the epithelium for epi-LASIK was performed by application of 50% ethanol in a trephine well for five seconds, followed by rinsing of the eye with balanced salt solution and epithelial separation using an epikeratome (Norwood EyeCare, Duluth, GA).
After stromal ablation was performed, 0.1% mitomycin C was applied on a cellulose sponge on the ablated corneal bed for high myopes (>−6 D) for a time equal to the laser ablation time. Topical prednisolone acetate and levofloxacin were applied once before a bandage contact lens (Acuvue Moist®, Johnson & Johnson Vision Care Inc, New Brunswick, NJ) was applied to the operated eye. Subjects were asked to refrain from using pain suppressants (escape medication), such as oral NSAIDs and/or narcotics. Ultraviolet protection was mandated postoperatively for three months to prevent scarring.
Efficacy outcome measures
Subjects completed a self-assessment questionnaire from day of surgery to postoperative day 4. On each day, the questionnaire asked the subject to evaluate the pain level and the visual blurriness in each eye based on the following scale: 0 = none, 1 = minimal, 2 = moderate, 3 = severe, and 4 = extreme. The questionnaire did not extend to postoperative day 6, when the patient returned to the clinic, because the NSAIDs were only instilled up to postoperative day 4. Moreover, it was preferable that the pain and visual blurriness scores were self-assessed before the postoperative day 6 visit to eliminate potential influence by the study coordinators.
The questionnaire was returned at the postoperative day 6 visit. Each patient was evaluated in the office on postoperative day 1 and day 6. Each patient was asked what medications were taken and at what dose to confirm compliance. Uncorrected visual acuity was also measured for each eye.
Efficacy analysis and statistical methods
The results were based on the total number of patients who completed all aspects of the study without premature termination. The Wilcoxon Signed-Rank test was performed to compare the pain scores and the visual blurriness scores between bromfenac-treated and ketorolac-treated eyes for each day. Characteristics, such as degree of myopia/hyperopia, degree of astigmatism, spherical equivalent, ablation depth, and uncorrected visual acuity in logMAR between bromfenac-treated and ketorolac-treated eyes, were compared using the paired Student’s t-test.
Safety outcome measures
During the office visits on postoperative day 1 and day 6, any complaints, such as dry eyes and irritation, were noted. A slit lamp examination was performed on each eye to confirm the presence of the bandage contact lens and to look for any corneal changes. On postoperative day 6, if the epithelium had healed, the bandage contact lenses were removed; otherwise, patients were given additional analgesics and were excluded from the study.
Results
One hundred eyes from 50 patients were initially enrolled in the study. Ten patients (20 eyes) were excluded from the study because patient-induced premature bandage contact lens removal from one or both eyes resulted in the need for escape medication(s). The mean average age of the per protocol cohort was 33.13 ± 9.34 years. There were no statistically significant surgical treatment differences between the two groups, as shown in Table 2. This was expected because the characteristics and treatments of two eyes from the same person are expected to be almost identical. Overall, in each group, 80% of the eyes underwent LASEK, 20% underwent epi-LASEK, and 75% were given mitomycin C. No subject had an immediate postoperative pain score that differed by more than one point, demonstrating that the two eyes were similar enough in postoperative pain to use as matched controls.
Table 2.
Participant characteristics
| Demographics | n (%) | ||
|---|---|---|---|
| Male | 18 (45) | ||
| Female | 22 (55) | ||
|
Ketorolac-treated eye n = 40 Mean ± SD |
Bromfenac-treated eye n = 40 Mean ± SD |
Pvalue | |
| Degree of myopia/hyperopia (preop) | −4.81 ± 3.81 | −4.86 ± 3.73 | 0.8 |
| Degree of astigmatism (preop) | −1.34 ± 0.85 | −1.26 ± 0.83 | 0.62 |
| Spherical equivalent (preop) | −5.48 ± 3.76 | −5.49 ± 3.75 | 0.95 |
| Ablation depth (in microns) | 76.4 ± 43.1 | 79.4 ± 40.0 | 0.46 |
Abbreviations: preop, preoperative; SD, standard deviation.
Postoperative pain relief and blurriness scores
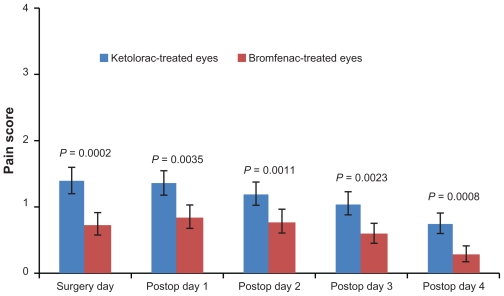
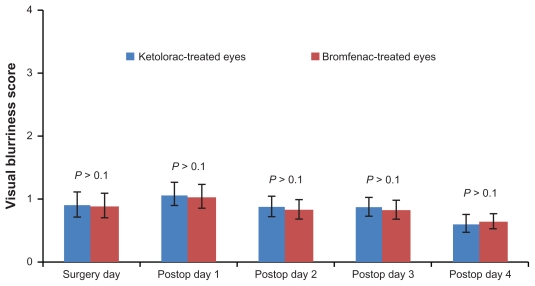
Patients assessed their pain and blurriness levels (using a scale from 0 to 4) daily in the clinic, from day of surgery through postoperative day 4. Eighty percent (32 of 40) of patients reported less pain in bromfenac-treated eyes than in ketorolac-treated eyes, as shown in Figure 1. Blurriness score differences did not reach statistical significance on any day, as shown in Figure 2. On the day of surgery, mean pain levels were 1.39 ± 0.19 in the ketorolac-treated eye and 0.74 ± 0.16 in the bromfenac-treated eye (P = 0.0002). Mean blurriness levels were 0.90 ± 0.19 in the ketorolac-treated eye and 0.88 ± 0.19 in the bromfenac-treated eye (P > 0.1). On postoperative day 1, mean pain levels were 1.36 ± 0.18 in the ketorolac-treated eye, and 0.85 ± 0.17 in the bromfenac-treated eye (P = 0.0035). Mean blurriness levels were 1.06 ± 0.18 in the ketorolac-treated eye and 1.03 ± 0.18 in the bromfenac-treated eye (P > 0.1). On postoperative day 2, mean pain levels were 1.19 ± 0.17 in the ketorolac-treated eye and 0.78 ± 0.17 in the bromfenac-treated eye (P = 0.0011). Mean blurriness levels were 0.87 ± 0.16 in the ketorolac-treated eye and 0.82 ± 0.15 in the bromfenac-treated eye (P > 0.1). On postoperative day 3, mean pain levels were 1.05 ± 0.16 in the ketorolac-treated eye and 0.60 ± 0.14 in the bromfenac-treated eye (P = 0.0023). Mean blurriness levels were 0.87 ± 0.16 in the ketorolac-treated eye and 0.82 ± 0.15 in the bromfenac-treated eye (P > 0.1). On postoperative day 4, mean pain levels were 0.75 ± 0.15 in the ketorolac-treated eye and 0.29 ± 0.11 in the bromfenac-treated eye (P = 0.0008). Mean blurriness levels were 0.60 ± 0.13 in the ketorolac-treated eye and 0.64 ± 0.13 in the bromfenac-treated eye (P > 0.1).
Figure 1.
Pain scores of ketorolac-treated eyes vs. bromfenac-treated eyes.
Note: Error bar = standard error
Figure 2.
Visual blurriness scores of ketorolac-treated eyes vs. bromfenac-treated eyes.
Note: Error bar = standard error
Efficacy of bromfenac and ketorolac on visual acuity
Uncorrected visual acuity was measured for each eye on postoperative day 1 and day 6. Data were converted to logMAR before statistical analysis. The uncorrected visual acuity in the ketorolac-treated eye was 0.50 ± 0.04 logMAR and 0.45 ± 0.04 in the bromfenac-treated eye on postoperative day 1, and 0.26 ± 0.03 and 0.28 ± 0.03, respectively, on postoperative day 6. These differences were not statistically significantly different.
Adverse events
There were no serious adverse events reported. In both sets of eyes, 20% (n = 8) reported temporary dry eye, and 5% (n = 2) reported temporary irritation. On postoperative day 6, epithelial regeneration was complete for all eyes in the study, thus permitting removal of bandage contact lenses during the clinic visit.
Discussion
Because the cornea is one of the most densely innervated tissues in the eye, it is also the most sensitive to pain.24 Each successive advancement in laser vision correction has sought to diminish postoperative pain, among other things. In LASIK, this postoperative pain is caused by the combination of corneal epithelial layer disruption and wound creation via the incision. 5 In LASEK and epi-LASEK, the pain is caused by the use of 50% ethanol solution in LASEK and an epikeratome in epi-LASEK to separate the epithelium at Bowman’s layer.25,26 Comparing the latter two procedures, epi-LASEK produces less postoperative pain than LASEK.3
This study examined the efficacy of two marketed NSAID ophthalmic solutions in their abilities to relieve pain following either epi-LASEK or LASEK surgery. The use of bromfenac 0.09% or ketorolac 0.5% for postoperative pain relief after laser vision correction surgery has been well documented.16,20,27,28 In this study, bromfenac 0.09% was found to be more effective in reducing overall postoperative pain than ketorolac 0.5%. The authors did not find any statistically significant differences in visual blurriness, uncorrected visual acuity, or adverse events between the two treatment groups. Stromal and epithelial variations between epi-LASEK procedures were eliminated by the application of 50% ethanol in a trephine well for five seconds, which loosened the corneal epithelial cells before the microkeratome was used for separation.
All topical NSAIDs work by eliminating the ability of COX-1 and COX-2 to produce prostaglandin.29 In oral form, bromfenac has been shown to have superior potency when compared with other NSAIDs, with no systemic side effects.30,31 In its topical form, bromfenac has been shown to have enhanced intraocular penetration and distribution in the cornea, iris, ciliary body, retina, and choroids, along with a longer duration of action that may last for up to 24 hours at these sites.8,23,32–34 Bromfenac has been cited as being more selective toward COX-2, with ketorolac being more selective towards COX-1.35–37 The inhibition of COX-2 has been associated with therapeutic effects of NSAIDs more than COX-1, in vivo and in humans.36,38,39 COX-1 is expressed in most tissues and organs, whereas COX-2 expression is localized primarily to inflammatory cells and tissues.40
Data have shown that inhibition of COX-2 was enough to produce a maximum analgesic effect in response to prostaglandin production.36,38 Our findings support the theories in those previously published studies, because bromfenac-treated eyes were subjectively evaluated to be less painful in the postoperative period than ketorolac-treated eyes.
Although this study was not double-masked, the patients evaluated the drugs in terms of pain and visual blurriness on their own in the absence of the study coordinators. Questionnaire responses were subjective and involved self-reported analysis of pain in both eyes individually, thereby eliminating potential biases. Even though the sample size was small (n = 80), the authors were able to show significant differences in pain scores between the treatment groups.
The authors suggest conducting future studies to determine whether inhibition of COX-1 or COX-2 reduces postoperative ocular inflammation and pain more effectively after epi-LASEK or LASEK. The authors also suggest that newer, commercially available ocular NSAIDs, such as once-daily bromfenac ophthalmic solution 0.09% (Bromday®, ISTA Pharmaceuticals Inc), ketorolac 0.4% or ketorolac 0.45% (Acular LS® and Acuvail®, respectively, Allergan Inc), and nepafenac ophthalmic suspension 0.1% (Nevanac®, Alcon Laboratories Inc) be evaluated for their analgesic capabilities after epi-LASEK or LASEK.
Acknowledgment
ISTA Pharmaceuticals Inc provided ketorolac tromethamine ophthalmic solution 0.5% and bromfenac sodium ophthalmic solution 0.09% free of charge for this study and monetary compensation to the subjects to encourage participation.
Footnotes
Disclosure
The authors have no financial interests in any materials or products discussed in the manuscript or any other disclosures to report.
References
- 1.Salceh TA, Almasri MA. A comparative study of post-operative pain in laser epithelial keratomileusis versus photorefractive keratectomy. Surgeon. 2003;1(4):229–232. doi: 10.1016/s1479-666x(03)80022-7. [DOI] [PubMed] [Google Scholar]
- 2.O’Keefe M, Kirwan C. Laser epithelial keratomileusis in 2010 – a review. Clin Experiment Ophthalmol. 2010;38(2):183–191. doi: 10.1111/j.1442-9071.2010.02198.x. [DOI] [PubMed] [Google Scholar]
- 3.Reilly CD, Panday V, Lazos V, Mittelstaedt BR. PRK vs LASEK vs Epi-LASIK: A comparison of corneal haze, postoperative pain and visual recovery in moderate to high myopia. Nepal J Ophthalmol. 2010;2(4):97–104. doi: 10.3126/nepjoph.v2i2.3715. [DOI] [PubMed] [Google Scholar]
- 4.Brilakis HS, Deutsch TA. Topical tetracaine with bandage soft contact lens pain control after photorefractive keratectomy. J Refract Surg. 2000;16(4):444–447. doi: 10.3928/1081-597X-20000701-07. [DOI] [PubMed] [Google Scholar]
- 5.Dougherty PJ. Acular LS before and during LASIK for the control of pain: a randomized, masked contralateral eye trial. J Refract Surg. 2009;25(2):210–213. doi: 10.3928/1081597X-20090201-06. [DOI] [PubMed] [Google Scholar]
- 6.Kaiser PK, Pineda R, Chynn EW. Corneal Abrasion Patching Study Group. A study of topical nonsteroidal anti-inflammatory drops and no pressure patching in the treatment of corneal abrasions. Ophthalmology. 1997;104(8):1353–1359. doi: 10.1016/s0161-6420(97)30135-3. [DOI] [PubMed] [Google Scholar]
- 7.Sher NA, Frantz JM, Talley A, et al. Topical diclofenac in the treatment of ocular pain after excimer photorefractive keratectomy. Refract Corneal Surg. 1993;9(6):425–436. [PubMed] [Google Scholar]
- 8.Cho H, Wolf KJ, Wolf EJ. Management of ocular inflammation and pain following cataract surgery: focus on bromfenac ophthalmic solution. Clin Ophthalmol. 2009;3:199–210. doi: 10.2147/opth.s4806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rowen S. Preoperative and postoperative medications used for cataract surgery. Curr Opin Ophthalmol. 1999;10(1):29–35. doi: 10.1097/00055735-199902000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Colin J, Paquette B. Comparison of the analgesic efficacy and safety of nepafenac ophthalmic suspension compared with diclofenac ophthalmic solution for ocular pain and photophobia after excimer laser surgery: a phase II, randomized, double-masked trial. Clin Ther. 2006;28(4):527–536. doi: 10.1016/j.clinthera.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 11.Donnenfeld ED, Holland EJ, Stewart RH, Gow JA, Grillone LR. Bromfenac ophthalmic solution 0.09% (Xibrom) for postoperative ocular pain and inflammation. Ophthalmology. 2007;114(9):1653–1662. doi: 10.1016/j.ophtha.2006.12.029. [DOI] [PubMed] [Google Scholar]
- 12.Eiferman RA, Hoffman RS, Sher NA. Topical diclofenac reduces pain following photorefractive keratectomy. Arch Ophthalmol. 1993;111(8):1022. doi: 10.1001/archopht.1993.01090080016002. [DOI] [PubMed] [Google Scholar]
- 13.Goyal R, Shankar J, Fone DL, Hughes DS. Randomised controlled trial of ketorolac in the management of corneal abrasions. Acta Ophthalmol Scand. 2001;79(2):177–179. doi: 10.1034/j.1600-0420.2001.079002177.x. [DOI] [PubMed] [Google Scholar]
- 14.Price MO, Price FW. Efficacy of topical ketorolac tromethamine 0.4% for control of pain or discomfort associated with cataract surgery. Curr Med Res Opin. 2004;20(12):2015–2019. doi: 10.1185/030079904x16759. [DOI] [PubMed] [Google Scholar]
- 15.Seitz B, Sorken K, LaBree LD, Garbus JJ, McDonnell PJ. Corneal sensitivity and burning sensation. Comparing topical ketorolac and diclofenac. Arch Ophthalmol. 1996;114(8):921–924. doi: 10.1001/archopht.1996.01100140129002. [DOI] [PubMed] [Google Scholar]
- 16.Sher NA, Golben MR, Bond W, Trattler WB, Tauber S, Voirin TG. Topical bromfenac 0.09% vs ketorolac 0.4% for the control of pain, photophobia, and discomfort following PRK. J Refract Surg. 2009;25(2):214–220. doi: 10.3928/1081597X-20090201-07. [DOI] [PubMed] [Google Scholar]
- 17.Solomon KD, Donnenfeld ED, Raizman M, et al. Safety and efficacy of ketorolac tromethamine 0.4% ophthalmic solution in post-photorefractive keratectomy patients. J Cataract Refract Surg. 2004;30(8):1653–1660. doi: 10.1016/j.jcrs.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 18.Szerenyi K, Sorken K, Garbus JJ, Lee M, McDonnell PJ. Decrease in normal human corneal sensitivity with topical diclofenac sodium. Am J Ophthalmol. 1994;118(3):312–315. doi: 10.1016/s0002-9394(14)72954-x. [DOI] [PubMed] [Google Scholar]
- 19.Yee RW. Analgesic efficacy and safety of nonpreserved ketorolac tromethamine ophthalmic solution following radial keratotomy. Ketorolac Radial Keratotomy Study Group. Am J Ophthalmol. 1998;125(4):472–480. doi: 10.1016/s0002-9394(99)80187-1. [DOI] [PubMed] [Google Scholar]
- 20.Durrie DS, Kennard MG, Boghossian AJ. Effects of nonsteroidal ophthalmic drops on epithelial healing and pain in patients undergoing bilateral photorefractive keratectomy (PRK) Adv Ther. 2007;24(6):1278–1285. doi: 10.1007/BF02877774. [DOI] [PubMed] [Google Scholar]
- 21.Ruiz J, Lopez M, Mila J, Lozoya E, Lozano JJ, Pouplana R. QSAR and conformational analysis of the antiinflammatory agent amfenac and analogues. J Comput Aided Mol Des. 1993;7(2):183–198. doi: 10.1007/BF00126444. [DOI] [PubMed] [Google Scholar]
- 22.Seward MS, Cooke DL, Grillone LR, Sacks RM, Group BS. Association for Research in Vision and Ophthalmology. Vol. 47. Fort Lauderdale, FL: Invest Ophthalmol Vis Sci; 2006. Topical Xibrom 0.09% significantly reduced ocular pain following cataract surgery; p. E-Abstract 679. [Google Scholar]
- 23.Perry HD, Donnenfeld ED. Bromfenac ophthalmic solution 0.09%: ocular role and systemic safety profile. Expert Rev Ophthalmol. 2008;3(2):121–129. [Google Scholar]
- 24.Muller LJ, Marfurt CF, Kruse F, Tervo TM. Corneal nerves: structure, contents and function. Exp Eye Res. 2003;76(5):521–542. doi: 10.1016/s0014-4835(03)00050-2. [DOI] [PubMed] [Google Scholar]
- 25.Kim JH, Lee J, Kim JY, Tchah H. Early postoperative pain and visual outcomes following epipolis-laser in situ keratomileusis and photorefractive keratectomy. Korean J Ophthalmol. 2010;24(3):143–147. doi: 10.3341/kjo.2010.24.3.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O’Doherty M, Kirwan C, O’Keeffe M, O’Doherty J. Postoperative pain following epi-LASIK, LASEK, and PRK for myopia. J Refract Surg. 2007;23(2):133–138. doi: 10.3928/1081-597X-20070201-05. [DOI] [PubMed] [Google Scholar]
- 27.Donnenfeld ED, Holland EJ, Durrie DS, Raizman MB. Double-masked study of the effects of nepafenac 0.1% and ketorolac 0.4% on corneal epithelial wound healing and pain after photorefractive keratectomy. Adv Ther. 2007;24(4):852–862. doi: 10.1007/BF02849978. [DOI] [PubMed] [Google Scholar]
- 28.Price FW, Price MO, Zeh W, Dobbins K. Pain reduction after laser in situ keratomileusis with ketorolac tromethamine ophthalmic solution 0.5%: a randomized, double-masked, placebo-controlled trial. J Refract Surg. 2002;18(2):140–144. doi: 10.3928/1081-597X-20020301-07. [DOI] [PubMed] [Google Scholar]
- 29.Jones J, Francis P. Ophthalmic utility of topical bromfenac, a twice-daily nonsteroidal anti-inflammatory agent. Expert Opin Pharmacother. 2009;10(14):2379–2385. doi: 10.1517/14656560903188425. [DOI] [PubMed] [Google Scholar]
- 30.Sancilio LF, Nolan JC, Wagner LE, Ward JW. The analgesic and antiinflammatory activity and pharmacologic properties of bromfenac. Arzneimittelforschung. 1987;37(5):513–519. [PubMed] [Google Scholar]
- 31.Walsh DA, Moran HW, Shamblee DA, et al. Antiinflammatory agents. 3. Synthesis and pharmacological evaluation of 2-amino-3-benzoylphenylacetic acid and analogues. J Med Chem. 1984;27(11):1379–1388. doi: 10.1021/jm00377a001. [DOI] [PubMed] [Google Scholar]
- 32.McNamara TR, Baklayan GA, Deshmukh HM, Patterson HM, Gow JA. Association for Research in Vision and Ophthalmology. Vol. 47. Fort Lauderdale, FL: Invest Ophthalmol Vis Sci; 2006. Concentrations of radioactivity in ocular tissues following a single topical ocular dose of 14c–bromfenac ophthalmic solution (Xibrom) p. E-Abstract 5086. [Google Scholar]
- 33.Ogawa T, Miyake K, McNamara TR, Gow JA. Association for Research in Vision and Ophthalmology. Fort Lauderdale, FL: Invest Ophthalmol Vis Sci; 2006. Pharmacokinetic profile of topically applied bromfenac sodium ophthalmic solution 0.1% in subjects undergoing cataract surgery; p. E-Abstract 687. [Google Scholar]
- 34.Miyake K, Ogawa T, Tajika T, Gow JA, McNamara TR. Ocular pharmacokinetics of a single dose of bromfenac sodium ophthalmic solution 0.1% in human aqueous humor. J Ocul Pharmacol Ther. 2008;24(6):573–578. doi: 10.1089/jop.2007.0132. [DOI] [PubMed] [Google Scholar]
- 35.Ahuja M, Dhake AS, Sharma SK, Majumdar DK. Topical ocular delivery of NSAIDs. AAPS J. 2008;10(2):229–241. doi: 10.1208/s12248-008-9024-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Waterbury LD, Silliman D, Jolas T. Comparison of cyclooxygenase inhibitory activity and ocular anti-inflammatory effects of ketorolac tromethamine and bromfenac sodium. Curr Med Res Opin. 2006;22(6):1133–1140. doi: 10.1185/030079906X112471. [DOI] [PubMed] [Google Scholar]
- 37.Alcon Laboratories Inc, assignee. Topically administrable compositions containing 3-benzoylphenylaceteic acid derivatives for treatment of ophthalmic inflammatory disorders. Dec 12, 1995. [Google Scholar]
- 38.Seibert K, Zhang Y, Leahy K, et al. Pharmacological and biochemical demonstration of the role of cyclooxygenase 2 in inflammation and pain. Proc Natl Acad Sci U S A. 1994;91(25):12013–12017. doi: 10.1073/pnas.91.25.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Masferrer JL, Zweifel BS, Manning PT, et al. Selective inhibition of inducible cyclooxygenase 2 in vivo is antiinflammatory and nonulcerogenic. Proc Natl Acad Sci U S A. 1994;91(8):3228–3232. doi: 10.1073/pnas.91.8.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Crofford LJ. COX-1 and COX-2 tissue expression: implications and predictions. J Rheumatol Suppl. 1997;49:15–19. [PubMed] [Google Scholar]