Abstract
Background
Oncogenic BRAF mutations have been found in diverse malignancies and activate RAF/MEK/ERK signaling, a critical pathway of tumorigenesis. We examined the clinical characteristics and outcomes of patients with mutant (mut) BRAF advanced cancer referred to phase 1 clinic.
Methods
We reviewed the records of 80 consecutive patients with mutBRAF advanced malignancies and 149 with wild-type (wt) BRAF (matched by tumor type) referred to the Clinical Center for Targeted Therapy and analyzed their outcome.
Results
Of 80 patients with mutBRAF advanced cancer, 56 had melanoma, 10 colorectal, 11 papillary thyroid, 2 ovarian and 1 esophageal cancer. Mutations in codon 600 were found in 77 patients (62, V600E; 13, V600K; 1, V600R; 1, unreported). Multivariate analysis showed less soft tissue (Odds ratio (OR) = 0.39, 95%CI: 0.20–0.77, P = 0.007), lung (OR = 0.38, 95%CI: 0.19–0.73, p = 0.004) and retroperitoneal metastases (OR = 0.34, 95%CI: 0.13–0.86, p = 0.024) and more brain metastases (OR = 2.05, 95%CI: 1.02–4.11, P = 0.043) in patients with mutBRAF versus wtBRAF. Comparing to the corresponding wtBRAF, mutBRAF melanoma patients had insignificant trend to longer median survival from diagnosis (131 vs. 78 months, p = 0.14), while mutBRAF colorectal cancer patients had an insignificant trend to shorter median survival from diagnosis (48 vs. 53 months, p = 0.22). In melanoma, V600K mutations in comparison to other BRAF mutations were associated with more frequent brain (75% vs. 36.3%, p = 0.02) and lung metastases (91.6% vs. 47.7%, p = 0.007), and shorter time from diagnosis to metastasis and to death (19 vs. 53 months, p = 0.046 and 78 vs. 322 months, p = 0.024 respectively). Treatment with RAF/MEK targeting agents (Hazard ratio (HR) = 0.16, 95%CI: 0.03–0.89, p = 0.037) and any decrease in tumor size after referral (HR = 0.07, 95%CI: 0.015–0.35, p = 0.001) correlated with longer survival in mutBRAF patients.
Conclusions
BRAF appears to be a druggable mutation that also defines subgroups of patients with phenotypic overlap, albeit with differences that correlate with histology or site of mutation.
Introduction
The RAS proteins regulate cell proliferation, survival and differentiation by activating a number of downstream effectors, including RAF protein kinase. Once activated, RAF stimulates a signaling cascade involving the MEK/ERK pathway. BRAF, a serine-threonine kinase, is one of three RAF protein kinase family members (ARAF, BRAF and CRAF) [1]. The BRAF proto-oncogene has recently been the focus of intensive research, as its mutation constitutively activates RAF/MEK signaling, a major driver of carcinogenesis in various malignancies, most notably in melanoma, colon cancer, and papillary thyroid cancer1. The most common BRAF mutation is a substitution of glutamic acid for valine in codon 600 (V600E) [2]–[3].
In recent years, a plethora of promising compounds that target the RAS/RAF/MEK pathway have entered clinical trials, some of them demonstrating promising clinical activity, mainly in cancers with BRAF mutations [4]–[6]. Consequently, testing for activating mutations in BRAF is becoming more common, especially if patients are to be treated with BRAF inhibitors, or other pathway modulators such as MEK inhibitors.
Oncogenic mutations such as BRAF occur across diverse tumor types. Herein, we examined clinical features and outcome associated with the presence of BRAF mutations, with the main objectives being to outline clinical and prognostic characteristics associated with the presence of BRAF mutations, whether or not specific BRAF mutations have a distinct clinical course, as well predictive impact of targeted treatment.
Methods
Patients
Starting in January 2006, we investigated the BRAF mutation status of patients with advanced tumors and available tissue referred to the Clinical Center for Targeted Therapy in the Department of Investigational Cancer Therapeutics (Phase I Clinical Trials Program) at The University of Texas MD Anderson Cancer Center. The registration of patients in the database, pathology assessment, and mutation analysis were performed at MD Anderson. In total, 80 consecutive patients with BRAF mutations were selected.
To define distinguishing features of mutant (mut) BRAF advanced cancers, we selected a control group of consecutive patients with wild-type (wt) BRAF advanced cancers seen at our center during the same time period and matched in a 1∶2 ratio by tumor type with mutBRAF patients.
The MD Anderson Cancer Center Institutional Review Board has approved the study. Written consent was given by the patients for their information to be stored in the hospital database and used for research.
Tissue samples and mutational analysis
Archival formalin-fixed, paraffin-embedded tissue blocks or material from fine-needle aspiration biopsy obtained from diagnostic and/or therapeutic procedures were used to test for BRAF mutations. All pathology was centrally confirmed at MD Anderson. BRAF mutation testing was performed in a Clinical Laboratory Improvement Amendment–certified Molecular Diagnostic Laboratory within the Division of Pathology and Laboratory Medicine at MD Anderson. DNA was extracted from micro-dissected paraffin-embedded tumor sections and analyzed using a polymerase chain reaction (PCR)-based DNA sequencing method for BRAF codons 595–600 mutations of exon 15 by pyrosequencing as previously described [7]. Substitution of glutamic acid for valine in codon 600 is denoted as V600E; V600K denotes substitution of lysine for valine; V600R, arginine for valine.
Whenever possible, we tested for other mutations such as EGFR (exons 18 and 21) [8], KIT (exons 11, 13 and 17) [9], PIK3CA (exons 9 and 20) [10], NRAS and KRAS (exon 2) [7], [11]. PTEN loss was assessed using immunohistochemistry (monoclonal mouse anti-human PTEN, clone 6H2.1, Dako®, Denmark) [12].
Clinical characteristics and treatment evaluation
All clinical variables were assessed by review of the electronic medical record. Treatment efficacy was evaluated from computed tomography (CT) scans and/or magnetic resonance imaging (MRI) at baseline before treatment initiation and then about every 6 to 8 weeks. All radiographs were read in the Department of Radiology at MD Anderson and reviewed in the Department of Investigational Cancer Therapeutics tumor measurement clinic.
Prognostic assessment was done using the Royal Marsden Hospital (RMH) [13] prognostic score as follows: 0 points, normal lactate dehydrogenase (LDH), albumin ≥3.5 g/dL, a ≤2 metastatic sites; 1 point- LDH>upper limit of normal, albumin <3.5 g/dL, >2 metastatic sites. Patients with 0–1 points had a good RMH score, and patients with 2–3 points had a poor RMH score.
Statistical Analysis
Statistical analysis was verified by our statistician (SW). The following covariates pertaining to patient characteristics were analyzed: type of cancer, age, gender, race, personal history of cancer, history of smoking or alcoholism, family history of cancer, site and number of metastases, presence of ascites, pleural effusion or deep venous thrombosis, tumor markers (CEA, CA 19-9, CA125, CA27.29), lactate dehydrogenase, albumin, hemoglobin, white blood cell count, platelet count, calcium level, site of mutation, presence of other aberrations (PIK3CA, NRAS or KRAS, KIT mutation and PTEN loss), date of diagnosis, locally advanced disease, distant metastases, referral, death or date of last follow-up, information about best standard systemic treatment for metastatic disease and treatment with phase 1 trial.
Descriptive statistics were used to summarize patient characteristics. The Fisher exact test was used to test for any association between two categorical variables. Mann-Whitney U test was used to test for association between age and BRAF mutation status.
Overall survival (OS) was measured (method of Kaplan-Meier) from the time of diagnosis, date of metastases, or date of referral to the date of death or last follow-up, whichever occurred first. Patients alive were censored at the last follow-up date. Progression-free survival (PFS) was defined as the time interval between the start of therapy to the first observation of disease progression (as determined by Response Evaluation Criteria in Solid Tumors (RECIST) [36] or death, whichever came first. Patients alive and without disease progression were censored at the last follow-up date. Disease-free survival was measured from time of diagnosis to first distant metastases. Log-rank test was used to compare OS or PFS among subgroups. Multivariate analysis with the Cox proportional hazards regression model was used to assess an independent association between a characteristics and PFS or OS. The “enter” method was used where all the variables are entered in the model without checking. Binary logistic regression method was used to test for any independent correlation between a categorical variable and BRAF mutational status. The “enter” method was used where all the variables are entered in the model without checking. All tests were two-sided. A p value less than 0.05 was considered statistically significant. Statistical analysis was performed using SPSS (version 17.0; SPSS, Chicago, IL, USA).
Results
Patient characteristics
A total of 80 patients with advanced tumors and mutBRAF were identified. The median age was 52 years (range, 18–78 years), and 43 were men (54%). The majority of patients had melanoma (n = 56, 70%) followed by papillary thyroid carcinoma (n = 11, 14%), colorectal cancer (n = 10, 13%), and other tumor types (ovarian cancer, n = 2, 2%; esophageal cancer, n = 1, 1%) (reflecting referral patterns to our clinic), (Table 1).
Table 1. Clinical characteristic of 80 patients with BRAF-mutant disease and 149 matched controls with BRAF-wild-type (Univariate Analysis).
| mutBRAF (N = 80) | wtBRAF (N = 149) | P value | |
| Age at diagnosis (median, range) | 52 (18–78) | 58 (24–87) | 0.002 |
| Age at diagnosis ≥60 years | 27 (34%) | 69 (46%) | 0.07 |
| Gender | |||
| Men | 43 (54%) | 100 (67%) | 0.06 |
| Women | 37 (46%) | 49 (33%) | 0.06 |
| Race | |||
| Caucasian | 67 (87%) | 130 (87%) | Not significant |
| Hispanic | 8 (10%) | 9 (6%) | Not significant |
| Asian | 2 (3%) | 1 (1%) | Not significant |
| African-American | 0 (0%) | 9 (6%) | Not significant |
| Type of Cancer | |||
| Melanoma | 56 (70%) | 112 (75%) | Not significant |
| Colorectal cancer | 10 (13%) | 20 (14%) | Not significant |
| Papillary thyroid cancer | 11 (14%) | 11 (7%) | Not significant |
| Ovarian cancer | 2 (2%) | 4 (3%) | Not significant |
| Esophageal cancer | 1 (1%) | 2 (1%) | Not significant |
| Personal history of cancer | 15 (19%) | 27 (18%) | 0.99 |
| Family history of cancer | 62 (78%) | 120 (81%) | 0.61 |
| First degree | 48 (60%) | 96 (64%) | 0.56 |
| Age<60 years | 25 (31%) | 43 (29%) | 0.76 |
| First degree & age<60 | 16 (20%) | 30 (20%) | 0.99 |
| Social history | |||
| Tobacco | 26 (33%) | 61 (41%) | 0.25 |
| Alcohol | 11 (14%) | 28 (19%) | 0.36 |
| Site of metastasis | |||
| Brain | 27 (34%) | 45 (30%) | 0.65 |
| Liver | 31 (39%) | 67 (45%) | 0.40 |
| Lung | 48 (60%) | 118 (79%) | 0.003 |
| Retroperitoneum | 9 (11%) | 37 (25%) | 0.004 |
| Bone | 20 (25%) | 41 (28%) | 0.75 |
| Superficial Lymph Node | 39 (49%) | 76 (51%) | 0.78 |
| Soft tissue | 26 (33%) | 75 (50%) | 0.01 |
| Peritoneum | 33 (41%) | 61 (41%) | 0.99 |
| Mediastinum | 19 (24%) | 38 (26%) | 0.87 |
| Stage at diagnosis | |||
| Early stage | 57 (71%) | 91 (61%) | 0.14 |
| Locally advanced stage | 13 (16%) | 36 (24%) | 0.18 |
| Metastatic stage | 10 (13%) | 22 (15%) | 0.69 |
| Pleural effusion | 11 (14%) | 17 (11%) | 0.60 |
| Ascites | 8 (10%) | 13 (9%) | 0.74 |
| Thrombosis | 15 (19%) | 30 (20%) | 0.80 |
| Site of mutation | |||
| C600/599 | 1 (V600E/T599S) | N/A | N/A |
| C600 | 77 ( 62 V600E, 13 V600K, 1 V600R, 1 unknown) | N/A | N/A |
| C601 | 2 (2 K601E) | N/A | N/A |
| PTEN loss | 2/71 (29%) | 2/20 (10%) | 0.27 |
| KRAS mutation | 0/24 (0%) | 13/45 (29%) | 0.002 |
| PIK3CA mutation | 1/26 (4%) | 4/46 (9%) | 0.64 |
| NRAS mutation | 1/17 (6%) | 42/108 (39%) | 0.006 |
| KIT mutation | 0/30 (0%) | 3/93 (3%) | 0.99 |
| EGFR mutation | 0/18 (0%) | 0/43 (0%) | 0.99 |
| Median time from diagnosis to metastases (months) (95%CI) | |||
| Melanoma | 44 (17–71) | 20 (16–24) | 0.058 |
| Colorectal cancer | 0 | 8 (0–28.4) | 0.96 |
| Papillary thyroid cancer | 37 (0–74.7) | 73 (29.8–116.1) | 0.45 |
| Combined | 28 (12.8–43.1) | 19 (14.5–23.5) | 0.13 |
| Time from diagnosis to metastasis ≥2 years | 45 (56%) | 63 (42%) | 0.052 |
Denominator refers to the number of patients tested.
The most common metastatic sites were lungs (n = 48, 60%), superficial lymph nodes (n = 39, 49%), peritoneum (n = 33, 41%), liver (n = 31, 39%), brain (n = 27, 34%), soft tissue (n = 26, 33%), bones (n = 20, 25%) and retroperitoneal lymph nodes (n = 9, 11%).
We identified 149 control patients with advanced cancers who tested negative for BRAF mutations in the same time period and who were matched on a 1∶2 basis by tumor type with mutBRAF patients. For papillary thyroid cancer, matching was done with a 1∶1 ratio due to an inadequate number of patients referred who had tests done and tested negative for BRAF mutation. The detailed patient characteristics are shown in Table 1.
Groups with mutBRAF and wtBRAF were similar in terms of median time from diagnosis to referral to the phase 1 clinic as calculated by log-rank method (12 vs. 12.7 months, p = 0.95). Initial cancer staging at diagnosis was also equally distributed among the two groups. Patients were treated on a clinical trial if they had failed to respond to conventional treatment. Whenever possible, patients with mutBRAF were offered treatment targeting the RAF/MEK pathway. Patients had a median of two prior treatments, regardless of BRAF status.
Types of BRAF mutations
Of the 80 patients with mutBRAF, 77 (96%) had mutations in codon 600 and two (3%) in codon 601. One (1%) patient had simultaneous mutations in codons 599 and 600. Of the 77 patients with codon 600 mutBRAF, 62 (81%) had V600E mutations (melanoma, n = 40; colorectal, n = 8; papillary thyroid cancer, n = 11; esophageal, n = 1 and ovarian, n = 2), 13 (17%) V600K mutations (melanoma, n = 12; colorectal cancer, n = 1), 1 (1%) V600R mutation (melanoma, n = 1) and one of unreported type (colorectal, n = 1) (Table 1).
BRAF mutations and clinical features
Univariate Analysis
Patient age at diagnosis was significantly younger for patients with mutBRAF (median age = 52 years) versus wtBRAF disease (median age = 58 years) (p = 0.002). Men were more commonly represented in both mutBRAF and wtBRAF groups, but the proportion of women trended towards being greater in the mutBRAF group (46% vs. 33%, p = 0.06). There were no significant differences between the mutBRAF and wtBRAF group for other characteristics, including ethnicity, personal, social and family history, complications including thrombosis, ascites and pleural effusion (Table 1).
Patients who had mutBRAF tumors had less frequent involvement of the lungs (60% vs. 79%; p = 0.003), retroperitoneal nodes (11% vs. 25%; p = 0.004), and soft tissue (33% vs. 50%; p = 0.01). In subgroup analysis, this pattern was also observed in each of the three major tumor types; however due to the small number of patients in the non-melanoma cohort, significance was only achieved for patients with melanoma (unshown data). There was no difference in involvement of other sites by metastases.
Multivariate Analysis
In multivariate analysis using a logistic regression model, patients with mutBRAF had less frequent metastases to (i) soft tissue (OR = 0.39, 95% CI: 0.20–0.77, p = 0.007); (ii) lung (OR = 0.38, 95% CI: 0.19–0.73, p = 0.004); and (ii) the retroperitoneum (OR = 0.34, 95% CI: 0.13–0.86, p = 0.024) (Table 2). Women were more likely to have mutBRAF than wtBRAF (OR = 1.92, 95% CI: 1.02–3.57, p = 0.045). Patients with mutBRAF compared with wtBRAF were more likely to have brain metastases (OR = 2.05, 95% CI: 1.02–4.11, p = 0.043). Patients younger than 60 years showed a trend towards higher likelihood of BRAF mutations (OR = 1.88, 95% CI: 0.99–3.70, p = 0.053). In subgroup analysis of melanoma, this trend was statistically significant (multivariate p value = 0.023) (Table 3). The smaller numbers of patients with other cancers precluded a separate analysis for this factor. An interval from diagnosis to distant metastases of ≥2 years was more likely to be associated with mutBRAF (Odds ratio (OR) = 2.84, 95% Confidence interval (CI): 1.18–4.14, p = 0.013) (Table 2). However, in disease specific analyses, in colorectal and papillary thyroid cancer, the proportion of patients with a disease-free interval from diagnosis to metastases of over two years was less for patients with mutBRAF disease, but this did not reach statistical significance because of the small number of patients in each subgroup (data not shown).
Table 2. Multivariate analysis by logistic regression model showing the clinico-pathological features correlated with the BRAF mutation.
| Clinical feature | Odds Ratio | Lower 95% | Upper 95% | P value |
| Age<60 years | 1.88 | 0.99 | 3.70 | 0.053 |
| Women | 1.92 | 1.02 | 3.57 | 0.045 |
| Metastatic site | ||||
| Soft tissue | 0.39 | 0.20 | 0.77 | 0.007 |
| Brain | 2.05 | 1.02 | 4.11 | 0.043 |
| Lung | 0.38 | 0.19 | 0.73 | 0.004 |
| Liver | 0.86 | 0.46 | 1.63 | 0.665 |
| Retroperitoneum | 0.34 | 0.13 | 0.86 | 0.024 |
| Bone | 1.10 | 0.53 | 2.26 | 0.78 |
| Peritoneum | 0.97 | 0.51 | 1.83 | 0.92 |
| Superficial lymph node | 0.91 | 0.47 | 1.75 | 0.79 |
| Time from diagnosis to metastasis ≥2 years | 2.21 | 1.18 | 4.15 | 0.013 |
Table 3. Multivariate analysis by logistic regression model showing the clinico-pathological features correlated with the BRAF mutation in melanoma patients.
| Clinical feature | Odds Ratio | Lower 95% | Upper 95% | P value |
| Age<60 years | 2.57 | 1.13 | 5.81 | 0.023 |
| Women | 2.27 | 1.01 | 5.12 | 0.047 |
| Metastatic site | ||||
| Soft tissue | 0.36 | 0.16 | 0.83 | 0.017 |
| Brain | 2.37 | 1.05 | 5.37 | 0.038 |
| Lung | 0.28 | 0.12 | 0.63 | 0.002 |
| Liver | 0.93 | 0.41 | 2.08 | 0.85 |
| Retroperitoneum | 0.32 | 0.11 | 0.95 | 0.04 |
| Bone | 1.39 | 0.57 | 3.42 | 0.46 |
| Peritoneum | 0.78 | 0.34 | 1.78 | 0.56 |
| Superficial lymph node | 0.74 | 0.34 | 1.62 | 0.45 |
| Time from diagnosis to metastasis ≥2 years | 2.96 | 1.36 | 6.45 | 0.006 |
Co-Existing Mutations/Molecular Aberrations
A subset of mutBRAF patients with available data also had PTEN loss (2/7; 29%) or PIK3CA mutations (1/26; 4%) (Table 1). In patients with wtBRAF, 2/20 (10%) had PTEN loss and 4/46 (9%) had PIK3CA mutation. There was no difference in the rates of PTEN loss or PIK3CA mutations between mutBRAF vs. wtBRAF groups, but the small numbers of patients may preclude firm conclusions, especially in the PTEN group.
As expected KRAS and NRAS mutations were significantly less common in the mutBRAF group compared to the wtBRAF group (KRAS: 0/24 (0%) vs. 13/45 (29%), p = 0.002; NRAS: 1/17 (6%) vs. 42/108 (39%), p = 0.006). Of interest, it should be noted that one patient had a concomitant BRAF and NRAS mutation.
BRAF status and Progression-free survival (PFS) on conventional standard treatment
We analyzed PFS on conventional treatment (before referral to phase 1 clinic) for metastatic disease according to BRAF status. We chose the longest PFS each patient had ever achieved on a conventional treatment.
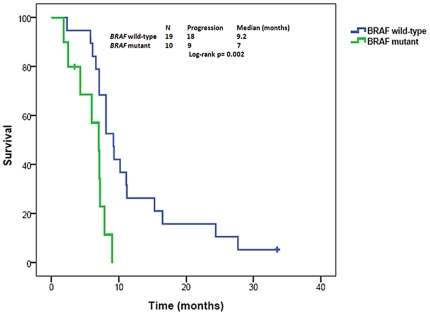
When analyzed with all patients included, there was no overall difference in median PFS between mutBRAF vs. wtBRAF disease (7.0 months, 95%CI 5.6–8.3 vs. 7.1 months, 95%CI 5.7–8.5; p = 0.49). However, patients with colorectal cancer and mutBRAF had a median PFS of 7 months (95%CI 5.3–8.6) compared to 9.2 months (95%CI 7.4–10.9) in wtBRAF (p = 0.002) (Figure 1). In multivariate analysis, mutBRAF was an independent prognostic factor for shorter PFS (HR: 3.76, 95% CI 1.22–11.49, p = 0.02) on the best standard systemic therapy in metastatic colorectal cancer.
Figure 1. Kaplan Meier curve showing progression-free survival on best standard systemic treatment comparing patients with mutBRAF vs. wtBRAF metastatic colorectal cancer.
(One patient with inadequate records on prior treatment was excluded).
In melanoma and papillary thyroid cancer, there was no difference in median PFS in patients with mutBRAF compared to wtBRAF (4.3 months, 95%CI 1.9–6.8 vs. 5.5 months, 95%CI 3.5–6.7; p = 0.29; 24 months, 95%CI 14.4–33.5 vs. 25 months, 95%CI 0–55.4; p = 0.65 respectively).
BRAF Status and Survival
Univariate Analysis
We analyzed OS from time of diagnosis and from time of metastasis. The median OS from time of diagnosis of mutBRAF patients was 322 months vs. 112 months (95%CI 58.2–165.7) for wtBRAF patients (p = 0.24). The median OS from time of metastasis of mutBRAF patients compared to wtBRAF was 99 months (95%CI 17.1–180.8) vs. 51 months (95%CI 38.7–63.2) (p = 0.58).
In disease specific subgroup analysis, the median OS from diagnosis and from metastasis was numerically longer in melanoma patients with mutBRAF compared to wtBRAF (131 months 95%CI 52.7–209.2 vs. 78 months, 95%CI 41.8–114.1; p = 0.14 and 35 months 95%CI 8.7–61.2 vs. 30 months, 95%CI 8.3–53.6; p = 0.63 respectively). In contrast, in colorectal cancer, the median OS from diagnosis and from metastasis was numerically shorter in mutBRAF patients compared to wtBRAF (48 months 95%CI 23.4–72.5 vs. 53 months, 95%CI 0–125.2; p = 0.22 and 30 months, 95%CI 14.5–45.4 vs. 53 months, 95%CI 38.8–67.1; p = 0.26 respectively). Small number of patients in disease specific subgroups precluded more definite conclusions and might explain the lack of statistical significance. The OS from time of diagnosis and metastasis did not differ between mutBRAF and wtBRAF patients with papillary thyroid cancer. The median OS from time of diagnosis was not reached after a follow-up of 133 and 138 months for mutBRAF and wtBRAF respectively. Also, the median OS from metastases was not reached with a median follow-up of 67 and 46 months respectively.
Further, we analyze the prognostic significance of NRAS in melanoma by stratifying our melanoma patients as follows: mutBRAF/wtNRAS, wtBRAF/mutNRAS, and wtBRAF/wtNRAS. A median OS from diagnosis in each of the 3 groups was 131 months (95%CI 81.6–180.3) (mutBRAF/wtNRAS), 67 months (95%CI 29–105) (wtBRAF/mutNRAS), and 109 months (95%CI 51.6–166.3) (wtBRAF/wtNRAS).The OS difference between mutBRAF/wtNRAS and wtBRAF/mutNRAS was of borderline statistical significance (p = 0.05). A median OS from time of metastasis was 35 months (95%CI 8.5–61.5), 20 months (95%CI 10.3–29.6), and 51 months (95%CI 4.8–97.1), respectively (p = 0.45). These data suggest that patients with mutBRAF melanoma survive longer than those with NRAS-mutant disease, but that the survival of mutBRAF melanoma is not different from that of melanoma patients with wtBRAF and wtNRAS.
Multivariate analysis
A multivariate analysis on all 229 patients based on age, gender, RAS (KRAS, NRAS) mutations, BRAF mutations, and disease type was conducted to determine whether any of these factors affects survival. NRAS mutation and male gender were the only independent factors associated with shorter OS from time of diagnosis (Hazard ratio (HR): 2.52, 95%CI 1.32–4.80, p = 0.005 and HR: 2.84, 95%CI 1.46–5.53, p = 0.002, respectively) whereas diagnosis of melanoma predicted a better OS from time of diagnosis (HR: 0.15, 95%CI 0.04–0.58, p = 0.005). Male gender was the only factor predicting poor OS from time of metastasis (HR: 2.79, 95%CI 1.42–5.45, p = 0.003).
A disease-specific multivariate analysis including age, gender, RAS (KRAS, NRAS) mutations and BRAF mutations was performed. In melanoma, only NRAS mutation and male gender were associated with shorter OS from time of diagnosis (HR: 2.16, 95% CI 1.11–4.18, p = 0.02 and HR: 2.64, 95% CI 1.28–5.41, p = 0.008, respectively). Male gender was the only prognostic factor for shorter OS from time of metastasis (HR: 2.84, 95% CI 1.35–5.97, p = 0.006). In colorectal cancer, only KRAS mutation was identified as an independent indicator for poor OS from time of diagnosis and metastasis (HR: 13.56, 95% CI 1.61–113.88, p = 0.016 and HR: 5.46, 95% CI 1.07–27.89, p = 0.04 respectively). We also detected a trend for mutBRAF to predict poor OS from diagnosis or first time of metastasis (HR: 8.31, 95% CI 0.95–72.56, p = 0.055 and HR: 4.05, 95% CI 0.75–21.76, p = 0.10, respectively).
In multivariate analysis, no prognostic factor was detected for papillary thyroid carcinoma, perhaps due to the low number of cases.
Survival in the Clinical Center for Targeted Therapy (phase I clinic) according to the BRAF status
We performed a univariate and multivariate analysis to examine the factors that might predict OS from time of referral to the Clinical Center for Targeted Therapy (Phase I Program) until death in mutBRAF patients. Factors included were: age (≥60 vs. <60 years, gender (male vs. female), tumor type, RMH prognostic score, age, Eastern Cooperative Oncology Group (ECOG) performance status (0–1 vs. ≥2), treatment with RAF/MEK targeting agents vs. never treated with RAF/MEK targeting agent, any decrease in target lesion size vs. no decrease after referral to the phase 1 trial.
Univariate analysis in mutBRAF patients
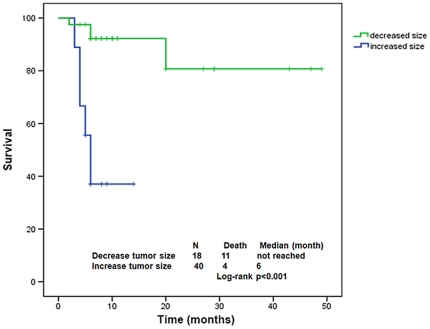
In univariate analysis, we observed a longer OS from referral in women vs. men (not reached in both groups, p = 0.015 and HR 2.62, 95%CI 1.14–6.01; p = 0.02), RMH score of 0–1 vs. 2–3 (not reached vs. 5 months, 95%CI 3–7; p<0.001 and HR 3.69, 95%CI 1.74–7.82; p = 0.001), performance status ≤1 vs. 2–4 (not reached vs. 6 months, 95%CI 2.1–9.9; p = 0.035 and HR 2.51, 95%CI 1.01–6.28; p = 0.048), treatment with RAF/MEK targeting agents (56 of the 80 patients received RAF/MEK targeting agents including 37 with melanoma, 10 with papillary thyroid, 8 with colon cancer and 1 with ovarian cancer) vs. treatment with any other agents or no treatment (not reached vs. 5 months, 95%CI 3.4–6.6; p<0.001 and HR 0.20, 95%CI 0.095–0.43; p<0.001), papillary thyroid cancer vs. other cancers (not reached in both groups, p = 0.018 and HR 0.09, 95%CI 0.10–0.89; p = 0.04), and any decrease in tumor size on any phase I clinical trial vs. no decrease (not reached vs. 6 months, 95%CI 4.7–7.2; p<0.001 and HR 0.09, 95%CI 0.025–0.32; p<0.001) (Figure 2 and Table 4).
Figure 2. Kaplan-Meier estimate of overall survival from time of referral to phase 1 clinic in patients with BRAF mutation who showed any decrease vs. no decrease in size of target lesions on phase 1 trial.
(Patients who did not have tumor measurements at the time of last follow-up (N = 9) or patients who were not enrolled in a phase 1 trial after referral (N = 13) were excluded).
Table 4. Univariate analysis of survival predictors after referral to phase 1 clinic in patients with mutBRAF advanced cancer.
| Predictor | Median OS (95% CI) | N | P value | HR | 95%CI | P value |
| Age≥60 | Unreached | 36 | 0.57 | 1.23 | 0.58–2.58 | 0.58 |
| Age<60 | Unreached | 44 | ||||
| Male | Unreached | 43 | 0.015 | 2.62 | 1.14–6.01 | 0.02 |
| Female | Unreached | 37 | ||||
| RMH score 1 2–3 | 5 (3–7) | 57 | <0.001 | 3.69 | 1.74–7.82 | 0.001 |
| RMH score 0–1 | Unreached | 23 | ||||
| Performance status >1 | 6 (2.1–9.9) | 11 | 0.035 | 2.51 | 1.01–6.28 | 0.048 |
| Performance status 0–1 | Unreached | 69 | ||||
| RAF/MEK targeting agents | Unreached | 56 | <0.001 | 0.20 | 0.095–0.43 | <0.001 |
| Other 2 | 5 (3.4–6.6) | 24 | ||||
| Brain metastasis | Unreached | 27 | 0.08 | 1.90 | 0.89–4.05 | 0.09 |
| No Brain metastasis | Unreached | 53 | ||||
| Time from diagnosis to metastases ≥2 years 3 | Unreached | 45 | 0.36 | 0.71 | 0.33–1.51 | 0.38 |
| Time from diagnosis to metastases <2 years | Unreached | 34 | ||||
| Melanoma | Unreached | 56 | 0.38 | 1.46 | 0.60–3.57 | 0.39 |
| Non melanoma | Unreached | 24 | ||||
| Colorectal cancer | 5 (2.1–7.9) | 10 | 0.11 | 2.13 | 0.80–5.69 | 0.12 |
| Non Colorectal cancer | Unreached | 70 | ||||
| Papillary thyroid cancer | Unreached | 11 | 0.018 | 0.09 | 0.10–0.89 | 0.04 |
| Non papillary thyroid cancer | Unreached | 69 | ||||
| Any decrease tumor size 4 | Unreached | 40 | <0.001 | 0.09 | 0.025–0.32 | <0.001 |
| Any increase tumor size | 6 (4.7–7.2) | 18 |
Royal Marsden Hospital (RMH) 13 prognostic score is determined as follows: 0 points, normal lactate dehydrogenase (LDH), albumin ≥3.5 g/dL, a ≤2 metastatic sites; 1 point- LDH>upper limit of normal, albumin <3.5 g/dL, >2 metastatic sites. Patients with 0–1 points had a good RMH score, and patients with 2–3 points had a poor RMH score.
Includes patients treated with other agents (N = 11) as well as patients who never started on phase 1 trial (N = 13).
One patient of whom the exact date of diagnosis was not documented was excluded only from the univariate analysis comparing the OSref between patients who had a time from diagnosis to metastasis less or more than 2 years.
Patients who never had a restaging at the last follow-up or who never started on a phase 1 trial were excluded in the univariate analysis (N = 22).
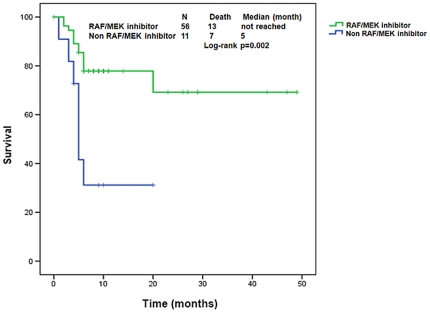
By excluding patients who did not get enrolled into a phase 1 trial after referral (13 patients total), we found that mutBRAF patients treated with RAF/MEK targeting agents has improved survival after referral compared to mutBRAF patients treated with any other agents (not reached vs. 5 months, 95%CI 4–6; p = 0.002 and HR 0.26, 95%CI 0.10–0.66; p = 0.005) (Figure 3).
Figure 3. Kaplan-Meier estimate of overall survival from time of referral to phase 1 clinic in patients with mutBRAF treated with RAF/MEK targeting agents or other phase 1 trials.
Tic marks represent patients still alive at the last follow-up. (Of 80 patients with BRAF mutations, 56 received a RAF/MEK targeting agents, 11 received a non RAF/MEK targeting agents and 13 were not enrolled on a phase 1 trial).
Multivariate analysis in mutBRAF patients
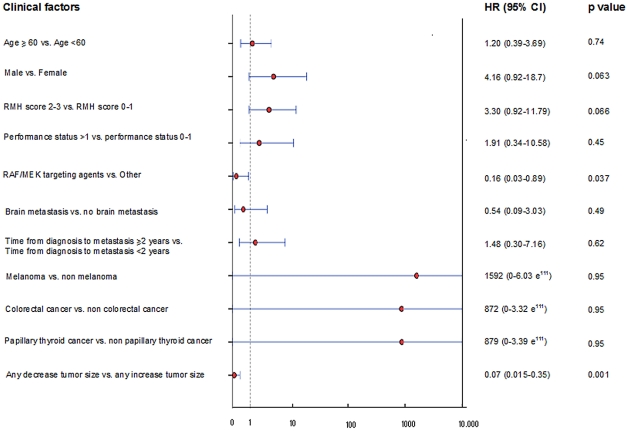
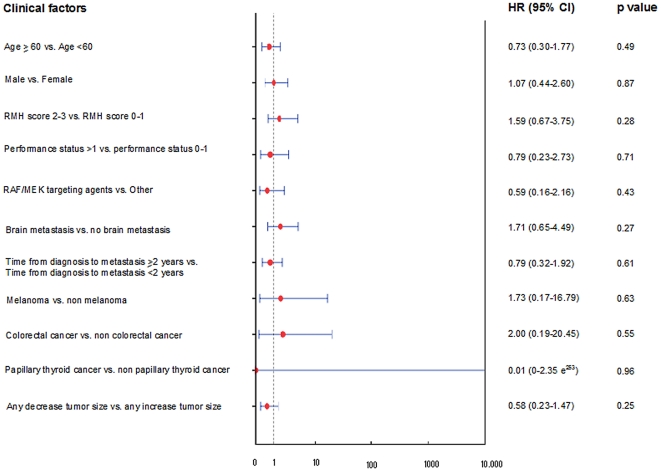
In multivariate analysis, the only two factors that predicted a superior OS after referral to the Phase I clinic in the mutBRAF group were treatment with any RAF/MEK targeting agents (HR 0.16, 95% CI, 0.03–0.89, p = 0.037) and any decrease in tumor size (RECIST measurement) on any phase I trial (HR 0.07, 95% CI, 0.015–0.35, p = 0.001) (Figure 4). Of note, the HR values of the following predictive factors “melanoma vs. non melanoma”, “colorectal cancer vs. non colorectal cancer” and “papillary thyroid cancer vs. non papillary thyroid cancer” are extremely high, compared to their HR calculated by univariate analysis (figure 4). This discrepancy could be explained by the difference in methodology used. Despite their high absolute values, this should be interpreted cautiously provided they don't have any statistical significance as demonstrated by a p value close to 1 and a 95% confidence interval that contains zero. Furthermore, their extremely wide 95CI% is indicative of the poor estimate of their value.
Figure 4. Forest plot summarizing the clinical factors affecting overall survival after referral and displaying their hazard ratio and 95% Confidence interval calculated by Cox proportional hazards regression model in patients with mutBRAF advanced cancer.
Univariate analysis in wtBRAF patients
A similar analysis was conducted in the wtBRAF group for the 104 patients referred. Univariate analysis revealed superior OS from referral associated with the following: RMH score of 0–1 compared to RMH score of 2–3 (50 months, 95%CI 6.4–93.3 vs. 6 months, 95%CI 2–10.3; p<0.001 and HR 2.94, 95%CI 1.56–5.56; p = 0.001), treatment with RAF/MEK targeting agents vs. treatment with any other agents or no treatment (51 months vs. 10 months, 95%CI 7.1–12.9; p = 0.014 and HR 0.32, 95%CI 0.12–0.83; p = 0.019), no brain metastases vs. brain metastases detected (15 months, 95%CI 0–34.3 vs. 7 months, 95%CI 3–10.3; p = 0.004 and HR 2.47, 95%CI 1.31–4.65; p = 0.005), non melanoma vs. melanoma (50 months vs. 10 months, 95%CI 6–13.9; p = 0.006 and HR 2.57, 95%CI 1.27–5.18; p = 0.008) and any decrease in tumor size vs. no decrease (50 months vs. 10 months, 95%CI 6.2–13.8; p = 0.006 and HR 0.32, 95%CI 0.13–0.75; p = 0.009) (Table 5).
Table 5. Univariate analysis of survival predictors after referral to phase 1 clinic in patients with wtBRAF advanced cancer.
| Predictor | Median OS (95% CI) | N4 | P value | HR | 95% CI | P value |
| Age≥60 | 13.5 (7–20) | 46 | 0.84 | 0.93 | 0.49–1.77 | 0.84 |
| Age<60 | 11.1 (4.6–17.5) | 58 | ||||
| Male | 10.3 (6.8–13.7) | 69 | 0.21 | 1.56 | 0.76–3.21 | 0.22 |
| Female | Unreached | 35 | ||||
| RMH score 1 2–3 | 6.2 (2–10.3) | 36 | <0.001 | 2.94 | 1.56–5.56 | 0.001 |
| RMH score 0–1 | 49.8 (6.4–93.3) | 68 | ||||
| Performance status >1 | Unreached | 31 | 0.20 | 0.62 | 0.29–1.30 | 0.21 |
| Performance status 0–1 | 9.5 (3.2–15.7) | 73 | ||||
| RAF/MEK targeting agents | 50.6 | 22 | 0.014 | 0.32 | 0.12–0.83 | 0.019 |
| Other 2 | 10 (7.1–12.9) | 82 | ||||
| Brain metastasis | 6.7 (3–10.3) | 33 | 0.004 | 2.47 | 1.31–4.65 | 0.005 |
| No Brain metastasis | 15.3 (0–34.3) | 71 | ||||
| Time from diagnosis to metastases ≥2 years | 15.3 (0–37) | 43 | 0.19 | 0.65 | 0.34–1.24 | 0.19 |
| Time from diagnosis to metastases <2 years | 9.5 (5.5–3.4) | 61 | ||||
| Melanoma | 10 (6–13.9) | 67 | 0.006 | 2.57 | 1.27–5.18 | 0.008 |
| Non melanoma | 49.8 | 37 | ||||
| Colorectal cancer | 8.9 (6.5–1.3) | 20 | 0.74 | 1.13 | 0.53–2.40 | 0.74 |
| Non Colorectal cancer | 13.7 (4.9–22.6) | 84 | ||||
| Papillary thyroid cancer | Unreached | 11 | <0.001 | 0.027 | 0.001–0.68 | 0.029 |
| Non papillary thyroid cancer | Unreached | 93 | ||||
| Any decrease tumor size 3 | 49.8 | 36 | 0.006 | 0.32 | 0.13–0.75 | 0.009 |
| Any increase tumor size | 10 (6.2–13.8) | 44 |
Royal Marsden Hospital (RMH) 13 prognostic score is determined as follows: 0 points, normal lactate dehydrogenase (LDH), albumin ≥3.5 g/dL, a ≤2 metastatic sites; 1 point- LDH>upper limit of normal, albumin <3.5 g/dL, >2 metastatic sites. Patients with 0–1 points had a good RMH score, and patients with 2–3 points had a poor RMH score.
Among the 149 patients with wtBRAF, 22 patients were treated with RAF/MEK targeting agents, 64 patients were treated with non RAF/MEK targeting agents, 18 patients never been enrolled in phase 1 trial after referral and 45 patients from melanoma department who were not referred to the phase 1 department the time of the analysis.
Patients who never had a restaging at the last follow-up or who never started on a phase 1 trial were excluded in the univariate analysis (N = 24).
Only patients who were referred to the phase 1 clinic were considered in this analysis (Overall survival from time of referral to the phase 1 clinic).
By excluding patients who did not get enrolled into a phase 1 trial after referral (18 patients total), we found that wtBRAF patients treated with RAF/MEK targeting agents has a trend towards improved survival after referral compared to wtBRAF patients treated with any other agents (51 months vs. 10 months, 95%CI 4.7–15.9; p = 0.052 and HR 0.39, 95%CI 0.14–1.04; p = 0.06)
Multivariate analysis in wtBRAF patients
In the multivariate analysis, none of these factors was significantly associated with a better OS from referral (Figure 5).
Figure 5. Forest plot summarizing the clinical factors affecting overall survival after referral and displaying their hazard ratio and 95% Confidence interval calculated by Cox proportional hazards regression model in patients with wtBRAF advanced cancer.
Characteristics of Melanoma Patients with V600K BRAF mutation
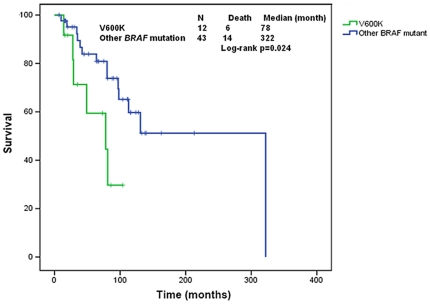
We further investigated the behavior of mutBRAF melanoma with V600K substitution compared to other subtypes of BRAF mutation. (There were 13 patients with V600K mutations including 12 with melanoma and one with colorectal cancer). In the melanoma group, we compared patients with V600K BRAF mutations vs. non-V600K BRAF mutations (the vast majority being V600E). We found that V600K was associated with more brain (75% vs. 36.3%, p = 0.02) and lung metastases (91.6% vs. 47.7%, p = 0.007). (The single patient with colorectal cancer and V600K also had brain and lung metastases). V600K melanomas metastasized earlier (median time to metastasis = 19 months, 95%CI 0–49 vs. 53 months, 95%CI 33–72, p = 0.046), and were associated with a shorter OS from time of diagnosis (median 78 months, 95%CI 10–146 vs. 322 months, p = 0.024) (Figure 6).
Figure 6. Kaplan Meier estimate of overall survival from time of diagnosis comparing patients with melanoma with V600K BRAF mutation vs. other BRAF mutations.
Tic marks represent patients who were alive and censored at time of last follow up. (One patient for whom the time of diagnosis was unknown was excluded.)
We also compared the OS from diagnosis and from metastases between V600K melanoma vs. wtBRAF melanoma and it was not statistically different (P = 0.53 and 0.54, respectively).
Among the 13 patients with V600K BRAF mutation, eight received RAF/MEK targeting agents (of which one was colorectal cancer), three did not receive treatment (only best palliative care) and two received other targeting agents. There were two patients with stable disease of over four months, but no partial or complete remissions.
Discussion
BRAF is one of the most frequently mutated protein kinase in cancer [14]. It has been reported in approximately 40 to 60% of melanoma, 40 to 70% of papillary thyroid carcinoma and 5 to 15% of colorectal cancer cases [6]. In this study we examined whether mutBRAF cancers exhibit any distinctive clinical features compared to wtBRAF cancer.
Overall, we found a higher frequency of women and younger patients with cancer harboring BRAF mutation compared to those without the mutation. These results are consistent with those in a smaller series (18 patients) with mutBRAF melanoma, in whom a higher frequency of patients younger than 60 and women was noted [15]. In our study, mutBRAF cancers were less likely to metastasize to the soft tissue, retroperitoneum and lungs and more likely to metastasize to the brain, suggesting that mutBRAF might affect the metastatic spread pattern of the disease. In the subset of patients with melanoma, the presence of mutBRAF is more likely associated with a time from diagnosis to distant metastasis beyond 2 years.
Kumar et al also reported a longer disease-free survival in mutBRAF melanoma compared to those without the mutation, although the difference was not statistically significant [16]. In a large Australian series of 207 patients with melanoma, mutBRAF was also associated with younger age; however, other clinical features, including time to metastases, response to chemotherapy and metastatic site were essentially indistinguishable [17]. In another report of 68 patients with melanoma, 30 of whom had mutant BRAF, an increase in the incidence of liver metastases was noticed in the mutBRAF group [18].
In mutBRAF colorectal cancer, Tran et al [19] observed a higher incidence of peritoneal disease and central nervous system involvement, but a lower incidence of lung metastases and a shorter OS from time of diagnosis. These data support our findings albeit without statistical significance, perhaps due to the small number of patients with colorectal cancer.
Some differences in the behavior of mutBRAF cancer were seen across histologies. Whereas mutBRAF vs. wtBRAF is associated with a trend towards longer OS from time of diagnosis in melanoma, OS from time of diagnosis tended to be shorter in colorectal cancer, albeit without reaching significance, perhaps due to the low number of patients. In multivariate analysis, NRAS and male gender were the only factors correlated with diminished OS from time of diagnosis in melanoma. Scoggins et al [20] found male gender associated with unfavorable survival in melanoma, however, gender difference did not appear to be a significant factor in a larger retrospective study [21]. Houben et al [22] showed a significantly decreased survival of mutBRAF metastatic melanoma, which is discordant with our data. In our population, the longer disease-free interval and possibly the introduction of new targeted therapy against mutBRAF melanoma [6] might explain, at least in part, the improvement in overall survival from time of diagnosis favoring the mutBRAF group.
mutBRAF was in general mutually exclusive with the presence of mutRAS (KRAS, NRAS). Interestingly, however, we observed one patient with a concomitant NRAS and BRAF mutation. This observation might be explained by different clones of cancer cells inside the tumor with distinct dual mutations. A similar finding was previously reported in familial melanoma, with CDKN2A as well as BRAF and NRAS mutations [23]. It is believed that BRAF and NRAS mutations can coexist within the same melanoma but not at the single-cell level [24].
We also examined the response to best standard systemic treatment. In melanoma, we noted that there was a trend towards a shorter PFS among patients with mutBRAF but this did not reach statistical significance. Findings in the published literature are conflicting. Joseph et al did not find an impact of NRAS or BRAF mutational status on response to high dose interleukin-2 in metastatic melanoma [25]. Similarly, Chang et al [18] reported no difference in response rate to systemic treatment between mutBRAF and wtBRAF melanoma. In another series by Kumar and colleagues, patients with mutBRAF melanoma had a diminished response to therapy [16]. In colorectal cancer, we showed that mutBRAF was independently associated with a shorter PFS on best standard systemic therapy. Our observations are consistent with those of others which have demonstrated that mutBRAF is an adverse predictor in colorectal cancer [26]–[29]. Further, it has been recently suggested that mutBRAF may also predict resistance to cetuximab-based regimens, though it is still unclear whether the mutation is indeed a predictor of resistance or a prognostic marker for a subgroup that simply does worse [30]–[32]. mutBRAF has also been linked to a shorter PFS on standard chemotherapy in few studies [33], although these findings have been disputed by others [28]. In regard to thyroid cancer, many series have demonstrated high-risk features associated with mutBRAF in papillary thyroid carcinoma. Xing et al [34] also reported an association between mutBRAF and the rate of tumor recurrence, though these results have not been confirmed by other studies [35]. The number of patients with papillary thyroid cancer in our study precluded making conclusions on this issue.
Our study demonstrated that treatment with RAF/MEK targeting agents and initial tumor shrinkage are independent factors associated with improved survival in patient with mutant BRAF. These findings support data from a series of published individual studies with molecules including but not limited to PLX4032, GSK2118436 (BRAF inhibitor), and GSK 1120212 (MEK1/2 inhibitor) in mutBRAF cancer [3]–[6]. Multivariate analysis conducted on patients with wtBRAF found no association between treatment with RAF/MEK targeting agents and survival.
Interestingly, we identified V600K BRAF mutation as a prognostic factor associated with more aggressive behavior in metastatic melanoma. Indeed, V600K associated with more brain metastases, shorter time for both disease-free survival and OS from diagnosis, and a trend towards a shorter OS following metastases in comparison to melanoma with other types of mutBRAF (Figure 6). Because of the small number of patients, it is unclear as to how this would impact BRAF- targeted therapies, other than the fact that treatment that penetrates the brain might be needed.
Our analysis has limitations: (i) the small number of patients in each histologic group; (ii) the absence of randomization in regard to the PFS and overall survival data; (iii)the possibility of selection bias based on treatment choice; (iv) selection bias because we only analyzed patients with metastatic disease and cannot therefore ascertain the behavior of patients whose disease never metastasized; (v) the retrospective nature of the study; and (vi) the fact that multiple tests were analyzed for significance. Taken together, this study must therefore be considered exploratory. Even so, several observations that merit further investigations emerge. First, some clinical features appear to differ between histologies despite the presence of BRAF mutation. For instance, patients with colorectal cancer and BRAF mutation showed a trend towards poor overall survival from diagnosis while, in patients with melanoma, the presence of a BRAF mutation was associated with a trend towards better survival. Other factors, including a higher frequency of women and younger patients with cancer harboring BRAF mutation compared to those without the mutation, as well as a lower likelihood to metastasize to the soft tissue, retroperitoneum and lungs was seen across histologic groups, albeit not always in a statistically significant manner. Overall, the only independent factors predicting survival in BRAF- mutant patients in our clinic was treatment with any RAF/MEK axis targeting agent and any initial tumor regression. Of interest, our preliminary data also suggest that the site of mutation may be important, since the subgroup with V600K BRAF mutation (as opposed to V600E) was associated with more brain metastases, and shorter time for both disease-free and overall survival from diagnosis in melanoma. These data support a role for BRAF as a driver mutation that influences phenotype and that provides a druggable target for patients with cancer.
Acknowledgments
We would like to thank Joann Aaron for editorial assistance.
Footnotes
Competing Interests: Dr Razelle Kurzrock received commercial research grants from GlaxoSmithKline, AstraZeneca, Genentech and Bayer. Dr Gerald Falchook received commercial research grant from GlaxoSmithKline. This does not alter the authors' adherence to all the PLoS ONE policies on sharing data and materials.
Funding: Supported in part by Grant Number RR024148 from the National Center for Research Resources, a component of the National Institutes of Health Roadmap for Medical Research. The funders had no role in the study design, data collection and analysis, decision to publish or preparation of the manuscript.
References
- 1.Flaherty KT, McArthur G. BRAF, a target in melanoma: implications for solid tumor drug development. Cancer. 2010;116(21):4902–4913. doi: 10.1002/cncr.25261. [DOI] [PubMed] [Google Scholar]
- 2.Rubinstein JC, Sznol M, Pavlick AC, Ariyan S, Cheng E, et al. Incidence of the V600K mutation among melanoma patients with BRAF mutations, and potential therapeutic response to the specific BRAF inhibitor PLX4032. J Transl Med. 2010;8:67. doi: 10.1186/1479-5876-8-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davies H, Bignell GR, Cox C, Stephens P, Edkins S, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417(6892):949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 4.Infante JR, Fecher LA, Nallapareddy S, Gordon MS, Flaferty KT, et al. Safety and efficacy results from the first-in-human study of the oral MEK1/2 inhibitor GSK1120212. J Clin Oncol. 2010;28:15s. (suppl; abstr 2503) [Google Scholar]
- 5.Kefford R, Arkenau H, Brown MP, Millward M, Infante JR, et al. Phase I/II study of GSK2118436, a selective inhibitor of oncogenic mutant BRAF kinase, in patients with metastatic melanoma and other solid tumors. J Clin Oncol. 2010;28:15s. (suppl; abstr 8503) [Google Scholar]
- 6.Flaherty KT, Puzanov I, Kim K, Ribas A, McArthur GA, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Eng J Med. 2010;363:908–819. doi: 10.1056/NEJMoa1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zuo Z, Chen SS, Chandra PK, Galbincea JM, Soape M. Application of COLD-PCR for improved detection of KRAS mutations in clinical samples. Mod Pathol. 2009;22:1023–1031. doi: 10.1038/modpathol.2009.59. [DOI] [PubMed] [Google Scholar]
- 8.Eberhard D, Johnson B, Amler L, Goddard AD, Heldens SL, et al. Mutations in the epidermal growth factor receptor and in KRAS are predictive and prognostic indicators in patients with non-small-cell lung cancer treated with chemotherapy alone and in combination with erlotinib. J Clin Oncol. 2005;23:5900–5909. doi: 10.1200/JCO.2005.02.857. [DOI] [PubMed] [Google Scholar]
- 9.Curtin J, Busam K, Pinkel D, Bastian B. Somatic activation of KIT in distinct subtypes of melanoma. J Clin Oncol. 2006;24:4340–4346. doi: 10.1200/JCO.2006.06.2984. [DOI] [PubMed] [Google Scholar]
- 10.Nosho K, Kawasaki T, Ohnishi M, Suemoto Y, Kirkner GJ, et al. PIK3CA kinase in colorectal cancer: Relationship with genetic and epigenetic alterations. Neoplasia. 2008;10:534–541. doi: 10.1593/neo.08336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Curtin J, Fridlyand J, Kageshita T, Patel HN, Busam KJ, et al. Distinct sets of genetic alterations in melanoma. N Eng J Med. 2005;353:135–147. doi: 10.1056/NEJMoa050092. [DOI] [PubMed] [Google Scholar]
- 12.Sakr R, Barbashina V, Morrogh M, Chandarlapaty S, Andrade VP, et al. Protocol for PTEN expression by immunohistochemistry in formalin-fixed paraffin-embedded human breast carcinoma. Appl Immunohistochem Mol Morphol. 2010;18:371–374. doi: 10.1097/PAI.0b013e3181d50bd5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arkenau HT, Barriuso J, Olmos D, Ang JE, de Bono J, et al. Prospective Validation of a Prognostic Score to Improve Patient Selection for Oncology Phase I Trials. J Clin Oncol. 2009;27:2692–2696. doi: 10.1200/JCO.2008.19.5081. [DOI] [PubMed] [Google Scholar]
- 14.Greenman C, Stephens P, Smith R, Dalgliesh GL, Hunter C, et al. Patterns of somatic mutation in human cancer genomes. Nature. 2007;446:153–158. doi: 10.1038/nature05610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shinozaki M, Fujimoto A, Morton DL, Hoon DS. Incidence of BRAF oncogene mutation and clinical relevance for primary cutaneous melanomas. Clin Cancer Res. 2004;10:1753–1757. doi: 10.1158/1078-0432.ccr-1169-3. [DOI] [PubMed] [Google Scholar]
- 16.Kumar R, Angelini S, Czene K, Sauroja I, Hahka-Kemppinen M, et al. BRAF mutations in metastatic melanoma: a possible association with clinical outcome. Clin Cancer Res. 2003;9:3362–3368. [PubMed] [Google Scholar]
- 17.Long GV, Menzies AM, Nagrial A, Haydu L, Hamilton AL, et al. Clinico-pathologic correlates of BRAF mutation status in 207 consecutive patients with metastatic melanoma. J Clin Oncol. 2010;28:15s. (suppl; abstr 8548) [Google Scholar]
- 18.Chang D, Panageas K, Osman I, Polsky D, Busam K, et al. Clinical significance of BRAF mutations in metastatic melanoma. J Transl Med. 2004;2:46. doi: 10.1186/1479-5876-2-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tran B, Kopetz S, Tie J, Gibbs P, Jiang Z, et al. Differences in sites of metastatic disease and outcomes observed in patients with BRAF mutant colorectal cancers. J Clin Oncol. 2010;28:15s. (suppl; abstr 3592) [Google Scholar]
- 20.Scoggins CR, Ross MI, Reintgen DS, Noyes RD, Goydos JS, et al. Gender-related differences in outcome for melanoma patients. Ann Surg. 2006;243:693–8. doi: 10.1097/01.sla.0000216771.81362.6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Balch CM, Soong SJ, Gershenwald JE, Thompson JF, Reintgen DS, et al. Prognostic factors analysis of 17,600 melanoma patients: validation of the American Joint Committee on Cancer melanoma staging system. J Clin Oncol. 2001;19:3622–3634. doi: 10.1200/JCO.2001.19.16.3622. [DOI] [PubMed] [Google Scholar]
- 22.Houben R, Becker JC, Kappel A, Terheyden P, Bröcker EB, et al. Constitutive activation of the Ras-Raf signaling pathway in metastatic melanoma is associated with poor prognosis. J Carcinog. 2004;3:6. doi: 10.1186/1477-3163-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jovanovic B, Egyhazi S, Eskandarpour M, Ghiorzo P, Palmer JM, et al. Coexisting NRAS and BRAF mutations in primary familial melanomas with specific CDKN2A germline alterations. J Invest Dermatol. 2010;130:618–620. doi: 10.1038/jid.2009.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sensi M, Nicolini G, Petti C, Bersani I, Lozupone F, et al. Mutually exclusive NRASQ61R and BRAFV600E mutations at the single-cell level in the same human melanoma. Oncogene. 2006;25:3357–3364. doi: 10.1038/sj.onc.1209379. [DOI] [PubMed] [Google Scholar]
- 25.Joseph RW, Sullivan RJ, Panka D, Manoukian G, Percy A, et al. Effect of mutational status on response, PFS, or OS after treatment with IL-2 for metastatic melanoma. J Clin oncol. 2010;28:15s. (suppl; abstr 8597) [Google Scholar]
- 26.Sougklakos I, Saridaki Z, Tzardi M, Papadatos D, Patsos, et al. Use of BRAF mutations, microsatellite instability status, and cyclin D1 expression to predict metastatic colorectal patients' outcome. 2010. 2010 Gastrointestinal Cancers Symposium, Abst # 355. [DOI] [PMC free article] [PubMed]
- 27.Zlobec I, Bihl MP, Schwarb H, Terraciano L, Lugli A. Clinicopathological and protein characterization of BRAF- and K-RAS-mutated colorectal cancer and implications for prognosis. Int J Cancer. 2010;127:367–380. doi: 10.1002/ijc.25042. [DOI] [PubMed] [Google Scholar]
- 28.Richman SD, Seymour MT, Chambers P, Elliott F, Daly CL, et al. KRAS and BRAF mutations in advanced colorectal cancer are associated with poor prognosis but do not preclude benefit from oxaliplatin or irinotecan: results from the MRC FOCUS trial. J Clin Oncol. 2009;27:5931–5937. doi: 10.1200/JCO.2009.22.4295. [DOI] [PubMed] [Google Scholar]
- 29.Tie J, Gibbs P, Lipton L, Christie M, Jorissen RN, et al. Optimizing targeted therapeutic development: Analysis of a colorectal cancer patient population with the BRAFV600E mutation. Int J Cancer. 2011;128(9):2075–2084. doi: 10.1002/ijc.25555. [DOI] [PubMed] [Google Scholar]
- 30.Loupakis F, Ruzzo A, Cremolini C, Vincenzi B, Salvatore L, et al. KRAS codon 61, 146 and BRAF mutations predict resistance to cetuximab plus irinotecan in KRAS codon 12 and 13 wild-type metastatic colorectal cancer. Br J Cancer. 2009;101:715–721. doi: 10.1038/sj.bjc.6605177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Roock W, Claes B, Bernasconi D, De Schutter J, et al. Effects of KRAS, BRAF, NRAS, and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy in chemotherapy-refractory metastatic colorectal cancer: a retrospective consortium analysis. Lancet Oncol. 2010;11:753–762. doi: 10.1016/S1470-2045(10)70130-3. [DOI] [PubMed] [Google Scholar]
- 32.Linot B, Capitain O, Metges J, Adenis A, Raoul J, et al. Impact of PI3K, BRAF, and KRAS mutations on efficacy intensified FOLFIRI+cetuximab regimen in advanced colorectal cancer. 2010. 2010 Gastrointestinal Cancers Symposium, Abst # 365.
- 33.Tol J, Nagtegaal ID, Punt CJ. BRAF mutation in metastatic colorectal cancer. N Engl J Med. 2009;361:98–99. doi: 10.1056/NEJMc0904160. [DOI] [PubMed] [Google Scholar]
- 34.Xing M, Westra WH, Tufano RP, Cohen Y, Rosenbaum E, et al. BRAF mutation predicts a poorer clinical prognosis for papillary thyroid cancer. J Clin Endocrinol Metab. 2005;90:6373–6379. doi: 10.1210/jc.2005-0987. [DOI] [PubMed] [Google Scholar]
- 35.Fugazzola L, Puxeddu E, Avenia N, Romei C, Cirello V, et al. Correlation between B-RAFV600E mutation and clinico-pathologic parameters in papillary thyroid carcinoma: data from a multicentric Italian study and review of the literature. Endocr Relat Cancer. 2006;13:455–464. doi: 10.1677/erc.1.01086. [DOI] [PubMed] [Google Scholar]
- 36.Therasse P, Arbuck SG, Eisnehauer EA, Wanders J, Kaplan RS, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the Unites States, National Cancer Institute of Canada. J Natl Cancer Ins. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]