Diarrhea kills nearly 650 children below the age of 5 years each day in India. Oral rehydration solution (ORS) and oral zinc have been recommended for the treatment of acute diarrhea in children by the Indian Academy of Paediatrics (IAP) National task force for use of ORS and zinc in the year 2003[1] and later endorsed in 2006.[2] Zinc was included in the National Programme for the treatment of Diarrhea in 2007.[3] The Integrated Management of Neonatal and Childhood Illness (IMNCI) advocates the use of these two drugs in the treatment of acute diarrhea.[4] The evidence for the use of ORS and zinc as first-line treatment in children in resource limited countries is overwhelming.[5,6] If given early on during an episode of diarrhea it would help save 50% of children who would otherwise die. The treatment is effective, safe, cheap, and easily tolerated by children.[6] More importantly, there are no other alternatives to this treatment. Recognizing the importance of this, the National Rural Health Mission (NRHM) has included ORS and zinc in the list of medicines to be available at the subcenters.[3]
Yet, unfortunately, it is hardly available in the public[7–9] and private[7,8] health facilities in India. Only 34.2% of children with diarrhea in India receive ORS[10] and a miniscule 1% are prescribed zinc during an episode of diarrhea.[11] The lack of availability in public health facilities[7–9] points to the fact that zinc was probably not procured at all, or if procured it was not done in sufficient quantities. Why does this state of affairs persist? We explore some of the regulatory and other issues which prevent access to zinc in India.
REGULATORY ISSUES
It is evident that in order to promote wide use of zinc and ORS these should be available as over the counter (OTC) drugs. The consequence of having it as OTC is that people will be able to buy these two medicines at any shop and not only from licensed chemists. Importantly, there are rules and regulations for that specific category. For example, the packaging is defined; OTC drugs have to be sold in the original packaging so that the consumer can easily identify the drug. Promotion is defined; they can usually be advertised in the general media but for specific conditions (e.g. headache, fever) and not for specific diseases. The generic name must appear in any advertisement. The sales channel is defined; in most countries they can be sold in general stores along with other consumer goods. The seller is simply a seller of goods and does not provide information on the OTC product. That is done through promotion in the media and the information on the package. This means that OTC as a specific group will have a clear and level “playing field”; which will obviously attract players, in this case the pharmaceutical manufacturers.
In India, the term OTC has no legal recognition. All drugs which are not “prescription-only” are by default considered OTC. Even the so-called OTC drugs like paracetamol (which come under schedule K) have to be sold by licensed drug stores.[12] Non-drug-licensed stores or shops can sell drugs which are under schedule K of the Drugs Control Rules (1945) which are known as “household remedies.”[12] These medicines can be sold without a license in villages with a population less than 1000 subject to some other conditions. This rather arbitrary and confusing rule is the first barrier to widespread availability of zinc. Nearly 700 million people in India live in rural areas, away from easy access to health facilities. It is in the interests of these people, the consumers, to have a legally recognized schedule such as OTC to promote access to medicines like ORS and zinc. Countries of the South East-Asia region, such as Thailand, have licensed zinc and ORS as OTC products. ORS is an OTC product in Sri Lanka. At this juncture we should remind ourselves that we are not speaking of a medicine which brings down fever by a few degrees or provides symptomatic relief for trivial conditions like cold and cough or a balm to soothe an itchy skin. We are speaking of simple remedies which have proven efficacy and safety and will potentially save more than 200,000 children dying each year. It is hard to believe, that despite the many meetings, task force reports, and hard core data to support a prescription to OTC switch, the government has not done so. The time is right for the government to seize the opportunity to define an OTC category and to have zinc and ORS as the first products.
THE “OFFICIAL” STATUS OF ZINC
Tablet zinc sulfate (or any other salt) either in dispersible form or otherwise did not find a place in the National Essential Drugs List 2003.[13] Only zinc oxide (as dusting powder) is mentioned. Even the Standard Treatment Guidelines (STG), brought out by the Armed Forces Medical College, Pune, in collaboration with Ministry of Health and Family Welfare, Govt. of India near the end of 2007, does not list zinc for treatment of diarrhea.[14] The Indian Pharmacopoeia (I.P.) 2007 and 2010 do not give standards for oral tablets (dispersible or otherwise) of zinc sulfate.[15,16] The recently released National Formulary of India (NFI) 2010 (preprint version) also fails to mention oral zinc[17] though hopefully it may be corrected in the revised version. However, the National List of Essential Medicines India (NLEMI) 2011 lists zinc sulfate syrup (20 mg/5 ml) for diarrhea.[18]
A high-level meeting to discuss the role of zinc in diarrhea was held on 20 March 2007 during which the government approved the use of 20 mg dispersible zinc tablets for use in acute diarrhea in children.[19] Despite this “official” status of zinc, none of the publications which have been dated after this meeting such as the STG, I.P., and NFI mention zinc for the management of diarrhea. The NLEMI, released in June 2011, lists only syrup zinc and not dispersible tablets. Such documents of public health importance should be subject to wider participation and greater scrutiny. Medicines in the national programs should be listed in the same formulation after consultation with these programs to avert a disconnect between the NLEMI and national programs. However, individual states like Chhattisgarh, Orissa, Tamil Nadu, and others have included dispersible zinc tablets in their recent essential drug lists, making a small but significant move in the right direction.
What makes the situation more confusing is the statement by the then Drugs Controller General of India (DCGI) at the March 2007 meeting that “zinc is already a non prescription drug and rules permit it for its use as an OTC drug.”[19] It was not clear what formulation of Zinc was a nonprescription drug and what exactly he meant by OTC. Zinc as a mineral supplement is a nonprescription drug, not oral zinc sulfate for diarrhea. This is an opportunity for the current DCGI to demonstrate through a clear statement, the rules and regulations for zinc tablets that will benefit public health.
MANUFACTURING CAPACITY FOR ZINC IN INDIA
In April 2008, the Union Minister for Science and Technology, Mr. Kapil Sibal, announced that Bharat Immunologicals and Biologicals Corporation Limited (BIBCOL), a public sector undertaking of the Dept. of Biotechnology, Govt. of India, has been equipped with a production capacity of 240 million tablets of BIBZinC (20 mg dispersible scored tablets of zinc sulfate) per year.[20] There have been efforts by the United States Agency for International Development (USAID) to improve the manufacture of zinc with aid up to 1,50,000 U.S. dollars as start-up funds.[21] Companies such as Zuventus (Zinconia), Dr. Reddy's (Z and D), Wallace (ZN), USV (Trustim), Emcure (Emzinc) have started production of oral zinc with some companies exporting the drug. The production capacity for zinc seems adequate to meet the demands of the country.
AVAILABILITY AND COST OF ZINC AND ORS
The ground reality, however, is quite different from what one would expect in a country with a sophisticated pharmaceutical industry. These drug companies (manufacturing zinc) do not come forward to quote in tenders for hospitals (personal experience from JIPMER, Pondicherry) and their products are not easily available in all states of India [Table 1]. The unclear regulations, lack of a clear market and want of a good business model maybe some of the reasons. Nonprescription drugs are not inherently unprofitable; a market for the “morning after pill” was created from virtually nothing, from the clear need for the product, an aggressive educational/promotional campaign by the pharmaceutical industry and extremely lucrative margins for the industry as well as the pharmacist. There certainly is a market for zinc tablets though it would be a high-volume low-margin product rather than the low-volume high-margin one like the “morning after” pill. The opportunity for pharmaceutical companies to be associated in the public mind (through advertising to the general public) with a proven useful product is not one they would miss.
Table 1.
Comparison of the availability of oral zinc sulfate 20 mg dispersible tablets and oral rehydration salt (one liter) in Chhattisgarh[7] and Orissa[8]
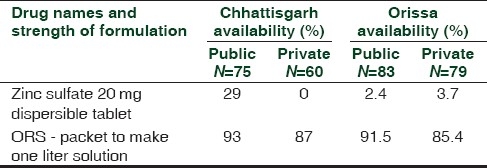
One hundred tablets of zinc sulfate are priced at Indian rupees (INR) 13.68 to be supplied to the Tamil Nadu Government for the current year.[22] Therefore, a 2-week course of zinc for a child will cost the Tamil Nadu Government less than two rupees. A single sachet of ORS (20.5 g) costs INR 1.58 to the Tamil Nadu Medical Services Corporation.[22] The cost of ORS and zinc for pooled procurement by the government is clearly well within the medicines budget for nearly all states. Why are public health facilities still not able to stock these medicines in sufficient quantities? Poor supply chain management, lack of prescribers prescribing them in diarrhea, and inadequate procurement at the central drug store all contribute.
In the private chemist shops of Tamil Nadu, a single sachet of ORS costs between INR 12-15 and a course of zinc sulfate tablets costs 30-40 rupees. This puts zinc and ORS out of the reach for those who go to public health facilities for treatment because they cannot afford private care. The National Pharmaceutical Pricing Authority should discuss the price of zinc tablets and ORS with the manufacturers. An adequate return to the manufacturer should be assured, but the public health importance should be the primary concern.
Pricing and availability surveys in Orissa and Chhattisgarh have shown that though the availability of ORS is relatively good it is pretty dismal for zinc [Table 1]. This was also reflected in a snap-shot survey of five essential pediatric medicines undertaken previously.[9] It is highly anomalous that the vastly developed pharmaceutical industry in India is unable to make available a simple product which is needed for millions of its population on a regular basis. Indian pharmaceutical companies have been praised extensively for their role in responding to patients with HIV-AIDs by making good quality, affordable antiretrovirals available globally. Surely these same companies should be able to relate to the needs of the children in India?
WHAT NEEDS TO BE DONE?
The Ishikawa's Fishbone diagram [Figure 1] illustrates the possible causes of poor availability of zinc and demonstrates the interwoven elements that facilitate and hinder the rational use of zinc. Perhaps the first thing the government needs to do is make sure that all the “official” documents, like NLEMI 2011, STG, I.P 2010, and NFI 2010, list oral zinc for diarrhea and are in alignment with each other regarding the formulation and strength. Departments within the government must consult and collaborate with each other and produce documents that are in alignment. Next, there is an urgent need to formally make zinc sulfate and ORS available widely, through distribution channels outside the pharmacies and medicines shops. As mentioned before this would provide the DCGI an opportunity define the “OTC” segment; it can be done through promulgation of regulations and does not require parliamentary action. This will ensure that these drugs can be sold in all shops (without a license), promoted by direct to consumer marketing and people will have easy access to them. These products should be advertised, ethically, using effective promotional measures targeting all sections of society.
Figure 1.
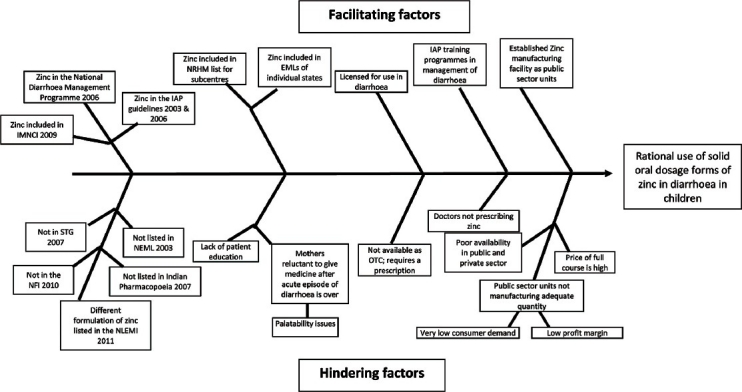
Fishbone diagram identifying factors facilitating and hindering the rational use of solid oral dosage forms of zinc in the treatment of diarrhea in children
Simultaneously, there has to be an advocacy campaign by the Ministry of Health to urge prescribers to comply with the STGs for diarrhea. A recent survey in Ujjain shows the pathetic concordance to STGs in acute diarrhea with only 6 out of the 843 prescriptions being compliant.[23] A media blitz of the kind which was used to promote ORS in the early 1990s should be planned to promote ORS and zinc to mothers and care-givers of children. Efforts should be made to convince care-givers to continue with zinc even after the episode of diarrhea is over and to caution them regarding palatability issues. Using FM radio stations, local cable networks, text messages on mobile phones, newspaper and other modalities, a concentrated effort to educate the public should be made. Companies manufacturing zinc should be permitted to ethically promote their products to the public. Irrational indications on promotional material[24] should be scanned and weeded out or else the wheat will get buried with the chaff [see Figure 2].
Figure 2.

Advertisement for syrup zinc with irrational indications
Pharmaceutical companies manufacturing zinc should be assured of regular orders from public and private health care facilities. This will happen only if the procurement lists of all states and major hospitals have zinc in them in adequate quantities. This by itself may not be sufficient for pharmaceutical companies to start producing more zinc, but could be a small motivator which may slowly turn the tide. But the writing on the wall is clear – the drug regulatory authority and the ministry of health must clarify the situation; the DCGI could have the “OTC” classification of ORS and zinc as an agenda item in his next meeting with the State Drug Controllers. Such a commitment to this issue will prevent the needless deaths of children. Zinc, ORS, and deaths in childhood diarrhea is a solution waiting to happen; each day the solution is delayed is one day too much for the children of India.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Bhatnagar S, Bhandari N, Mouli UC, Bhan MK. IAP National Task Force. Consensus Statement of IAP National Task Force: Status report on management of acute diarrhea. Indian Pediatr. 2004;41:335–48. [PubMed] [Google Scholar]
- 2.Bhatnagar S, Lodha R, Choudhury P, Sachdev HP, Shah N, Narayan S, et al. IAP Guidelines 2006 on management of acute diarrhea. Indian Pediatr. 2007;44:380–9. [PubMed] [Google Scholar]
- 3.Bhatnagar S, Alam S, Gupta P. Management of Acute Diarrhea: From Evidence to Policy. Indian Paediatr. 2010;47:215–7. doi: 10.1007/s13312-010-0049-7. [DOI] [PubMed] [Google Scholar]
- 4.Physician Chart Booklet. New Delhi: Ministry of Health and Family Welfare, Government of India; 2009. Integrated management of neonatal and childhood illness. [Google Scholar]
- 5.Lazzerini M, Ronfani L. Oral zinc for treating diarrhoea in children. Cochrane Database Syst Rev. 2008;(3):CD005436. doi: 10.1002/14651858.CD005436.pub2. [DOI] [PubMed] [Google Scholar]
- 6.Bajait C, Thawani V. Role of zinc in pediatric diarrhea. Indian J Pharmacol. 2011;43:232–5. doi: 10.4103/0253-7613.81495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Antony KR, Jain V, Puni KK, Puni K Jain. Proceedings of the 43rd Annual Conference of the Indian Pharmacological Society. Hyderabad: 2010. Dec, Survey of the availability and prices of children's medicines in Chattisgarh state; p. 28. [Google Scholar]
- 8.Swain TR. Proceedings of the rd Annual Conference of the Indian Pharmacological Society. Hyderabad: 2010. Dec, What children's medicines are on our shelves and how much do they cost? (The Orissa Story) p. 26. [Google Scholar]
- 9.Gitanjali B, Manikandan S. Availability of five essential medicines for children in public health facilities in India: A snapshot survey. J Pharmacol Pharmacother. 2011;2:95–9. doi: 10.4103/0976-500X.81900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.District Level Household and Facility Survey 2007-08. Ministry of Health and Family Welfare, Government of India, New Delhi. Fact sheets India. [Last accessed on 2011 June 22]. Available from: http://www.rchiips.org/pdf/rch3/state/India.pdf .
- 11.New Delhi: UNICEF; 2009. [Last accessed on 2011 July 5]. Management practices for childhood diarrhoea in India. Survey of 10 districts. Available from: http://www.unicef.org/india/Management_Practices_for_Childhood_Diarrhoea_in_India2009.pdf . [Google Scholar]
- 12.The drugs and cosmetics act and rules; Government of India, Ministry of Health and Family Welfare. The drugs and cosmetics act, 1940 the drugs and cosmetics rules 1945. [Last accessed on 2011 June 25]. Available from: http://cdsco.nic.in/html/Copy%20of%201.%20DandCAct121.pdf .
- 13.The National Essential Drug List of India 2003. Ministry of Health and Family Welfare. Govt. of India. [Last accessed on 2011 Jul 7]. Available from: http://whoindia.org/LinkFiles/Essential_Medicine_List_Essential-Medicine-2003.pdf .
- 14.Standard Treatment Guidelines. Medical Management and Costing of Select Conditions. Armed Forces Medical College, Pune, in collaboration with Ministry of Health and Family Welfare, Government of India and WHO Country Office, India. 2007 [Google Scholar]
- 15.Government of India, Ministry of Health and Family Welfare. The Ghaziabad: Indian Pharmacopoeia Commission; 2007. Indian Pharmacopoeia. [Google Scholar]
- 16.Government of India, Ministry of Health and Family Welfare. The Ghaziabad: Indian Pharmacopoeia Commission; 2010. Indian Pharmacopoeia. [Google Scholar]
- 17.Government of India, Ministry of Health and Family Welfare. 4th ed. India: Indian Pharmacopoeia Commission; 2010. National Formulary of India. [Google Scholar]
- 18.National Essential List of Medicines 2011. Ministry of Health and Family Welfare, Government of India, New Delhi. [Last accessed on 2011 Jun 22]. Available from: http://www.mohfw.nic.in/showfile.php?lid=785 .
- 19.Minutes of the high level committee meeting held on 20.3.2007 to discuss role of zinc in management of diarrhoea and new guidelines for treatment of diarrhoea in children. [Last accessed on 2011 Jun 14]. Available from: http://www.whoindia.org/LinkFiles/Child_Health_in_India_Child_Health_GOI_Guidelines_002.pdf .
- 20.Zinc Dispersible tablets for Management of Diarrhoea in Infants and Children. 2008. Apr 07, [Last accessed on 2011 Jun 22]. Available from: http://www.dst.gov.in/whats_new/press-release08/tablets-diarrhoea.htm .
- 21.Million dollar pharma industry investment in diarrhoea treatment. [Last accessed on 2011 Jun 14]. Available from: http://www.usaid.gov/in/about_us/pdfs/India_Pharma.pdf .
- 22.Finalized rates and suppliers for the tender for the supply of Drugs and Medicines to TNMSC for the year 2010-2011. [Last accessed on 2011 Jun 29]. Available from: http://www.tnmsc.com/tnmsc/new/html/pdf/drug.pdf .
- 23.Pathak D, Pathak A, Marrone G, Diwan V, Lundborg CS. Adherence to treatment guidelines for acute diarrhoea in children up to 12 years in Ujjain, India - a cross-sectional prescription analysis. BMC Infect Dis. 2011;11:32. doi: 10.1186/1471-2334-11-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. [Last accessed on 2011 Jun 29]. Available from: http://www.mapra.com/Main/frmProductDetails.aspx?id=109 mapra .


