Abstract
Background:
Pulmonary arterial hypertension (PAH) is a progressive and fatal disorder. Despite the emergence of effective therapy, PAH is commonly at an advanced stage when recognized. Factors associated with a prolonged symptomatic period before the recognition of PAH have not been fully evaluated.
Methods:
The Registry to Evaluate Early and Long-term PAH Disease Management (REVEAL Registry) enrolled 2,967 US adult patients with PAH from March 2006 to September 2007. Patients were considered to have delayed disease recognition if > 2 years elapsed between symptom onset and the patient receiving a PAH diagnosis, starting on PAH-specific therapy, or receiving a diagnosis by right-sided heart catheterization.
Results:
In 21.1% of patients, symptoms were experienced for > 2 years before PAH was recognized. Patients with onset of PAH symptoms before age 36 years showed the highest likelihood of delayed disease recognition (OR, 3.07; 95% CI, 2.03-4.66). History of obstructive airways disease (OR, 1.93; 95% CI, 1.5-2.47) and sleep apnea (OR, 1.72; 95% CI, 1.33-2.22) were independently associated with delayed PAH recognition. Six-minute walk distance < 250 m (OR, 1.91; 95% CI, 1.16-3.13), right atrial pressure < 10 mm Hg (OR, 1.77; 95% CI, 1.26-2.48), and pulmonary vascular resistance < 10 Wood units (OR, 1.28; 95% CI, 1.02-1.60) were also associated with delayed disease recognition, but sex, race/ethnicity, and geographic region showed no association.
Conclusions:
One in five patients in the REVEAL Registry who were diagnosed with PAH reported symptoms for > 2 years before their disease was recognized. Younger individuals and patients with histories of common respiratory disorders were most likely to experience delayed PAH recognition.
Trial registry:
ClinicalTrials.gov; No.: NCT00370214; URL: www.clinicaltrials.gov
The Bottom Line
How does this work advance the field?
Despite progress in understanding the cellular and genetic basis of pulmonary arterial hypertension, the time to recognition of the disease has not improved over the past 2 decades. This article builds upon previous research by identifying factors that are associated with delayed recognition of pulmonary arterial hypertension.
What are the clinical implications?
Research suggests that younger patients and those with common respiratory disorders are more likely to experience delayed recognition of pulmonary arterial hypertension. Interventions to promote earlier disease recognition should focus on these populations. Clinicians caring for younger patients and those diagnosed with common respiratory illnesses should consider pulmonary arterial hypertension if a patient’s severity of symptoms or response to therapy are inadequately explained by the existing diagnosis.
Pulmonary arterial hypertension1 (PAH) is an uncommon disorder characterized by abnormal increases in pulmonary artery pressure (PAP), normal pulmonary capillary wedge pressure (PCWP), and increased pulmonary vascular resistance (PVR).2 PAH results in right ventricular pressure/volume overload leading to right ventricular failure and death.3 Patients with PAH are often diagnosed late in the course of the disease when the pathologic changes are advanced and irreversible.4-7 Diagnosis of PAH at this stage is associated with poor prognosis for survival,8,9 underscoring the importance of early disease recognition and treatment.
In 1987, National Institutes of Health Registry investigators identified the common presenting symptoms of PAH as dyspnea on exertion, edema, fatigue, and chest pain; in this registry, the median time between the onset of these symptoms and the performance of a right-sided heart catheterization (RHC) was 1.3 years.6 More recently, patients enrolled in the ongoing Registry to Evaluate Early and Long-term Pulmonary Arterial Hypertension Disease Management (REVEAL Registry) had a median time from PAH symptom onset to the performance of an RHC of 1.1 years.10 Multiple effective treatments have been developed utilizing the National Institutes of Health and REVEAL Registries,11-15 and rapid diagnosis maximizes these new opportunities to improve survival. Studies published to date have provided little insight regarding which patients are at greatest risk for delayed recognition of PAH. Studies in other disease states indicate that patient characteristics such as age, sex, and race can result in delays in diagnosis and treatment initiation.16-19 Whether similar characteristics exist among patients with PAH has not been examined. Such information is crucial if interventions to promote earlier disease recognition and treatment are to be successfully implemented in the PAH population. The purpose of this study is to identify factors associated with a > 2-year interval between the onset of PAH-attributable symptoms to recognition of the disease.
Materials and Methods
Design Overview
We conducted a cohort study among patients enrolled in the REVEAL Registry between March 1, 2006, and September 30, 2007. The study was approved by the institutional review board at each participating center (e-Appendix 1). Subjects provided written informed consent for collection of baseline and follow-up data. The design of the REVEAL Registry has been described in detail previously.20 Briefly, patients with PAH who were either previously diagnosed or newly diagnosed (defined as having a diagnostic RHC within 90 days of enrollment) were included. Patients were excluded if RHC was not performed, hemodynamic criteria were not met, or the clinical presentation was inconsistent with the diagnosis of PAH. Data collection was Web-based and was performed by a trained research associate at each site who reviewed medical records and recorded prespecified variables electronically.
The hemodynamic criteria necessary for enrollment into the REVEAL Registry differed from those conventionally required for a diagnosis of PAH. A mean PAP > 25 mm Hg (> 30 mm Hg with exercise) and a PVR ≥ 3 Wood units were compulsory. However, the PCWP and left ventricular end-diastolic pressure (LVEDP) requirements were liberalized to include patients with values > 15 to ≤ 18 mm Hg. The cohort for this analysis excluded patients with PCWP > 15 mm Hg. Analysis of the entire population, including patients with PCWP > 15 mm Hg, is available in e-Tables 1-5. The designation of patients as having primarily group 1 PAH (rather than group 2-4 pulmonary hypertension)1 was at the discretion of the principal investigator at each of the participating sites.
Time to Disease Recognition
Time to disease recognition was measured from the date of the onset of symptoms attributable to PAH (ascertained from the medical record) to the earliest of three indicators for disease recognition: physician announcement to a patient of a diagnosis of PAH, initiation of PAH-specific therapy, or RHC confirmation of the diagnosis. The REVEAL Registry records each of these indicators of enrollment.
Statistical Analysis
Among many covariates, the REVEAL Registry records PAH subgroup classification, demographic variables, physician subspecialty consulted at symptom onset/initial symptoms, comorbid diagnoses, and disease severity at diagnosis as defined by functional classification, 6-min walk distance (6MWD), and hemodynamic variables. Patients were stratified by time to disease recognition (≤ 2 years or > 2 years) after the onset of PAH symptoms. In the descriptive analyses, we used the χ2 test or Fisher exact test to screen covariates for associations with delay in diagnosis. Characteristics with statistically significant associations were evaluated by unadjusted logistic regression models wherein the outcome was delay in disease recognition. The parameters that were statistically significant in the unadjusted models were entered into a multivariate logistic regression model and subjected to stepwise model selection.
Two sensitivity analyses were conducted as part of this study. First, the analysis was repeated excluding previously diagnosed patients (defined as having an RHC > 90 days prior to enrollment into REVEAL). Second, the analysis was repeated using the time from symptom onset to RHC as the end point. Statistical analysis was performed using SAS software, version 9.1.3 (SAS Institute, Inc; Cary, North Carolina).
Results
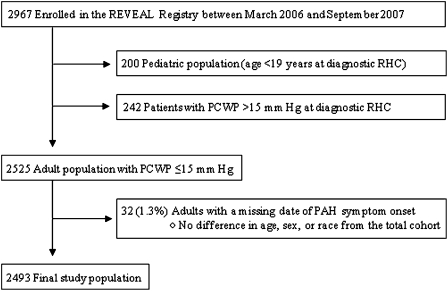
Of the 2,967 patients with PAH enrolled in the REVEAL Registry (Fig 1), we excluded 200 patients younger than 19 years at the time of diagnostic RHC. A total of 242 patients (8.2% of the enrolled population) had a PCWP or LVEDP > 15 to ≤ 18 mm Hg. A total of 2,525 patients met the conventional hemodynamic criteria for PAH. Thirty-two of these patients were excluded because of a missing date of PAH symptom onset. The final study population consisted of 2,493 patients, of whom 526 (21.1%) had recognition of PAH > 2 years after the onset of symptoms. display baseline characteristics of patients with and without delayed disease recognition.
Figure 1.
Study flow diagram. PAH = pulmonary arterial hypertension; PCWP = pulmonary capillary wedge pressure; REVEAL = Registry to Evaluate Early and Long-term PAH Disease Management; RHC = right-sided heart catheterization.
Table 1.
—PAH Subgroup According to Time to Disease Recognition
| PAH Subgroup | ≤ 2 y (n = 1,871) | > 2 y (n = 496) | P Valuea |
| IPAH | 863 (46.1) | 225 (45.4) | Reference |
| Associated with PAH | |||
| Collagen vascular disease | 467 (25.0) | 139 (28.0) | .11 |
| Congenital heart disease | 176 (9.4) | 51 (10.3) | .58 |
| Portal hypertension | 98 (5.2) | 21 (4.2) | .35 |
| Drugs and toxins | 105 (5.6) | 22 (4.4) | .83 |
| HIV | 40 (2.1) | 2 (0.4) | .010b |
| Other PAHc | 60 (3.2) | 24 (4.8) | .091 |
| Familial PAH | 53 (2.8) | 9 (1.8) | .19 |
| Pulmonary venoocclusive disease | 7 (0.4) | 3 (0.6) | .71b |
| Pulmonary capillary hemangiomatosis | 2 (0.1) | 0 (0.0) | > .99b |
Values are given as No. (%). Of the final study population of 2,493 patients, 2,367 had a PAH subgroup assigned at the time of diagnostic right-sided heart catheterization. IPAH = idiopathic pulmonary arterial hypertension; PAH = pulmonary arterial hypertension.
Unless otherwise stated, P values were obtained from χ test evaluating the equality of proportions of the select category vs the IPAH reference group.
P value was obtained from Fisher exact test evaluating the equality of the proportions of the select category vs the IPAH reference group.
Other conditions associated with PAH (2003 Venice classifications): thyroid disorders, glycogen storage disease, Gaucher disease, hereditary hemorrhagic telangiectasia, hemoglobinopathies, myeloproliferative disorders, and splenectomy.
Table 2.
—Patient Demographic Characteristics According to Time to Disease Recognition
| Characteristic | ≤ 2 y (n = 1,967) | > 2 y (n = 526) | P Valuea |
| Age at initial symptoms, y | |||
| < 36 | 428 (21.8) | 160 (30.4) | < .001 |
| 36 to < 46 | 454 (23.1) | 109 (20.7) | .049 |
| 46 to < 56 | 491 (25.0) | 118 (22.4) | .046 |
| 56 to < 65 | 315 (16.0) | 93 (17.7) | .003 |
| ≥ 65 | 279 (14.2) | 46 (8.7) | Reference |
| Sex | |||
| Male | 405 (20.6) | 105 (20.0) | .53b |
| Female | 1,562 (79.4) | 421 (80.0) | |
| Race/ethnicity | |||
| White | 1,422 (72.3) | 393 (74.7) | Reference |
| Black | 242 (12.3) | 67 (12.7) | .99 |
| Hispanic | 173 (8.8) | 42 (8.0) | .47 |
| Asian or Pacific Islander | 67 (3.4) | 16 (3.0) | .61 |
| Native American or Native Alaskan | 14 (0.7) | 0 (0.0) | .052c |
| Other | 16 (0.8) | 4 (0.8) | .86 |
| Unknown | 33 (1.7) | 4 (0.8) | .11 |
| US geographic region | |||
| Northeast | 442 (22.5) | 117 (22.2) | Reference |
| Midwest | 441 (22.4) | 106 (20.2) | .52 |
| South | 566 (28.8) | 158 (30.0) | .70 |
| West | 518 (26.3) | 145 (27.6) | .69 |
Values are given as No. (%). Numbers may not sum to the total number of patients within each variable due to missing data.
Unless otherwise stated, P values were obtained from χ test evaluating the equality of proportions of the select category vs the reference group.
P value was obtained from overall χ test.
P value was obtained from Fisher exact test evaluating the equality of proportions of the select category vs the reference group.
Table 3.
—Patient Comorbid Conditions, Patient Presenting Symptoms, and Physician Specialty Consulted at Symptom Onset According to Time to Disease Recognition
| Characteristic | ≤ 2 y (n = 1,967) | > 2 y (n = 526) | P Valuea |
| Comorbid conditions at diagnosis of PAH | |||
| History of obstructive airways diseaseb | 368 (19.2) | 156 (30.2) | < .001 |
| History of thromboembolic disease | 175 (9.1) | 64 (12.5) | .020 |
| Sleep apnea | 365 (19.7) | 139 (27.7) | < .001 |
| Obesity (BMI ≥ 30 kg/m2) | 526 (32.2) | 170 (37.9) | .025 |
| Diabetes | 224 (11.6) | 71 (13.8) | .18 |
| Cancer (excluding skin cancer) | 124 (6.4) | 21 (4.1) | .044 |
| Presenting symptom(s) attributable to PAH | |||
| Abdominal distention | 81 (4.1) | 14 (2.7) | .12 |
| Chest pain/discomfort | 436 (22.2) | 111 (21.1) | .60 |
| Cough | 271 (13.8) | 78 (14.8) | .54 |
| Dizziness/ lightheadedness | 295 (15.0) | 84 (16.0) | .58 |
| Dyspnea at rest | 226 (11.5) | 44 (8.4) | .041 |
| Dyspnea on exertion | 1,693 (86.1) | 449 (85.4) | .68 |
| Edema | 431 (21.9) | 90 (17.1) | .016 |
| Fatigue | 525 (26.7) | 138 (26.2) | .83 |
| Presyncope/syncope | 325 (16.5) | 96 (18.3) | .35 |
| Palpitations | 253 (12.9) | 61 (11.6) | .44 |
| Physician specialty consulted at symptom onset | |||
| Cardiologist | 555 (28.2) | 126 (24.0) | .58c |
| Pulmonologist | 447 (22.7) | 110 (20.9) | Reference |
| Internist | 263 (13.4) | 73 (13.9) | .48c |
| Rheumatologist | 70 (3.6) | 27 (5.1) | .071c |
| Other | 255 (13.0) | 63 (12.0) | .98c |
| Unknown | 376 (19.1) | 127 (24.1) | .032c |
Values are given as No. (%). Numbers may not sum to the total number of patients within each variable due to missing data. See Table 1 legend for expansion of abbreviation.
Unless otherwise stated, P values were obtained from overall χ test.
History of obstructive airways disease was defined as having a history of obstructive lung disease and/or reactive airway disease.
P value was obtained from χ test evaluating the equality of proportions of the select category vs the reference group.
Table 4.
—Patient Functional Classification, Exercise Tolerance, and RHC Variables According to Time to Disease Recognition
| Characteristic | ≤ 2 y (n = 1,967) | > 2 y (n = 526) | P Valuea |
| NYHA/WHO functional class at PAH diagnosisb | |||
| I | 56 (3.9) | 7 (1.9) | .056 |
| II | 325 (22.5) | 85 (22.9) | .87 |
| III | 881 (61.0) | 236 (63.6) | Reference |
| IV | 182 (12.6) | 43 (11.6) | .50 |
| 6MWD at PAH diagnosis, mb,c | |||
| < 250 | 143 (23.7) | 45 (30.8) | .011 |
| 250 to < 410 | 314 (52.0) | 56 (38.4) | Reference |
| ≥ 410 | 147 (24.3) | 45 (30.8) | .015 |
| mPAP, mm Hg | |||
| < 55 | 1,261 (64.1) | 347 (66.0) | .43d |
| ≥ 55 | 706 (35.9) | 179 (34.0) | … |
| mRAP, mm Hg | |||
| < 10 | 1,016 (56.4) | 303 (64.6) | .003 |
| 10 to < 15 | 456 (25.3) | 108 (23.0) | .16 |
| ≥ 15 | 329 (18.3) | 58 (12.4) | Reference |
| PCWP or LVEDP, mm Hg | |||
| < 12 | 1,409 (71.6) | 358 (68.1) | Reference |
| 12 to < 15 | 430 (21.9) | 124 (23.6) | .28 |
| 15 | 128 (6.5) | 44 (8.4) | .048 |
| Cardiac index, L/min × m2)b | |||
| < 2 | 573 (39.1) | 119 (31.9) | .011d |
| ≥ 2 | 893 (60.9) | 254 (68.1) | … |
| PVR, Wood units | |||
| < 10 | 956 (48.6) | 289 (54.9) | .010d |
| ≥ 10 | 1,011 (51.4) | 237 (45.1) | … |
| Vasoreactivitye | |||
| Yes | 92 (9.4) | 29 (11.3) | .37d |
| No | 885 (90.6) | 228 (88.7) | … |
Values are given as No. (%). Numbers may not sum to the total number of patients within each variable due to missing data. 6MWD = 6-min walking distance; LVEDP = left ventricular end-diastolic pressure; mPAP = mean pulmonary artery pressure; mRAP = mean right atrial pressure; NYHA = New York Heart Association; PCWP = pulmonary capillary wedge pressure; PVR = pulmonary vascular resistance; RHC = right-sided heart catheterization; WHO = World Health Organization. See Table 1 legend for expansion of other abbreviation.
Unless otherwise stated, P values were obtained from χ test evaluating the equality of proportions of the select category vs the reference group.
Missing data: functional classification of 694 patients; 6MWD of 1,770 patients, and cardiac index of 657 patients.
Categorization was based on the quartiles of the distribution; the second and third quartiles were combined.
P value was obtained from overall χ test.
Defined as patients with a decrease in mPAP ≥ 10 mm Hg to a level < 40 mm Hg without a decrease in cardiac output.
Of enrolled patients, 87% were younger than 65 years at symptom onset. In unadjusted analyses (Table 5), the patient’s age at symptom onset was associated with delayed recognition of PAH, with the largest difference observed in those < 36 years (vs ≥ 65 years) at symptom onset (OR, 2.27; 95% CI, 1.58-3.25). The risk was similarly elevated after adjustment (Table 5) for age at symptom onset, comorbid diagnoses, 6MWD, and hemodynamic variables at the time of diagnosis (OR, 3.07; 95% CI, 2.03-4.66).
Table 5.
—Unadjusted and Adjusted Logistic Regression of Factors Associated With a Time to Disease Recognition > 2 Years
| Risk Factor | Unadjusted OR (95% CI) | Unadjusted P Valuea | Adjusted OR (95% CI)b | Adjusted P Valuea |
| Age at initial symptoms, y | ||||
| < 36 | 2.27 (1.58-3.25) | < .001 | 3.07 (2.03-4.66) | < .001 |
| 36 to < 46 | 1.46 (1.00-2.12) | .050 | 1.85 (1.20-2.84) | .005 |
| 46 to < 56 | 1.46 (1.01-2.11) | .047 | 1.72 (1.13-2.61) | .012 |
| 56 to < 65 | 1.79 (1.21-2.64) | .003 | 2.07 (1.34-3.20) | .001 |
| ≥ 65 | Reference | Reference | Reference | Reference |
| Comorbid conditions | ||||
| History of obstructive airways diseasec | 1.82 (1.46-2.27) | < .001 | 1.93 (1.50-2.47) | < .001 |
| History of thromboembolic disease | 1.43 (1.06-1.94) | .021 | ||
| Sleep apnea | 1.56 (1.25-1.96) | < .001 | 1.72 (1.33-2.22) | < .001 |
| Obesity | 1.28 (1.03-1.59) | .025 | … | … |
| Diabetes | 1.22 (0.91-1.62) | .18 | … | … |
| Cancer (noncutaneous) | 0.62 (0.39-0.99) | .046 | … | … |
| 6MWD at PAH diagnosis, m | ||||
| < 250 | 1.76 (1.14-2.74) | .011 | 1.91 (1.16-3.13) | .010 |
| 250 to < 410 | Reference | Reference | Reference | Reference |
| ≥ 410 | 1.72 (1.11-2.66) | .016 | 1.42 (0.87-2.31) | .16 |
| mRAP, mm Hg | ||||
| < 10 | 1.69 (1.24-2.30) | < .001 | 1.77 (1.26-2.48) | < .001 |
| 10 to < 15 | 1.34 (0.95-1.90) | .097 | 1.41 (0.98-2.04) | .068 |
| ≥ 15 | Reference | Reference | Reference | Reference |
| Cardiac index, L/min × m2) | ||||
| < 2 | Reference | Reference | … | … |
| ≥ 2 | 1.37 (1.08-1.74) | .011 | … | … |
| PCWP or LVEDP, mm Hg | ||||
| < 12 | Reference | Reference | … | … |
| 12 to < 15 | 1.13 (0.90-1.43) | .28 | … | … |
| ≥ 15 | 1.35 (0.94-1.94) | .10 | … | … |
| PVR, Wood units | ||||
| < 10 | 1.29 (1.06-1.56) | .010 | 1.28 (1.02-1.60) | .034 |
| ≥ 10 | Reference | Reference | Reference | Reference |
P values from Wald χ test.
Adjusted model contains the following covariates: age at initial symptoms attributable to PAH, history of obstructive airways disease, sleep apnea, 6MWD at PAH diagnosis, mRAP, and PVR.
History of obstructive airways disease was defined as having a history of obstructive lung disease and/or reactive airway disease.
We evaluated whether the presence of a comorbid diagnosis was associated with a delayed recognition of PAH. Patients diagnosed with the common respiratory disorders of obstructive airways disease (adjusted OR, 1.93; 95% CI, 1.50-2.47) or sleep apnea (adjusted OR, 1.72; 95% CI, 1.33-2.22) were more likely to have > 2 years elapsed from first symptom to disease recognition. In the unadjusted model, patients with a history of thromboembolic disease and obesity (BMI ≥ 30 kg/m2) showed a significant delay in disease recognition. However, these variables were not included in the final adjusted model.
The severity of comorbid diseases was assessed in patients with relevant, available data. Spirometry results were recorded for 349 of the 524 patients (66.6%) with a history of obstructive airway disease. Of patients with obstruction, 28.1% had mild disease, 57.6% had moderate disease, 12.3% had severe disease, and 2% had very severe disease according to the GOLD (Global Initiative for Chronic Obstructive Lung Diseases) stages of COPD severity.21 The apnea-hypopnea indices of patients with sleep apnea were not recorded in the REVEAL Registry. The mean continuous positive airway pressure setting recorded for 164 of 504 patients (32.5%) with a history of sleep apnea was 9.6 cm H2O. Results of CT scan pulmonary angiography were available for 125 of 239 patients (52.3%) with a history of thromboembolic disease. No evidence of pulmonary embolism was found in 85.6% of patients.
Hemodynamic variables obtained during RHC that are associated with poor survival are higher right atrial pressures (RAPs) and higher PVRs.22,23 Analysis of RHC data in the REVEAL Registry showed that RAP < 10 mm Hg vs ≥ 15 mm Hg (adjusted OR, 1.77; 95% CI, 1.26-2.48) and PVR < 10 Wood units (OR, 1.28; 95% CI, 1.02-1.60) were each associated with delayed disease recognition. In adjusted analyses, 6MWD < 250 m was also associated with delayed PAH recognition (OR, 1.91; 95% CI, 1.16-3.13).
Several demographic characteristics were evaluated in regard to time to disease recognition, including sex, race, and US geographic region as determined by residential zip codes. Women are significantly more likely than men to be diagnosed with PAH10; however, there was no association between gender and a delayed recognition of PAH. Both race and geographic distributions of patients in the REVEAL Registry were similar to the results of the 2000 census. No association between either race or geographic region and delayed disease recognition was identified.
Finally, we examined whether the specialty of the physician consulted at the time of PAH symptom onset was associated with a delayed recognition of PAH. Patients sought evaluation by specialists in internal medicine (13.9% of patients), pulmonology (20.9%), cardiology (24%), and rheumatology (5.1%). No medical subspecialty was identified as being associated with a delay in recognition of PAH.
The sensitivity analysis excluding previously diagnosed patients led to greater variation due to the decreased sample size. The directionality of all of the effects remained constant. The model remained stable when the outcome measure was time to RHC instead of time to disease recognition.
Discussion
Hemodynamic measurements are required to make a diagnosis of PAH.24 However, the REVEAL Registry is designed to capture “real-world” clinical practice where patients, ultimately having a valid diagnosis of PAH, may be told of a diagnosis of PAH, or started on PAH-specific therapy prior to the performance of the RHC that qualified them for the REVEAL Registry. This may have been due to an incomplete assessment of PAH, but occasionally the hemodynamic parameters required for REVEAL Registry entry were incomplete or inaccessible. Because of these issues, the time from symptom onset to disease recognition was the end point of this study instead of the time to diagnosis by RHC alone.
We found that 21.1% of patients enrolled in the REVEAL Registry had > 2 years between the onset of symptoms attributable to PAH and recognition of PAH. A 2-year delay in starting treatment potentially worsens clinical outcome or survival. Strategies to diagnose PAH more quickly require identification of risk factors associated with delayed disease recognition. Based on data from the REVEAL Registry, we determined that those characteristics include younger age at symptom onset and diagnoses of other respiratory diseases. Additional factors associated with delayed disease recognition are less severely impaired right ventricular function at the time of RHC and 6MWD < 250 m.
In this study, patients younger than 36 years had the highest likelihood of delayed recognition of PAH. When younger patients present to physicians for evaluation of nonspecific symptoms, such as dyspnea, a more common disorder, such as asthma, may be suspected. In addition, younger patients with a higher activity level may be more likely to notice symptoms earlier in the course of disease. This sensitivity may cause a prolonged period from when abnormalities are noticed by the patient to the time when the symptoms are sufficiently severe to merit clinical investigation. In older patients, a complaint of breathlessness is likely to raise concern for cardiovascular disease (eg, cardiomyopathy or valvular abnormalities) that would lead to the performance of an echocardiogram, the main screening test for PAH.25 With older age, and perhaps a lower activity level, a loss of exercise tolerance may not be noticed until overt signs of right ventricular failure (eg, edema) occur. The period of time from symptom awareness to disease recognition by a health-care provider may consequently be shorter. Finally, uneven access to health care in the United States may be a contributing factor. Young patients are one of the largest groups of uninsured Americans.26,27 Uninsured patients who reach the enrollment age for Medicare have demonstrated improvement in health trends, such as decreased adverse cardiovascular outcomes once insurance coverage is acquired at age 65 years.28 This finding is consistent with our observation that patients with PAH who were > 65 years of age were least likely to experience a delay in disease recognition.
We also found that patients diagnosed with the common respiratory disorders of obstructive lung disease and sleep apnea were more likely to have delayed recognition of PAH. Our study cannot differentiate between misdiagnosis of PAH as another disorder (eg, PAH being mistakenly diagnosed as asthma)29 or the masking of the development of PAH due to the coexistence of another disease with respiratory symptoms. The high prevalence of obstructive airways disease and sleep apnea in the general population, combined with the familiarity of physicians with these more common disorders, makes identifying an uncommon disorder like PAH more difficult.
Patients with milder hemodynamic impairment (lower RAP and PVR) were less likely to have PAH recognized within 2 years of their first symptoms. The prolonged symptomatic period prior to disease recognition in these patients may reflect the fact that without overt signs of right ventricular failure, a diagnostic catheterization is less likely to occur promptly at symptom onset. Delayed disease recognition due to the absence of overt signs of right ventricular failure denies the opportunity for earlier initiation of PAH therapy. Initial reports suggest that treating patients with PAH when they are less symptomatic improves PVR and increases the time to clinical worsening.30
In contrast to the above findings in which less impaired right ventricular function was associated with delays in disease recognition, more impaired 6MWD (≤ 250 m) was associated with delay to PAH recognition. The explanation for this association may rest in comorbid conditions that limit the distance covered during a 6MWD test. For example, a patient may have a poor walk distance attributed to an orthopedic impairment, thus masking a cardiopulmonary limitation.
The observational study design is an important potential limitation of this study because of the possibility of uncorrected biases associated with enrollment. However, observational studies allow initial evaluations of multiple hypotheses and may assist in formulation of specific questions more amenable to prospective randomized study. In the current case, no prospective experimental design to detect delays in disease recognition would be ethical or feasible. Missing data were noted in a number of patients, but sensitivity analyses that compared the effect of excluding patients with missing data to treatment of missing data as a distinct category showed similar results.
In conclusion, our findings reinforce those results published by previous investigators that many patients suffer from symptoms of PAH for prolonged periods prior to the recognition of the disease. However, this study builds upon prior results by exploring patient characteristics and factors associated with delayed disease recognition. Efforts should focus on younger, symptomatic patients and those with suspected or established diagnoses of obstructive lung disease and sleep apnea whose symptoms are out of proportion to their underlying disease or who are not responding to therapy. Finally, all physicians, independent of their specialty, should be encouraged to consider PAH in the differential diagnosis of patients with exertional dyspnea and fatigue.
Supplementary Material
Acknowledgments
Author contributions: Dr Brown: contributed to the study design; collection, analysis and interpretation of data; drafting and critical review of the manuscript; and has seen and approved the final version.
Dr Chen: contributed to the study design; collection, analysis and interpretation of data; drafting and critical review of the manuscript; and has seen and approved the final version.
Dr Halpern: contributed to the study design; collection, analysis and interpretation of data; drafting and critical review of the manuscript; and has seen and approved the final version.
Dr Taichman: contributed to the study design; collection, analysis and interpretation of data; drafting and critical review of the manuscript; and has seen and approved the final version.
Dr McGoon: contributed to the study design; collection, analysis and interpretation of data; drafting and critical review of the manuscript; and has seen and approved the final version.
Dr Farber: contributed to the study design; collection, analysis and interpretation of data; drafting and critical review of the manuscript; and has seen and approved the final version.
Dr Frost: contributed to the study design; collection, analysis and interpretation of data; drafting and critical review of the manuscript; and has seen and approved the final version.
Dr Liou: contributed to the study design; collection, analysis and interpretation of data; drafting and critical review of the manuscript; and has seen and approved the final version.
Ms Turner: contributed to the study design; collection, analysis and interpretation of data; drafting and critical review of the manuscript; and has seen and approved the final version.
Dr Feldkircher: contributed to the study design; collection, analysis and interpretation of data; drafting and critical review of the manuscript; and has seen and approved the final version.
Mr Miller: contributed to the study design; collection, analysis and interpretation of data; drafting and critical review of the manuscript; and has seen and approved the final version.
Dr Elliott: contributed to the study design; collection, analysis and interpretation of data; drafting and critical review of the manuscript; and has seen and approved the final version.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following conflicts of interest: Dr Brown is employed by Intermountain Healthcare and is on the speaker’s bureau for United Therapeutics. Dr Chen serves as a consultant for United Therapeutics Corporation. Dr Halpern has received research grant support from Actelion and Pfizer. Dr Taichman receives institutional research support for participation in REVEAL Registry, which is funded by Actelion. Dr McGoon serves as a consultant with Actelion/CoTherix, Gilead/Myogen, Lung Rx, and Medtronic. Dr McGoon has received grants from Gilead/Myogen and Medtronic, and has received honoraria for service on the REVEAL Registry Steering Committee, which is supported by Actelion. Dr Farber serves as a consultant and is on the speaker’s bureau for Actelion. Dr Farber has received honoraria for service on the REVEAL Registry Steering Committee, which is supported by Actelion. Dr Frost serves as a consultant for Gilead and Actelion. Dr Frost has received honoraria from Gilead, Actelion, and Pfizer and grants to Baylor for IRB-approved research from Gilead, Pfizer, Bayer, United Therapeutics, Actelion, Lilly, and Novartis. Dr Frost has received honoraria for service on the REVEAL Registry Steering Committee, which is supported by Actelion. Dr Liou has received grants from the National Institutes of Health/National Heart, Lung, and Blood Institute, the Margolis Family Foundation of Utah, and the Cystic Fibrosis Foundation. He has been the site principal investigator for studies of cystic fibrosis and its treatment of the Therapeutic Developments Network of the Cystic Fibrosis Foundation, Altus, Axcan, Scandipharm, Bayer, Boehringer Ingelheim, Genentech, Inspire, Kalobios, MPEX, Novartis, and Vertex. Dr Liou has received honoraria for service on the REVEAL Registry Steering Committee, which is supported by Actelion. Ms Turner is employed by ICON Clinical Research, a company that receives research support from Actelion and other pharmaceutical companies. Mr Miller is employed by ICON Clinical Research. Dr Feldkircher is employed by Actelion Pharmaceuticals US, Inc. Dr Elliott is employed by Intermountain Healthcare. Intermountain Healthcare, with Dr Elliott as principal investigator, has received grant support during the last 5 years from Actelion, Pfizer, Encysive Pharmaceuticals, and United Therapeutics. Dr Elliott has received honoraria for service on the REVEAL Registry Steering Committee, which is supported by Actelion.
Role of sponsors: The sponsor (Actelion Pharmaceuticals US, Inc) has provided funding to support data collection, quality control, data analysis, and partial support for preparation of the manuscript.
Other contributions: Editorial support for the preparation of the manuscript was provided by Jennifer M. Kulak, PhD, and Carol A. Lewis, PhD, from inScience Communications, a Wolters Kluwer business. We thank the principal investigators and their study coordinators for their participation in the REVEAL Registry. A list of names is available in e-Appendix 2.
Additional information: The e-Appendices and e-Tables can be found in the Online Supplement at http://chestjournal.chestpubs.org/content/140/1/19/suppl/DC1.
Abbreviations
- 6MWD
6-min walk distance
- LVEDP
left ventricular end-diastolic pressure
- PAH
pulmonary arterial hypertension
- PAP
pulmonary artery pressure
- PCWP
pulmonary capillary wedge pressure
- PVR
pulmonary vascular resistance
- RAP
right atrial pressure
- REVEAL
Registry to Evaluate Early and Long-term Pulmonary Arterial Hypertension Disease Management
- RHC
right-sided heart catheterization
Footnotes
For editorial comment see page 4
Funding/Support: The REVEAL Registry is sponsored by Actelion Pharmaceuticals US, Inc.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (http://www.chestpubs.org/site/misc/reprints.xhtml).
References
- 1.Simonneau G, Robbins IM, Beghetti M, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2009;54(suppl 1):S43–S54. doi: 10.1016/j.jacc.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 2.McGoon MD, Torbicki A, Oudiz RJ. Diagnosis and Assessment of Pulmonary Arterial Hypertension. West Sussex, England: John Wiley and Sons, Ltd; 2008. [Google Scholar]
- 3.Voelkel NF, Quaife RA, Leinwand LA, et al. National Heart, Lung, and Blood Institute Working Group on Cellular and Molecular Mechanisms of Right Heart Failure Right ventricular function and failure: report of a National Heart, Lung, and Blood Institute working group on cellular and molecular mechanisms of right heart failure. Circulation. 2006;114(17):1883–1891. doi: 10.1161/CIRCULATIONAHA.106.632208. [DOI] [PubMed] [Google Scholar]
- 4.Tueller C, Stricker H, Soccal P, et al. Swiss Society for Pulmonary Hypertension Epidemiology of pulmonary hypertension: new data from the Swiss registry. Swiss Med Wkly. 2008;138(25-26):379–384. doi: 10.4414/smw.2008.11915. [DOI] [PubMed] [Google Scholar]
- 5.Humbert M, Sitbon O, Chaouat A, et al. Pulmonary arterial hypertension in France: results from a national registry. Am J Respir Crit Care Med. 2006;173(9):1023–1030. doi: 10.1164/rccm.200510-1668OC. [DOI] [PubMed] [Google Scholar]
- 6.Rich S, Dantzker DR, Ayres SM, et al. Primary pulmonary hypertension. A national prospective study. Ann Intern Med. 1987;107(2):216–223. doi: 10.7326/0003-4819-107-2-216. [DOI] [PubMed] [Google Scholar]
- 7.Jing ZC, Xu XQ, Han ZY, et al. Registry and survival study in Chinese patients with idiopathic and familial pulmonary arterial hypertension. Chest. 2007;132(2):373–379. doi: 10.1378/chest.06-2913. [DOI] [PubMed] [Google Scholar]
- 8.McLaughlin VV, Shillington A, Rich S. Survival in primary pulmonary hypertension: the impact of epoprostenol therapy. Circulation. 2002;106(12):1477–1482. doi: 10.1161/01.cir.0000029100.82385.58. [DOI] [PubMed] [Google Scholar]
- 9.Sitbon O, Humbert M, Nunes H, et al. Long-term intravenous epoprostenol infusion in primary pulmonary hypertension: prognostic factors and survival. J Am Coll Cardiol. 2002;40(4):780–788. doi: 10.1016/s0735-1097(02)02012-0. [DOI] [PubMed] [Google Scholar]
- 10.Badesch DB, Raskob GE, Elliott CG, et al. Pulmonary arterial hypertension: baseline characteristics from the REVEAL Registry. Chest. 2010;137(2):376–387. doi: 10.1378/chest.09-1140. [DOI] [PubMed] [Google Scholar]
- 11.Galiè N, Ghofrani HA, Torbicki A, et al. Sildenafil Use in Pulmonary Arterial Hypertension (SUPER) Study Group Sildenafil citrate therapy for pulmonary arterial hypertension. N Engl J Med. 2005;353(20):2148–2157. doi: 10.1056/NEJMoa050010. [DOI] [PubMed] [Google Scholar]
- 12.Galiè N, Olschewski H, Oudiz RJ, et al. Ambrisentan in Pulmonary Arterial Hypertension, Randomized, Double-Blind, Placebo-Controlled, Multicenter, Efficacy Studies (ARIES) Group Ambrisentan for the treatment of pulmonary arterial hypertension: results of the ambrisentan in pulmonary arterial hypertension, randomized, double-blind, placebo-controlled, multicenter, efficacy (ARIES) study 1 and 2. Circulation. 2008;117(23):3010–3019. doi: 10.1161/CIRCULATIONAHA.107.742510. [DOI] [PubMed] [Google Scholar]
- 13.Rubin LJ, Badesch DB, Barst RJ, et al. Bosentan therapy for pulmonary arterial hypertension. N Engl J Med. 2002;346(12):896–903. doi: 10.1056/NEJMoa012212. [DOI] [PubMed] [Google Scholar]
- 14.Barst RJ, Rubin LJ, Long WA, et al. The Primary Pulmonary Hypertension Study Group A comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertension. N Engl J Med. 1996;334(5):296–302. doi: 10.1056/NEJM199602013340504. [DOI] [PubMed] [Google Scholar]
- 15.Barst RJ, Galie N, Naeije R, et al. Long-term outcome in pulmonary arterial hypertension patients treated with subcutaneous treprostinil. Eur Respir J. 2006;28(6):1195–1203. doi: 10.1183/09031936.06.00044406. [DOI] [PubMed] [Google Scholar]
- 16.Chang AM, Mumma B, Sease KL, Robey JL, Shofer FS, Hollander JE. Gender bias in cardiovascular testing persists after adjustment for presenting characteristics and cardiac risk. Acad Emerg Med. 2007;14(7):599–605. doi: 10.1197/j.aem.2007.03.1355. [DOI] [PubMed] [Google Scholar]
- 17.Bond M, Bowling A, McKee D, et al. Does ageism affect the management of ischaemic heart disease? J Health Serv Res Policy. 2003;8(1):40–47. doi: 10.1177/135581960300800109. [DOI] [PubMed] [Google Scholar]
- 18.Mehta RH, Bufalino VJ, Pan W, et al. American Heart Association Get With the Guidelines Investigators Achieving rapid reperfusion with primary percutaneous coronary intervention remains a challenge: insights from American Heart Association’s Get With the Guidelines program. Am Heart J. 2008;155(6):1059–1067. doi: 10.1016/j.ahj.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 19.Gorin SS, Heck JE, Cheng B, Smith SJ. Delays in breast cancer diagnosis and treatment by racial/ethnic group. Arch Intern Med. 2006;166(20):2244–2252. doi: 10.1001/archinte.166.20.2244. [DOI] [PubMed] [Google Scholar]
- 20.McGoon MD, Krichman A, Farber HW, et al. Design of the REVEAL registry for US patients with pulmonary arterial hypertension. Mayo Clin Proc. 2008;83(8):923–931. doi: 10.4065/83.8.923. [DOI] [PubMed] [Google Scholar]
- 21.Gold PM. The 2007 GOLD guidelines: a comprehensive care framework. Respir Care. 2009;54(8):1040–1049. [PubMed] [Google Scholar]
- 22.Rich S, Levy PS. Characteristics of surviving and nonsurviving patients with primary pulmonary hypertension. Am J Med. 1984;76(4):573–578. doi: 10.1016/0002-9343(84)90279-1. [DOI] [PubMed] [Google Scholar]
- 23.D’Alonzo GE, Barst RJ, Ayres SM, et al. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann Intern Med. 1991;115(5):343–349. doi: 10.7326/0003-4819-115-5-343. [DOI] [PubMed] [Google Scholar]
- 24.Badesch DB, Champion HC, Sanchez MA, et al. Diagnosis and assessment of pulmonary arterial hypertension. J Am Coll Cardiol. 2009;54(1) suppl:S55–S66. doi: 10.1016/j.jacc.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 25.McLaughlin VV, Archer SL, Badesch DB, et al. American College of Cardiology Foundation Task Force on Expert Consensus Documents American Heart Association American College of Chest Physicians American Thoracic Society, Inc Pulmonary Hypertension Association ACCF/AHA 2009 expert consensus document on pulmonary hypertension a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association developed in collaboration with the American College of Chest Physicians; American Thoracic Society, Inc.; and the Pulmonary Hypertension Association. J Am Coll Cardiol. 2009;53(17):1573–1619. doi: 10.1016/j.jacc.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 26.Ayanian JZ, Weissman JS, Schneider EC, Ginsburg JA, Zaslavsky AM. Unmet health needs of uninsured adults in the United States. JAMA. 2000;284(16):2061–2069. doi: 10.1001/jama.284.16.2061. [DOI] [PubMed] [Google Scholar]
- 27.Martin S, Ulrich C, Munsell M, Taylor S, Lange G, Bleyer A. Delays in cancer diagnosis in underinsured young adults and older adolescents. Oncologist. 2007;12(7):816–824. doi: 10.1634/theoncologist.12-7-816. [DOI] [PubMed] [Google Scholar]
- 28.McWilliams JM, Meara E, Zaslavsky AM, Ayanian JZ. Health of previously uninsured adults after acquiring Medicare coverage. JAMA. 2007;298(24):2886–2894. doi: 10.1001/jama.298.24.2886. [DOI] [PubMed] [Google Scholar]
- 29.Hayes D., Jr Idiopathic pulmonary arterial hypertension misdiagnosed as asthma. J Asthma. 2007;44(1):19–22. doi: 10.1080/02770900601125243. [DOI] [PubMed] [Google Scholar]
- 30.Valerio CJ, Coghlan JG. Bosentan in the treatment of pulmonary arterial hypertension with the focus on the mildly symptomatic patient. Vasc Health Risk Manag. 2009;5:607–619. doi: 10.2147/vhrm.s4713. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.