Abstract
Sugar is an inseparable part of the food we consume. But too much sugar is not ideal for our teeth and waistline. There have been some controversial suggestions that excessive sugar may play an important role in certain degenerative diseases. So artificial sweeteners or artificially sweetened products continue to attract consumers. A sugar substitute (artificial sweetener) is a food additive that duplicates the effect of sugar in taste, but usually has less food energy. Besides its benefits, animal studies have convincingly proven that artificial sweeteners cause weight gain, brain tumors, bladder cancer and many other health hazards. Some kind of health related side effects including carcinogenicity are also noted in humans. A large number of studies have been carried out on these substances with conclusions ranging from “safe under all conditions” to “unsafe at any dose”. Scientists are divided in their views on the issue of artificial sweetener safety. In scientific as well as in lay publications, supporting studies are often widely referenced while the opposing results are de-emphasized or dismissed. So this review aims to explore the health controversy over perceived benefits of sugar substitutes.
Keywords: Artificial sweetener, aspartame, sugar substitute
INTRODUCTION
In the recent years the trend towards health, figure and fitness has increased. Energy imbalance between calories consumed on one hand, and calories expended on the other hand, due to urbanization, sedentary lifestyles and excessive consumption of sugary foods along with increased fat consumption, especially saturated fats is leading the Indian population to obesity. Obesity being a primary factor behind type II diabetes is leading India towards becoming a diabetic capital of the world by 2030. So the growing health awareness today has increased the demand for food products that support better health. Consumers are demanding a greater variety of low-calorie products as they strive to make healthier food choices. A sugar substitute is a food additive that duplicates the effect of sugar in taste, but usually has less food energy. It is about 200 times sweeter than sugar. Some sugar substitutes are natural and some are synthetic. Those that are not natural are, in general, referred to as artificial sweeteners.[1] The food and beverage industry is increasingly replacing sugar or corn syrup with artificial sweeteners in a range of products traditionally containing sugar. Artificial sweeteners cost the food industry only a fraction of the cost of natural sweeteners in spite of the extremely high profit margins for manufacturers of artificial sweeteners.[2]
The US Food and Drug Administration regulates artificial sweeteners as food additives.[3] Food Additives must be approved by the FDA, which publishes a Generally Recognized as Safe (GRAS) list of additives.[4] To date, the FDA has not been presented with scientific information that would support a change in conclusions about the safety of the five approved artificial sweeteners (saccharin, aspartame, sucralose, neotame, and acesulfame potassium). The safe conclusions are based on a detailed review of a large body of information, including hundreds of toxicological and clinical studies.[5]
SUGAR SUBSTITUTES
Natural sugar substitutes, artificial sugar substitutes and their potency are listed in Tables 1 and 2.[6]
Table 1.
Natural sugar substitutes
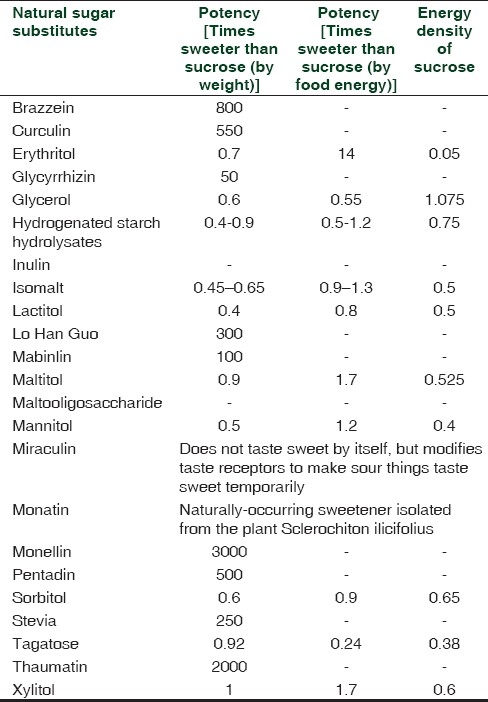
Table 2.
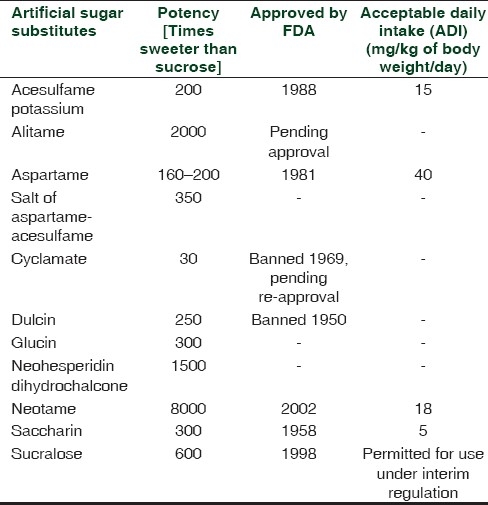
THERAPEUTIC USES
To assist in weight loss
Some people choose to limit their food energy intake by replacing high energy sugar or corn syrup with other sweeteners having little or no food energy (sugar substitutes). This allows them to eat the same foods they normally would, while allowing them to lose weight and avoid other problems associated with excessive calorie intake.[8]
Dental care
Although liquid preparations are particularly suitable for children, many contain sucrose which encourages dental decay. Unlike sugar , sugar substitutes are not fermented by the microflora of the dental plaque. In view of this harmful effect, doctors have been recommended to prescribe sugar-free (having sugar substitutes) medicines whenever possible.[9,10]
Diabetes mellitus
People with diabetes have difficulty in regulating their blood sugar levels. By limiting their sugar intake by substituting sugar with artificial sweeteners, they can enjoy a varied diet also, some sugar substitutes do release energy, but are metabolized more slowly, allowing blood sugar levels to remain more stable over time.[11]
Reactive hypoglycemia
Individuals with reactive hypoglycemia will produce an excess of insulin after quickly absorbing glucose into the bloodstream. This causes their blood glucose levels to fall below the amount needed for physiological function. As a result, like diabetics, they must avoid intake of high-glycemic foods like white bread, and often choose artificial sweeteners as an alternative.[12]
NON THERAPEUTIC USES
As sugar substitute
Studies conducted with taste-test panels show that aspartame's taste is very similar to the taste of sugar.[11] So it is used instead of sugar in various foods.
Enhances and extends flavors
Aspartame has the ability to intensify and extend fruit flavors, such as cherry and orange, in foods and beverages. For example, aspartame makes chewing gum taste sweet and more flavorful longer than sugar-sweetened gum.[11]
Avoiding processed foods
Individuals may opt to substitute refined white sugar with less-processed sugars such as fruit juice or maple syrup.
Cost
Many sugar substitutes are cheaper than sugar.
HEALTH HAZARDS
There is some ongoing controversy over whether artificial sweetener usage poses health risks. A study done in 2005 by the University of Texas Health Science Center at San Antonio showed that, rather than promoting weight loss, the use of diet drinks was a marker for increasing weight gain and obesity. Those who consumed diet soda were more likely to gain weight than those who consumed naturally-sweetened soda.[13] Animal studies have convincingly proven that artificial sweeteners cause body weight gain. A sweet taste induces an insulin response, which causes blood sugar to be stored in tissues, but because blood sugar does not increase with artificial sweeteners, there is hypoglycemia and increased food intake. So in the experiment, after a while, rats given artificial sweetener have steadily increased caloric intake, increased body weight, and increased adiposity.[14,15]
Other adverse effects or health hazards will be discussed with individual compounds.
Aspartame
Aspartame, discovered in 1965 is a low-calorie sweetener with a sugar-like taste but is approximately 200 times sweeter than sucrose.[16] It is unique among low-calorie sweeteners in that it is completely broken down by the body to its components - the amino acids, aspartic acid, phenylalanine and a small amount of ethanol.[17–19] These components are found in much greater amounts in common foods, such as meat, milk, fruits, and vegetables, and are used in the body in the same way whether they come from aspartame or common foods. It was approved by the US FDA in1981. Food Standards Australia New Zealand (FSANZ) and other international regulatory agencies have approved aspartame for general use in a range of foods including tabletop sweeteners, carbonated soft drinks, yoghurt and confectionery.[20]
Some animal studies have shown that aspartame poses antipyretic, analgesic and anti-inflammatory action.[21,22] Interference of aspartame with rheumatoid factor activity has been proposed to alleviate the pain and immobility resulting from chronic inflammation of joints.[23]
It has also been suggested that the components of aspartame can lead to a number of health problems.[3] Double-blind trials have been carried out with aspartame at Duke University and in one of the best-designed of these studies, the effects of a single large dose of aspartame in people who had claimed to be sensitive to the substance was investigated. The results showed no difference in headache frequency, blood pressure, or blood histamine concentrations (a measure of the allergenic potential) between the experimental and control groups.[24] In another study, at the University of Illinois, which involved diabetics, subjects in the placebo group actually had more reactions than those in the aspartame group. Reported anecdotal experiences are not confirmed by carefully controlled scientific studies. This, of course, does not mean that the problems are not real, but it does imply that in many cases the symptoms may not be caused by aspartame itself. One study has, however, confirmed allergic symptoms such as hives and swelling in sensitive individuals.[19] It is unclear how the allergy comes about, since none of the components of aspartame are believed to be capable of producing allergic reactions. It has been suggested that diketopiperazine, a compound which forms when aspartame decomposes, may be responsible.[16] When the temperature of Aspartame exceeds 86 degrees F, the wood alcohol in aspartame coverts to formaldehyde and then to formic acid, which in turn causes metabolic acidosis. The methanol toxicity mimics multiple sclerosis; thus people may be misdiagnosed with having multiple sclerosis. Multiple sclerosis does not lead to death whereas methanol toxicity does.[25] In some case reports associations have been made between aspartame intake, in particular the subsequent exposure to the aspartame metabolite formaldehyde, and Type IV Delayed Type Hypersensitivity (DTH) reactions in patients with proven contact sensitization to formaldehyde.[26,27] However, to confirm the associations observed in these two case studies with only a limited number of patients (seven in total), larger studies would be needed involving double-blind placebo-controlled challenges with aspartame and placebo exposures and the inclusion of well-defined control-patient groups. Although in in vivo studies by Parthasarathy et al., effects of the aspartame metabolite methanol on organs/tissues/cells and function of the immune system were described,[28,29] these observations are considered more likely to result from an indirect stress-effect due to the high methanol levels used, in addition these high dose levels are not considered relevant for aspartame exposure. In addition, the quality of the studies was poor.
Further experiments show that aspartame is no more likely to cause an allergic reaction than a placebo.[30] The three breakdown products of aspartame are all toxic in high doses. Phenylalanine is an essential amino acid which must be included in the diet for normal growth and maintenance, but sustained high blood levels can lead to brain damage. This is of major concern to the one out of roughly 20,000 children who are born with an inherited condition called “phenylketonuria” (PKU). These children cannot metabolize phenylalanine, which then builds up to dangerous levels in their brains. The condition, therefore, necessitates a severe curtailment of phenylalanine intake for at least the first six years of life. This means that aspartame, due to its phenylalanine content, is not suitable for PKU sufferers and consequently requires a warning to that effect on products in which it is an ingredient. It was suggested that some of the untoward effects of aspartame may be caused by a sudden increase in brain phenylalanine levels, especially when the sweetener is consumed along with foods high in carbohydrates. Carbohydrates trigger insulin release into the bloodstream which, in turn, makes it easier for phenylalanine to cross the blood-brain barrier.[31]
Aspartame has seizure-promoting activity in animal models that are widely used to identify compounds affecting (i.e., usually protecting against) seizure incidence. In a similar manner, it is possible that doses of the sweetener that cause a sufficient increase in brain phenylalanine might increase seizure frequency among susceptible humans, or might allow seizures to occur in people who are vulnerable but without prior episodes.[31] On the other hand, human studies disprove it. It was shown that there was no difference between the results for aspartame and those for the placebo.[32,33] In a crossover design by Ralph et al., they concluded that individuals with mood disorders are particularly sensitive to this artificial sweetener and its use in this population should be discouraged.[34]
The effect of aspartame during reproduction, development and lactation has been evaluated in rats, mice, hamsters, and rabbits. No-effect levels of exposure during reproduction and gestation have been reported to range from 1,600 mg/kg bw/day in rabbits to 4,000 mg aspartame/kg bw/day in rodents. Human clinical studies with daily doses of 75 mg/kg bw/day (more than 15 times the estimated daily average intake and 1.5 times the established Acceptable Daily Intake ( ADI) by the FDA) of aspartame for 24 weeks were not associated with any significant changes in clinical measures or adverse effects. The effect of aspartame on behavior, cognitive function, and seizures has been studied extensively in animals, and in healthy children, hyperactive children, sugar-sensitive children, healthy adults, individuals with Parkinson's disease, and individuals suffering from depression.[35]
The effects of aspartic acid, another aspartame breakdown product, have also been rigorously examined. Administration of extremely large amounts to non-human primates produced no damage even though blood levels were greatly elevated. It is a fact that in large doses, methanol can lead to blindness and even to death. Methanol occurs naturally in foods. In fact, the “natural” methanol content of fruit juice is about 2.5 times higher than from aspartame-sweetened drinks. Even at the 99th percentile level of 34 mg per kg of body weight consumed per day, blood levels of methanol are undetectable. A study published in 1996, claimed that a 10% increase in brain tumors noted in the 1980s was associated with the introduction of aspartame.[36] The suggestion was that aspartame or its diketopiperazine breakdown product may combine with nitrites in the diet to form nitrosated compounds. Nitrosoureas are indeed known to produce brain tumors in animals.[18] In 2005, researchers at the Ramazzini Foundation in Bologna, Italy, conducted a study which showed a significant dose dependent increase in incidence of lymphomas/leukemias in both male and female rats. They have stated that aspartame is a multi-potential carcinogenic compound whose carcinogenic effects are evident even at a daily dose of 20 mg/kg body weight, much less than the current ADI for humans in Europe (40 mg/kg bw) and in the United States (50 mg/kg bw). However, in 2009 the European Food Safety Authority reviewed the study and concluded that the tumors probably occurred just by chance.[37] In a study conducted in 2006, the U.S. National Cancer Institute researchers studied a large number of adults 50 to 69 years of age over a five-year period. There was no evidence that aspartame posed any risk. However, the study was limited in three major regards: It did not involve truly elderly people (the rat studies monitored the rats until they died a natural death), the subjects had not consumed aspartame as children, and it was not a controlled study (the subjects provided only a rough estimate of their aspartame consumption, and people who consumed aspartame might have had other dietary or lifestyle differences that obscured the chemical's effects).[38] In 2007, the same Italian researchers published a follow-up study that began exposing rats to aspartame in utero. This study found that aspartame caused leukemias/lymphomas and mammary (breast) cancer.[39] It is likely that the new studies found problems and the earlier company-sponsored studies did not because the Italian researchers monitored the rats for three years instead of two. The Italian tests remain controversial, with the industry contending that they were flawed in several ways and with the FDA stating that its scientists could not evaluate the studies because the researchers refused to provide their original data.
The Advisory Forum of EFSA (European Food Safety Authority) has reviewed the information on aspartame with national experts. The objectives of the Organizing Team were to identify, collect and review all published papers since the review carried out by the SCF (Scientific Committee on Food) in 2002. In addition the Organizing Team considered available non-peer-reviewed information and anecdotal evidence. They analyzed 26 reviews. The areas which were considered include exposure data, brain function, satiation and appetite, allergenicity and immunotoxicity, metabolic aspects and diabetes, carcinogenicity (including cancer epidemiology) and genotoxicity and reported on.[40] The review relating to brain function includes reports on the direct and indirect cellular effects of aspartame or its metabolites on the nervous system including neurotoxicity and functional aspects published or accessible after 2002. Several studies, in vitro or in vivo, indicate that aspartame or its metabolites may affect certain enzyme activities in the brain, for example, acetylcholinesterase,[41] Na+/K+-ATPase,[42] or cytochrome P450 (CYP) enzymes.[43] The National Experts consider that the biological relevance of such findings is not clear, particularly the relevance of findings in in vitro studies in which the toxicokinetic and toxicodynamic behavior of aspartame in vivo is not fully reflected. The National Experts consider, however, that the scientific literature needs to be monitored for further research and mechanistic explanations related to this area. The National Experts note that no new publications were identified reporting a link between aspartame intake and enhanced susceptibility to seizures, behavior, mood and cognitive function, and conclude that there is still no substantive evidence that aspartame can induce such effects, as earlier concluded by the SCF.
A number of studies focused on the effects of aspartame on appetite/hunger and food intake,[44–46] as it has been suggested that aspartame may have modulating effects on these body responses, even resulting in the converse effect than that intended, namely obesity rather than body weight maintenance or loss. The National Experts have noted that there is little or no substantive data suggesting that aspartame affects appetite/hunger, food intake. A study focusing on aspartame, such as that performed by Just et al., which looked at cephalic insulin response in healthy fasting volunteers after taste stimulation, comparing sucrose, starch and saccharin, warrants further consideration.[47]
In the literature reviewed by the Organizing Team it has been observed that the metabolites of aspartame (aspartic acid, phenylalanine and methanol) could affect the metabolism of endogenous and exogenous compounds. Amino acids per se have an influence on metabolic pathways and it is known that high doses of aspartame may increase plasma levels of the metabolites of aspartame. High levels of specific amino acids can also affect transporters and protein synthesis. The National Experts note that there is very little new information about the effects of aspartame and its metabolites on the metabolism of endogenous and exogenous compounds. The available reports mainly used high doses, and focused on plasma changes in aspartic acid, phenylalanine and methanol.[48] One reference showed, in patients with Type II diabetes, that the reduction of plasma glucose and insulin levels during exercise was similar after a sucrose meal compared to an aspartame-sweetened meal.[49] These results were obtained even though the aspartame meal contained 22% less calories and 10% less carbohydrates. The National Experts considered that research investigating whether aspartame and its metabolites affect gene expression, protein synthesis and enzyme activities of Cytochrome P450 enzymes in the brain could be useful to extend knowledge in this area. While the use of novel techniques such as metabolomics has not been considered in previous evaluations of aspartame, as they were not available at the time, it is recognized that such research is at the forefront of toxicological science and the results of such work may usefully increase the evidence base. There is no evidence to suggest that aspartame is carcinogenic (as discussed earlier).
Overall, National experts of EFSA in 2009 have not identified any new evidence regarding the safety of aspartame. The current weight of evidence is that aspartame is safe at current levels of consumption as a nonnutritive sweetener.[40]
Saccharin
Saccharin was discovered over a century ago and has been used as a non-caloric sweetener in foods and beverages for more than 100 years. Apart from Sugar of lead, Saccharin was the first artificial sweetener and was originally synthesized in 1879 by Remsen and Fahlberg. It had been created in an experiment with toluene derivatives. A process for the synthesis of saccharin from phthalic anhydride was developed in 1950 and currently, saccharin is synthesized by this process as well as the original process by which it was discovered. It is 300 to 500 times sweeter than sugar and is often used to improve the taste of toothpastes, dietary foods, and dietary beverages.
One animal study has shown that consumption of products containing saccharin may lead to increased body weight and obesity by interfering with fundamental homeostatic and physiological processes[15] Fear about saccharin increased when a study in 1960 showed that high levels of saccharin may cause bladder cancer in laboratory rats. In 1977, Canada banned saccharin due to the adverse effects reported in animal studies. In the United States, the FDA considered banning it in 1977, but Congress stepped in and placed a moratorium on such a ban which required a warning label and also mandated further study of saccharin safety. Subsequently, it was discovered that saccharin causes cancer in male rats by a mechanism not found in humans. At high doses, it forms a precipitate in rat urine. This precipitate damages the cells lining the bladder and a tumor forms when the cells regenerate. According to the International Agency for Research on Cancer, part of the World Health Organization, “Saccharin and its salts were downgraded from Group 2B, possibly carcinogenic to humans, to Group 3, not classifiable as carcinogenic to humans, despite sufficient evidence of carcinogenicity to animals, because it is carcinogenic by a non-DNA-reactive mechanism that is not relevant to humans because of critical interspecies differences in urine composition.”[11]
In May 2000, the U.S. Department of Health and Human Services removed saccharin from its list of cancer-causing chemicals. Later that year, Congress passed a law removing the warning notice that likely will result in increased use in soft drinks and other foods and in a slightly greater incidence of cancer. In 2001, the United States repealed the warning label requirement, while the threat of an FDA ban had already been lifted in 1991. Most other countries also permit saccharin but restrict the levels of its use, while other countries have outright banned it.[38]
Sucralose
Sucralose was discovered by British researchers in 1976. It is the only non-caloric sweetener made from sugar and considered as a latest international Zero-Calorie sugar substitute. It is a chlorinated sugar that is about 600 times as sweet as sugar. It is produced from sucrose when three chlorine atoms replace three hydroxyl groups. Its unique combination of sugar-like taste and excellent stability allow sucralose to be used as a replacement for sugar in virtually every type of food (more than 4,000 food products) and beverages, frozen desserts, chewing gum, baked goods, and other foods. Unlike other artificial sweeteners, it is stable when heated and can therefore be used in baked and fried goods. Sucralose is minimally absorbed by the body and most of it passes out of the body unchanged.[50] The FDA approved sucralose for use in 15 types of foods and beverages in 1998[5] and approved it for general purpose in 1999. In India, PFA (Prevention of Food Adulteration) also permits its use in certain products i.e. ice cream, dairy, beverages, bakery, Indian sweets, etc. It is helpful especially for Indian people who enjoy sweet food but are trying to reduce the number of calories that they consume or the ‘amount of sugar’ in their diet by reducing the amount of added sugar and calories. The establishment of an ADI for sucralose required some additional research beyond the routine studies ordinarily carried out to evaluate the safety of a food additive.[7]
Safety concerns pertaining to sucralose revolve around the fact that it belongs to a class of chemicals called organic chlorides, some types of which are toxic or carcinogenic; however, the presence of chlorine in an organic compound does not in any way ensure toxicity. The way sucralose is metabolized may suggest a reduced risk of toxicity. For example, sucralose is extremely insoluble in fat and thus does not accumulate in fat as do some other organic chlorides; sucralose also does not break down and dechlorinates only under conditions that are not found during regular digestion.[50]
Sucralose is considered safe for all segments of the population, including people with chronic health problems such as diabetes. A three-month study of 128 people with diabetes, in which sucralose was administered at a dose approximately three times the maximum estimated daily intake, showed no adverse effects on any measure of blood glucose control.[51]
Acesulfame K
Acesulfame potassium is a non-caloric sweetener with a clean, quickly perceptible sweet taste. It has excellent stability under high temperatures and has good solubility. So it is suitable for numerous products.
In 1998, acesulfame K was approved by the US FDA for use in liquid non-alcoholic beverages and in 2003, general use approval was granted. The Joint Expert Committee on Food Additives (JECFA), the scientific advisory body to the World Health Organization and the Food and Agriculture Organization of the United Nations, reviewed the available research on acesulfame K and stated that it is safe.[11,38]
Neotame
Neotame is a no-calorie sweetener, which is a derivative of the dipeptide composed of the amino acids, aspartic acid and phenylalanine. The components of neotame are joined together to form a uniquely sweet ingredient. Neotame is about 8,000 times sweeter than table sugar and 40 times sweeter than aspartame. Neotame is chemically related to aspartame, but it is chemically more stable, enabling the new sweetener to be used in baked foods. It is used mostly in low-calorie foods, but may also be used as a flavoring agent in other foods. It was approved by the U.S. FDA in 2002, but is still rarely used.[38]
Stevia/Rebaudioside A
Stevia is derived from Stevia rebaudiana, a South American plant, and it has been used for centuries to sweeten beverages and make tea in the plant's native Paraguay. Rebaudioside A is one compound within the stevia plant that provides sweetness. The steviol glycosides meet purity criteria established by the JECFA (WHO). The clinical studies show that they have no effect on either blood pressure or blood glucose response, indicating stevia sweeteners are safe for use by individuals with diabetes. Recent studies, including human studies on intake, metabolism and toxicity, support the safety of stevia sweetener. Based on the published research, independent scientific experts in both the U.S. and globally have concluded that stevia sweeteners are safe for people of all ages and an Acceptable Daily Intake (ADI) of 4 mg/kg body weight (expressed as steviol) has been established.[10] Stevia has a very low acute toxicity, and no allergic reactions to it seem to exist.[52]
Just because a substance is natural, does not mean that it is safe. Many natural plant components are toxic. And while a long history of use does indicate that a substance is free from severe, immediate toxic effects, it does not guarantee that the substance is entirely safe. Rare adverse effects, delayed effects, or effects that occur only with long-term use may not be identified initially. One study showed that high dosages fed to rats reduced sperm production and increased cell proliferation in their testicles, which could cause infertility or other problems. In the laboratory, steviol can be converted into a mutagenic compound, which may promote cancer by causing mutations in the cells’ DNA.[38] In the 1990s, the U.S. FDA rejected stevia for use as a food ingredient. In 1995, the FDA issued a statement allowing stevia to be used as a dietary supplement, and so it has to be labeled. Likewise, Canada and a European Community scientific panel did not approve it and declared that stevia was unacceptable for use in food.[53]
Tagatose
This new synthetic additive is chemically related to fructose, but is poorly absorbed by the body. That's why it yields only about one-third as many calories. Large amounts cause diarrhea, nausea, and flatulence. Although it is chemically a sugar, it does not promote tooth decay.[38] In 2004, JECFA assigned tagatose an ADI of “not specified” (the meaning of this type of ADI is used to refer to a food substance of very low toxicity which, on the basis of the available data and the total dietary intake of the substance arising from its use at the levels necessary to achieve the desired effect and from its acceptable background levels in food, does not represent a hazard to health. Under these circumstances, JECFA does not deem it necessary to establish an ADI expressed in numerical form.). Tagatose is approved in Australia, New Zealand, Korea, and the European Union.[54]
CONCLUSIONS
Sugar substitutes in various food and beverages are very popular in most of the countries. Extensive scientific research has demonstrated the safety of the six low-calorie sweeteners currently approved for use in foods in the U.S. and Europe (stevia, acesulfame-K, aspartame, neotame, saccharin and sucralose) each with an acceptable daily intake. A number of studies have been carried out to confirm the safety of artificial sweeteners. A number of studies have also shown the adverse effects of the same. But most of the studies have limitations such as effects shown only in animals not in human, small sample size, high doses, statistically non-significant or borderline significant, etc. The sugar substitutes are thoroughly investigated for safety with hundreds of scientific studies and then approved by different regulatory authorities like the U.S. FDA, JECFA and FSANZ. Some agents are approved with warning labels too. So further exploration is required with well-designed large-scale studies in the general population. On the anecdotal evidence, it has been concluded that based on analysis of the database of case histories, there are a number of symptoms that are recurrently reported by individuals who believe that they are caused by sugar substitute ingestion. The information gathered in this analysis can be useful in guiding the design and format of any investigative study that may be undertaken to determine individual sensitivity to sugar substitutes.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.FDA No Calories. Sweet! [Last accessed on 2011 Feb 1]. Available from: http://www.fda.gov/fdac/features/2006/406_sweeteners.html .
- 2.Sugar demand rising at expense of sweeteners, claims sugar industry. [Last accessed on 2011 Feb 1]. Available from: http://www.foodnavigator-usa.com/
- 3. [Last accessed on 2011 Feb 1]. Available from: http://WWW.sweetpoison.com/articles/0706/aspartame-syrup .
- 4.US FDA Website Guidance Documents. [Last accessed on 2011 Feb 4]. Available from: http://www.cfsan.fda.gov/~dms/grasguid.html#Q1 .
- 5.FDA's response to European Aspartame Study. [Last accessed on 2011 Feb 4]. Available from: http://www.fda.gov/bbs/topics/NEWS/2006/NEW01369.html .
- 6. [Last accessed on 2011 Feb 1]. Available from: http://leda.law.harvard.edu/leda/data/816/Burnett_07.html .
- 7.Kroger M, Meister K, Kava R. Low calorie sweetners and other sugar substitutes: A review of the safety issues. Compr Rev Food Sci Food Saf. 2006;5:35–47. [Google Scholar]
- 8.Bellisle F, Drewnowski A. Intense sweeteners, energy intake and the control of body weight. Eur J Clin Nutr. 2007;61:691–700. doi: 10.1038/sj.ejcn.1602649. [DOI] [PubMed] [Google Scholar]
- 9.Mackie IC. Children's dental health and medicines that contain sugar. BMJ. 1995;15:141–2. doi: 10.1136/bmj.311.6998.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bentley EM, Mackie IC. A qualitative investigation into general practitioners’ views on prescribing sugar-free medicines for children prior to a dental health education campaign. Health Educ Res. 1993;8:519–24. [Google Scholar]
- 11.The Calorie Control Council.mht. [Last accessed on 2011 Feb 10].
- 12.Reactivehypoglycemia. [Last accessed on 2011 Feb 15].
- 13.DeNoon, Daniel J. Reviewed by Charlotte Grayson Mathis MD. “Drink More Diet Soda, Gain More Weight? Overweight Risk Soars 41% with Each Daily Can of Diet Soft Drink”, Web MD Medical News (2005) [Last accessed on 2011 Feb 11]; [Google Scholar]
- 14.Swithers SE, Davidson TL. “A role for sweet taste: Calorie predictive relations in energy regulation by rats”. Behav Neurosci. 2008;122:161–73. doi: 10.1037/0735-7044.122.1.161. [DOI] [PubMed] [Google Scholar]
- 15.Hampton T. Sugar substitutes linked to weight gain. JAMA. 2008;299:2137–8. doi: 10.1001/jama.299.18.2137. [DOI] [PubMed] [Google Scholar]
- 16.Prodolliet J, Bruelhart M. “Determination of aspartame and its major decomposition products in foods”. J AOAC Int. 1993;76:275–82. [PubMed] [Google Scholar]
- 17.Stegink LD. “The aspartame story: A model for the clinical testing of a food additive”. Am J Clin Nutr. 1987;46:204–15. doi: 10.1093/ajcn/46.1.204. [DOI] [PubMed] [Google Scholar]
- 18.Lin SY, Cheng YD. “Simultaneous formation and detection of the reaction product of solid-state aspartame sweetener by FT-IR/DSC microscopic system.”. Food Addit Contam. 2000;17:821–7. doi: 10.1080/026520300420385. [DOI] [PubMed] [Google Scholar]
- 19.Rastogi S, Zakrzewski M, Suryanarayanan R. Investigation of solid-state reactions using vari-able temperature X-ray powder diffractrometry. I. Aspartame hemihydrate. Pharm Res. 2001;18:267–73. doi: 10.1023/a:1011086409967. [DOI] [PubMed] [Google Scholar]
- 20.Food Standards Australia New Zealand: “Food Standards Australia New Zealand: Aspar-tame (Septemb2007)”. [Last accessed on 2011 Jan 19].
- 21.Pradhan S, Shah UH, Mathur A, Sharma S. Experimental evaluation of antipyretic and an-algesic activity of aspartame. Indian J Pharmacol. 2011;43:89–90. doi: 10.4103/0253-7613.75683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.LaBuda CJ, Fuchs PN. A comparison of chronic aspartame exposure to aspirin on inflammation, hyperalgesia and open field activity following carrageenan-induced monoarthritis. Life Sci. 2001;69:443–54. doi: 10.1016/s0024-3205(01)01136-5. [DOI] [PubMed] [Google Scholar]
- 23.Ramsland PA, Movafagh BF, Reichlin M, Edmundson AB. Interference of rheumatoid factor activity by aspartame, a dipeptide methyl ester. J Mol Recognit. 1999;12:249–57. [PubMed] [Google Scholar]
- 24.Schiffman SS, Buckley CE, 3rd, Sampson HA, Massey EW, Baraniuk JN, Follett JV, et al. Aspartame a Susceptibility to Headache. N Engl J Med. 1987;317:1181–5. doi: 10.1056/NEJM198711053171903. [DOI] [PubMed] [Google Scholar]
- 25.Markle N. Contra Aspartam. [Last accessed on 2011 June 2]. Available from: http://www.ever.ch/medizinwissen/aspartam.php .
- 26.Jacob SE, Stechschulte S. Formaldehyde, aspartame and migraines: A possible connection. Dermatitis. 2008;19:10–1. [PubMed] [Google Scholar]
- 27.Hill AM, Belsito DV. Systemic contact dermatitis of the eyelids caused by formaldehyde derived from aspartame? Contact Dermatitis. 2003;49:258–9. doi: 10.1111/j.0105-1873.2003.0225a.x. [DOI] [PubMed] [Google Scholar]
- 28.Parthasarathy NJ, Kumar RS, Manikandan S, Narayanan GS, Kumar RV, Devi RS. Effect of methanol-induced oxidative stress on the neuroimmune system of experimental rats. Chem Biol Interact. 2006;161:14–25. doi: 10.1016/j.cbi.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 29.Parthasarathy NJ, Srikumar R, Manikandan S, Narayanan GS, Devi RS. Effect of methanol intoxication on specific immune functions of albino rats. Cell Biol Toxicol. 2007;23:117–87. doi: 10.1007/s10565-006-0151-8. [DOI] [PubMed] [Google Scholar]
- 30.Geha R, Buckley CE, Greenberger P, Patterson R, Polmar S, Saxon A, et al. Aspartame is no more likely than placebo to cause urticaria/angioedema: Results of a multicenter, randomized, double-blind, placebo-controlled, crossover study. J Allergy Clin Immunol. 1993;92:513–20. doi: 10.1016/0091-6749(93)90075-q. [DOI] [PubMed] [Google Scholar]
- 31.Wurtman RJ, Mahe TJ. New York: Naylor-Dana Foundation; 1987. Effects of aspartame on the brain: Neurologic effects of aspartame. Presented at the symposium: “Sweeteners: Health Effects,”; pp. 18–20. [Google Scholar]
- 32.Rowan AJ, Shaywitz BA, Tuchman L, French JA, Luciano D, Sullivan CM. Aspartame and seizure susceptibility: Results of a clinical study in reportedly sensitive individuals. Epilepsia. 1995;36:270–5. doi: 10.1111/j.1528-1157.1995.tb00995.x. [DOI] [PubMed] [Google Scholar]
- 33.Shaywitz BA, Anderson GM, Novotny EJ, Ebersole JS, Sullivan CM, Gillespie SM. Aspartame has no effect on seizures or epileptiform discharges in epileptic children. Ann Neurol. 1994;35:98–103. doi: 10.1002/ana.410350115. [DOI] [PubMed] [Google Scholar]
- 34.Walton RG, Hudak R, Green-Waite RJ. Green-Waite Adverse Reactions to Aspartame: Double-Blind Challenge in Patients from a Vulnerable Population. Biol Psychiatry. 1993;34:13–7. doi: 10.1016/0006-3223(93)90251-8. [DOI] [PubMed] [Google Scholar]
- 35.Magnuson BA, Burdock GA, Doull J, Kroes RM, Marsh GM, Pariza MW, et al. Aspar-tame: A Safety Evaluation Based on Current Use Levels, Regulations, and Toxicological and Epi-demiological Studies. Crit Rev Toxicol. 2007;37:629–727. doi: 10.1080/10408440701516184. [DOI] [PubMed] [Google Scholar]
- 36.Olney JW, Farber NB, Spitznagel E, Robins LN. Increasing brain tumor rates: Is there a link to aspartame? J Neuropathol Exp Neurol. 1996;55:1115–23. doi: 10.1097/00005072-199611000-00002. [DOI] [PubMed] [Google Scholar]
- 37.Soffritti M, Belpoggi F, Degli Esposti D, Lambertini L, Tibaldi E, Rigano A. First Experimental Demonstration of the Multipotential Carcinogenic Effects of Aspartame Administered in the Feed to Sprague-Dawley Rats. Environ Health Perspect. 2006;114:379–85. doi: 10.1289/ehp.8711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. [Last accessed on 2011 Feb 28]. Available from: http://www.cspinet.org/reports/chemcuisine.htm .
- 39.Zwillich T. Aspartame Safety Study Stirs Emotions. Italian Study Shows Sweetener Promotes Cancer in Rats; FDA Says It's Safe. 2007. [Last accessed on 2011 Mar 8]. Available from: http://www.webmd.com/diet/news/20070626/aspartame-safety-studystirs-emotions .
- 40.Reports of the meetings on Aspartame with national experts. ON-1641 Noted at the 36th Advisory Forum Meeting. 2010. May 19-20, [Last accessed on 2011 June 8]. Available from: http://www.efsa.europa.eu/en/supporting/doc/1641.pdf .
- 41.Simintzi I, Schlupis KH, Angelogianni P, Liapi C, Tsakiris S. The effect of aspartame metabolites on the suckling rat frontal cortex acetylcholinesterase. An in vitro study. Food Chem Toxicol. 2007;45:2397–401. doi: 10.1016/j.fct.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 42.Christian B, McConnaughey K, Bethea E, Brantley S, Coffey A, Hammond L, et al. Chronic aspartame affects T-maze performance, brain cholinergic receptors and Na+, K+-ATPase in rats. Pharmacol Biochem Behav. 2004;78:121–7. doi: 10.1016/j.pbb.2004.02.017. [DOI] [PubMed] [Google Scholar]
- 43.Tutelyan VA, Kravchenko LV, Kuzmina EE. The effect of aspartame on the activity of rat liver xenobiotic-metabolizing enzymes. Drug metabolism and Disposition: Drug Metab Dispos. 1990;18:223–5. [PubMed] [Google Scholar]
- 44.Appleton KM, Rogers PJ, Blundell JE. Effects of a sweet and non-sweet lunch on short term appetite: Differences in female high and low consumers of sweet/low-energy beverages. J Hum Nutr Diet. 2004;17:425–34. doi: 10.1111/j.1365-277X.2004.00548.x. [DOI] [PubMed] [Google Scholar]
- 45.Reid M, Hammersley R, Hill AJ, Skidmore P. Long-term dietary compensation for added sugar: Effects of supplementary sucrose drinks over a 4-week period. Br J Nutr. 2007;97:193–203. doi: 10.1017/S0007114507252705. [DOI] [PubMed] [Google Scholar]
- 46.van Wymelbeke V, Béridot-Thérond ME, de La Guéronnière V, Fantino M. Influence of repeated consumption of beverages containing sucrose or intense sweeteners on food intake. Eur J Clin Nutr. 2004;58:154–61. doi: 10.1038/sj.ejcn.1601762. [DOI] [PubMed] [Google Scholar]
- 47.Just T, Pau HW, Engel U, Hummel T. Cephalic phase insulin release in healthy humans after taste stimulation? Appetite. 2008;51:622–7. doi: 10.1016/j.appet.2008.04.271. [DOI] [PubMed] [Google Scholar]
- 48.Magnuson BA, Burdock GA, Doull J, Kroes RM, Marsh GM, Panza W, et al. Aspartame: A safety evaluation based on current use levels, regulations, and toxicological and epidemiological studies. Crit Rev Toxicol. 2007;37:629–727. doi: 10.1080/10408440701516184. [DOI] [PubMed] [Google Scholar]
- 49.Ferland A, Brassard P, Poirier P. Is aspartame really safer in reducing the risk of hypogly-caemia during exercise in patients with type 2 diabetes? Diabetes Care. 2007;30:e59. doi: 10.2337/dc06-1888. [DOI] [PubMed] [Google Scholar]
- 50.Daniel JW, Renwick AG, Roberts A, Sims J. “The metabolic fate of sucralose in rats”. Food Chem Tox. 2000;38:115–21. doi: 10.1016/s0278-6915(00)00034-x. [DOI] [PubMed] [Google Scholar]
- 51.Grotz VL, Henry RR, McGill JB, Prince MJ, Shamoon H, Trout JR, et al. Lack of effect of sucralose on glucose homeostasis in subjects with type 2 diabetes. J Am Diet Assoc. 2003;103:1607–12. doi: 10.1016/j.jada.2003.09.021. [DOI] [PubMed] [Google Scholar]
- 52.Geuns JM. Stevioside. Phytochemistry. 2007;64:913–21. doi: 10.1016/s0031-9422(03)00426-6. [DOI] [PubMed] [Google Scholar]
- 53.Sweet on Stevia: Sugar Substitute Gains Fans, Columbia Daily Tribune. [Last accessed on 2011 Mar 4];2008 Mar 23; [Google Scholar]
- 54.Geneva: WHO; [Last accessed on 2011 June 10]. [JFECFA] Joint FAO/WHO Expert Committee on Food Additives. 2004. Sixty-third meeting, 8 to 17 June. Available from: http://www.who.int/ipcs/publications/jecfa/en/Summary63final.pdf. publications/jecfa/en/Summary63final.pdf . [Google Scholar]


