Abstract
Objective:
To evaluate and compare the effects on high-sensitivity C-reactive protein (hs-CRP) levels and lipid profile of atorvastatin and rosuvastatin in obese type 2 diabetes mellitus (T2DM) patients.
Materials and Methods:
A total of 40 subjects with 20 in each group were randomly allocated to two groups. Group 1 patients received atorvastatin and that of Group 2 rosuvastatin treatment for 6 months. The patients were administered atorvastatin (40-80 mg) and rosuvastatin(10-40 mg) in accordance to their LDL-C status as per NCEP-ATP III guidelines. The parameters studied were, hs-CRP and lipid profile comprising LDL-C, HDL-C, TG and TC.
Results:
Results obtained from the study, clearly indicate that atorvastatin (A) as well as rosuvastatin(R) have significant effect on lowering of hs-CRP levels (for A P=0.001; for R P=0.002), reducing LDL-C levels (for A P=0.008; for R P=0.001), elevating HDL-C levels (for A P=0.02; for R P=0.001) along with reducing TC (for A P=0.003; for R P=0.002) and TG (for A P=0.000; for R P=0.000) levels in obese T2DM patients. It is also seen that there is no significant (P>0.05) difference in effect of atorvastatin and rosuvastatin in lowering of hs-CRP levels, elevating HDL-C levels and reducing TG levels in obese T2DM patients. However, percentage lowering of LDL-C (P=0.000) and TC (P=0.001) by rosuvastatin is to a greater extent than that caused by atorvastatin in these patients.
Conclusions:
Thus this study throws light on the fact that rosuvastatin should be preferred over atorvastatin in obese T2DM patients in whom LDL-C and TC levels are deviated from normal reference values. In rest of obese T2DM either of atorvastatin or rosuvastatin can be employed to lower hs-CRP levels, to elevate HDL-C levels or to reduce TG levels.
Keywords: Diabetes mellitus, hs-CRP, obese, statins
INTRODUCTION
The diabetes prevalence for 2010 has risen to 285 million, representing 6.6% of the world's adult population, with a prediction that by 2030 the number of people with diabetes will have risen to 438 million.[1] Increasing numbers of patients with diabetic complications will impose an enormous burden on the healthcare system.[2,3] It is characterised by clustered metabolic abnormalities including hyperglycemia, elevated triglycerides (TG) and total cholesterol (TC), low high-density lipoprotein cholesterol (HDL-C) and central obesity.[4] Cardiovascular diseases (CVD), account for the majority of deaths in these patients. Low-grade inflammation has a pivotal role in atherosclerosis, an important risk factor for CVDs. The inflammatory marker high-sensitivity C-reactive protein (hs-CRP) has emerged as a strong predictor for cardiovascular events.[5] Elevated baseline concentrations of hs-CRP are associated with the risk of atherosclerotic events and show a predictive value in terms of secondary prevention of CVD.
The global epidemic of DM is in large part due to obesity and sedentary lifestyle. Serum hs-CRP levels have also been correlated positively with adipocyte size[6] as well as body mass index (BMI).[7]
By previous studies[8–11] statins, the most widely used drugs for the lipid management have also been found to cause significant reduction in CRP concentrations, unrelated to the magnitude of low-density lipoprotein cholesterol (LDL-C) reduction.
Keeping these points in mind along with the facts that obesity is strongly associated with elevated plasma lipid levels and that actually 60-90% of cases of type 2 DM (T2DM) now appear to be related to obesity, it was thought worthwhile to evaluate and compare the effects on hs-CRP levels and lipid profile of various statins in obese T2DM patients.
According to the studies done previously[12–14] atorvastatin and rosuvastatin are two potent statins which can efficiently lower hs-CRP levels. So, in the present study effects of atorvastatin and rosuvastatin were evaluated and compared on the basis of the effects on hs-CRP levels and lipid profile in obese T2DM patients. Such study is hoped to be useful in choosing a statin out of plethora of statins available, suitable for lowering risk of atherosclerosis and hence chances of CVD specifically in obese T2DM patients. To best of our knowledge, no other study has been taken up as yet so as to evaluate and compare effects on hs-CRP levels and lipid profile of atorvastatin and rosuvastatin in obese T2DM patients.
MATERIALS AND METHODS
The present study was planned as prospective randomized open-labelled, parallel group, comparative study of 6-months duration held from August 2010 to February 2011. Study subjects were allocated randomly to two groups as follows:
Group 1: Obese T2DM patients administered atorvastatin (40-80 mg) in accordance to their LDL-C status as per NCEP-ATP III guidelines.
Group 2: Obese T2DM patients administered rosuvastatin (10-40 mg) in accordance to their LDL-C status as per NCEP-ATP III guidelines.
A total of 40 subjects with 20 in each group were enrolled. Inclusion and exclusion criteria, treatment allocation and follow-up of patients in the study are shown in Figure 1. The study subjects were randomized into respective groups by block permuted randomization. Approval of the Institutional Ethics Committee was taken prior to the start of the study. Forty patients were enrolled in the study after satisfying the inclusion and exclusion criteria. Included patients were explained in detail about the study protocol and related hazards. Informed written consent was obtained from all the patients.
Figure 1.
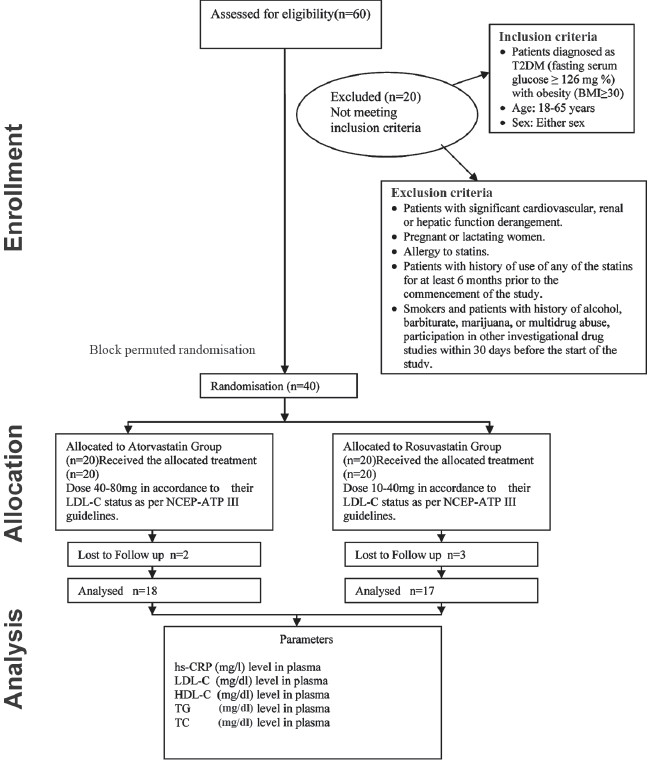
CONSORT statement of the study (Prospective randomized open-labeled parallel study)
Lipid profile was measured using standard methods. Serum hs-CRP levels were measured by ELISA using commercial kit (Accu-Bind Elisa Microwells, Monobind Inc., USA). Lipid profile comprised of LDL-C, HDL-C, TG and TC.
Each patient in the respective group was provided with the drug supplies for 15 days and was asked to visit the diabetic clinic for follow-up and for collection of drugs. As suggested by previous studies,[15] pill-count method was utilised to measure medication adherence by patient. Pill counts were calculated as the number of pills taken (the number of pills dispensed – the number of pills counted). The number of pills expected to have been taken was calculated by multiplying the daily dose (1/2, 1 or 2 tablets) by the number of days since the date dispensed. Pill count was 85–100% for all patients in our study. So, in accordance with previous studies[16] there was successful adherence of the medication. At each follow-up visit, patients were also assessed for glycemic control, and history pertaining to adverse drug effects was asked. All patients were given advice about diet and exercise.
Statistical methods
Sample size was calculated taking into consideration the mean values and standard deviation from study done by Lam et al.[17] Power of study = 80%, α = 0.05 and = 0.05 and β = 0.20. The data obtained at day 0 and after 6 months were entered into Case Record form and analyzed statistically. To analyze the results, paired t-test was used to assess within–subjects change across all study variables. Independent samples t-test was employed for analyzing inter-group variation across all study variables. Normal distribution of data was checked before applying statistical tests. Skewness and Kurtosis values were found to be between –2 and +2. All statistical analysis was done using SPSS 17.0 software. P-value <0.05 was taken as significant.
RESULTS
All variables in the study were equally distributed (P>0.05) in both groups before starting drug treatment. During the study no adverse drug reaction was encountered in any of the study subjects. In group 1 and in group 2, two and three patients were lost to follow-up, respectively. The results obtained following analysis are shown in Tables 1 and 2.
Table 1.
Baseline characteristics of patients in the atorvastatin and rosuvastatin groups
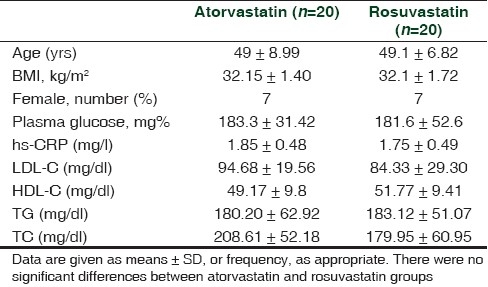
Table 2.
Analysis of effects of atorvastatin and rosuvastatin on hs-CRP and lipid profile
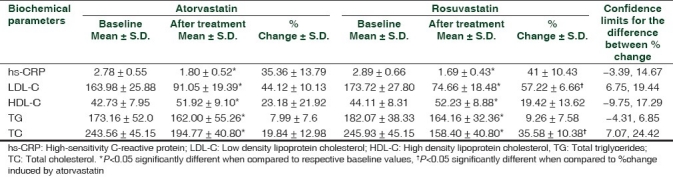
On statistically analyzing the tables, it is clearly indicated that atorvastatin (A) as well as rosuvastatin (R) have significant effect on lowering of hs-CRP levels (for A P=0.001; for R P=0.002), reducing LDL-C levels (for A P=0.008; for R P=0.001), elevating HDL-C levels (for A P=0.02; for R P=0.001) along with reducing TC (for A P=0.003; for R P=0.002) and TG (for A P=0.000; for R P=0.000) levels in obese T2DM patients. It is also seen that there is no significant (P>0.05) difference in effect of atorvastatin and rosuvastatin in lowering of hs-CRP levels, elevating HDL-C levels and reducing TG levels in obese T2DM patients. However, percentage lowering of LDL-C (P=0.000 confidence interval 6.75-19.44) and TC (P=0.001 confidence interval 7.07 to 24.42) by rosuvastatin is to a greater extent than that caused by atorvastatin in obese T2DM patients.
DISCUSSION
In the present study, the study subjects were obese type 2 diabetic patients. The criteria for evaluation were serum hs-CRP levels and lipid profile parameters, namely, LDL-C, HDL-C, TG and TC. The present study shows that both atorvastatin and rosuvastatin have significant effect on lowering of hs-CRP levels, reducing LDL-C levels, elevating HDL-C levels along with reducing TC and TG levels in obese T2DM patients.
Further both these agents are equally effective in lowering of hs-CRP levels, elevating HDL-C levels and reducing TG levels in obese T2DM patients. However, rosuvastatin lowers LDL-C and TC levels to a greater extent than atorvastatin in these patients. Atorvastatin reduced LDL-C levels from 163.98 ± 25.88 to 91.05 ± 19.39 whereas rosuvastatin reduced LDL-C from 173.72 ± 27.80 to 74.66 ± 18.48. Similarly, atorvastatin lowered TC from 243.56 ± 45.15 to 194.77 ± 40.80 and rosuvastatin lowered TC from 245.93 ± 48.15 to 158.40 ± 40.80.
The results of this study are similar to other studies where in patients with T2 DM, statin therapy has been shown to significantly reduce LDL -C, reduce elevated triglycerides, and modestly increase HDL cholesterol.[18,19–22]
Similar to present study, in the Use of Rosuvastatin Versus Atorvastatin in T2DM study, rosuvastatin reduced lipid and lipoprotein fractions compared with atorvastatin during, including LDL -C, non-HDL cholesterol, and apolipoprotein (apo) B to a significantly (P< 0.0001) greater extent.[21]
Both rosuvastatin and atorvastatin significantly reduced LDL-C, non-HDL cholesterol, and the apoB/apoA1 ratio (all, P<0.001 vs. placebo) in the compare rosuvastatin with atorvastatin on ApoB/ApoA1 Ratio in Patients with T2DM and dyslipidemia study.[22]
Differing from interpretation of present study, a study carried out in T2DM patients with dyslipidemia had shown that atorvastatin and rosuvastatin are equally effective in reducing LDL-C levels along with reducing TC and TG levels but rosuvastatin elevated HDL-cholesterol levels more significantly.[23]
In general, LDL cholesterol levels in people with diabetes are not higher than those in people without diabetes who are matched for age, sex and body weight.[24] In fact, the most common LDL cholesterol level in diabetes is “borderline high” (130-159 mg/dl).[24] Moreover, high LDL cholesterol levels (≥160 mg/dl) do not occur at higher-than-average rates in people with diabetes. Nonetheless, LDL cholesterol does not play less of a role in cardiovascular risk in people with T2DM. In fact, LDL cholesterol levels may underestimate cardiovascular risk in diabetes.[25] Small, dense LDL particles are considered more atherogenic than the larger, buoyant LDL particles because they are more readily oxidized and glycated, which make them more likely to invade the arterial wall.[26,27] This can initiate atherosclerosis or lead to increased migration and apoptosis of vascular smooth muscle cells in existing atherosclerotic lesions.[26,27] As a consequence, elevated or “normal” LDL cholesterol may be more pathogenic in people with diabetes. The strong association between increased small, dense LDL particles and elevated triglycerides, for example, appears to be linked to the altered insulin sensitivity common in the metabolic syndrome and T2DM.[28,29]
Limitation of study
This study was carried with the aim to evaluate effects of two commonly used statins, namely atorvastatin and rosuvaststin, on hs-CRP and lipid profile in obese T2DM. Such study is expected to guide physicians to choose a statin from plethora of statins available which can prevent or cure atherosclerosis and thus CVDs in T2DM patients particularly in those who are obese. The present study gives results of only 6-month treatment and that too only of two statins. Further, only surrogate markers have been studied. Hence, studies of longer duration and involving study of greater number of statins and those involving primary end points like prevention of CVDs as well as secondary end points like prevention of atherosclerosis need to done to strengthen clinical implications of the present study.
CONCLUSIONS
This study throws light on the fact that rosuvastatin should be preferred over atorvastatin in obese type 2 diabetic patients in whom LDL-C and TC levels are deviated from normal reference values. In rest of obese T2DM either of atorvastatin or rosuvastatin can be employed to lower hs-CRP levels, to elevate HDL-C levels or to reduce TG and TC levels. More elaborate research is required to study the pathophysiology in obese T2DM patients along with attempt to understand the pharmacological aspect of statin therapy in such patients.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.International diabetes federation. [Last assessed on 2011 May 20]. Available from: http://www.idf.org .
- 2.Alberti G, Zimmet P, Shaw J, Bloomgarden Z, Kaufman F, Silink M. Type 2 diabetes in the young: The evolving epidemic: The international diabetes federation consensus workshop. Diabetes Care. 2004;27:1798–811. doi: 10.2337/diacare.27.7.1798. [DOI] [PubMed] [Google Scholar]
- 3.Booth FW, Chakravarthy MV, Gordon SE, Spangenburg EE. Waging war on physical inactivity: Using modern molecular ammunition against an ancient enemy. J Appl Physiol. 2002;93:3–30. doi: 10.1152/japplphysiol.00073.2002. [DOI] [PubMed] [Google Scholar]
- 4.Belalcazar LM, Reboussin DM, Haffner SM, Hoogeveen RC, Kriska AM, Schwenke DC, et al. A One-Year Lifestyle Intervention for Weight Loss in Persons with Type 2 Diabetes Reduces High C-Reactive Protein Levels and Identifies Metabolic Predictors of Change, from the Look AHEAD(Action for Health in Diabetes) Study. Diabetes Care. 2010;33:2297–303. doi: 10.2337/dc10-0728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ridker PM, Rifai N, Bose L, Burning JE, Cook NR. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002;347:1557–65. doi: 10.1056/NEJMoa021993. [DOI] [PubMed] [Google Scholar]
- 6.Bahceci M, Gokalp D, Bahceci S, Tuzcu A, Atmaca S, Arikan S. The correlation between adiposity and adiponectin, tumor necrosis factor alfa, interleukin-6 and high sensitivity C-reactive protein levels.Is adipocyte size associated with inflammation in adults? J Endocrinol Invest. 2007;30:210–4. doi: 10.1007/BF03347427. [DOI] [PubMed] [Google Scholar]
- 7.Lajunen T, Vikatmaa P, Bloiqu A, Ikonen T, Le pantalo M, Pussinen PJ, et al. Chlamydial LPs and high senstivity CRP levels in serum are associated with an elevated body mass index in patients with cardiovascular disease. Innate Immune. 2008;14:375–82. doi: 10.1177/1753425908099172. [DOI] [PubMed] [Google Scholar]
- 8.Ridker PM, Rifai N, Clearfield M, Downs JR, Weis SE, Miles JS, et al. Air Force/Texas Coronary Atherosclerosis Prevevtion Study Investigators.Measurement of C-reactive protein for the targeting of statin therapy in the primary prevention of acute coronary events. N Engl J Med. 2001;344:1959–65. doi: 10.1056/NEJM200106283442601. [DOI] [PubMed] [Google Scholar]
- 9.Ridker PM, Rifai N, Pfeffer MA. Inflammation, pravastatin, and the risk of coronary events after myocardial infarction in patients with average cholesterol levels. Circulation. 1998;98:839–44. doi: 10.1161/01.cir.98.9.839. [DOI] [PubMed] [Google Scholar]
- 10.Ridker PM, Morrow DA, Rose LM, Rifai N, Cannon CP, Braunwald E. Relative efficacy of atorvastatin 80 mg and pravastatin 40 mg in achieving the dual goals of low-density lipoprotein cholesterol <70 mg/dL and C-reactive protein <2 mg/L: An analysis of the PROVE-IT TIMI-22 trial. J Am Coll Cardiol. 2005;45:1644–8. doi: 10.1016/j.jacc.2005.02.080. [DOI] [PubMed] [Google Scholar]
- 11.Ridker PM, Rifai N, Pfeffer MA, Sacks F, Braunwald E. Long-term effects of pravastatin on plasma concentration of C-reactive protein.The Cholesterol and Recurrent Events (CARE) Investigators. Circulation. 1999;100:230–5. doi: 10.1161/01.cir.100.3.230. [DOI] [PubMed] [Google Scholar]
- 12.Watson KE. The JUPITER trial: How will it change clinical practice? Rev Cardiovasc Med. 2009;10(2):91–6. [PubMed] [Google Scholar]
- 13.Ukinc K, Ersoz HO, Erem C, Hacihasanoglu AB, Karti SS. Effects of one year simvastatin and atorvastatin treatments on acute phase reactants in uncontrolled type 2 diabetic patients. Endocrine. 2009;35:380–8. doi: 10.1007/s12020-009-9157-3. [DOI] [PubMed] [Google Scholar]
- 14.Parson HK, Bundy MA, Dublin CB, Boyd AL, Paulson JF, Vinik AI. Pleiotropic effects of rosuvastatin on microvascular function in type 2 diabetes. Diabetes Metab Syndr Obes. 2010;3:19–26. [PMC free article] [PubMed] [Google Scholar]
- 15.Lee JK, Grace KA, Foster TG, Crawley MJ, Erowele GI, Sun HJ, et al. How should we measure medication adherence in clinical trials and practice? Ther Clin Risk Manag. 2007;3:685–90. [PMC free article] [PubMed] [Google Scholar]
- 16.Krueger KP, Felkey BG, Berger BA. Improving adherence and persistence: A review and assessment of interventions and description of steps toward a national adherence initiative. J Am Pharm Assoc (Wash DC) 2003;43:668–78. doi: 10.1331/154434503322642598. [DOI] [PubMed] [Google Scholar]
- 17.Lam HC, Chu CH, Wei MC, Keng HM, Lu CC, Sun CC, et al. The effects of different doses of Atorvastatin on plasma endothelin-1 levels in type 2 diabetic patients with dyslipidemia. Exp Biol Med. 2006;231:1010–5. [PubMed] [Google Scholar]
- 18.Colhoun HM, Betteridge DJ, Durrington PN, Hitman GA, Niel HA, Livingstone SJ, et al. and CARDS investigators.Primary Prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetic Study (CARDS): Multicentre randomized placebo-controlled trial. Lancet. 2004;364:685–96. doi: 10.1016/S0140-6736(04)16895-5. [DOI] [PubMed] [Google Scholar]
- 19.Collins R, Armitage J, Parish S, Sleigh P, Peto R. Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: A randomised placebo-controlled trial. Lancet. 2003;361:2005–16. doi: 10.1016/s0140-6736(03)13636-7. [DOI] [PubMed] [Google Scholar]
- 20.Knopp RH, D’Emden M, Smilde JG, Pocock SJ. Efficacy and safety of atorvastatin in the prevention of cardiovascular end points in subjects with type 2 diabetes: The Atorvastatin Study for Prevention of Coronary Heart Disease Endpoints in Non-Insulin-Dependent Diabetes Mellitus (ASPEN) Diabetes Care. 2006;29:1478–85. doi: 10.2337/dc05-2415. [DOI] [PubMed] [Google Scholar]
- 21.Berne C, Siewert-Delle A. The URANUS Study Investigators. Comparison of rosuvastatin and atorvastatin for lipid lowering in patients with type 2 diabetes mellitus: Results from the URANUS study. Cardiovasc Diabetol. 2005;4:7. doi: 10.1186/1475-2840-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wolffenbuttel BH, Franken AA, Vincent HH. Dutch CORALL Study Group. Cholesterol-lowering effects of rosuvastatin compared with atorvastatin in patients with type 2 diabetes: CORALL study. J Intern Med. 2005;257:531–9. doi: 10.1111/j.1365-2796.2005.01499.x. [DOI] [PubMed] [Google Scholar]
- 23.Adsule SM, Baig MS, Gade PR, Khandelwal PN. A comparative evaluation of safety and efficacy of rosuvastatin, simvastatin, and atorvastatin in patients of type 2 diabetes mellitus with dyslipidemia. Int J Diabetes Dev Ctries. 2009;29:74–9. doi: 10.4103/0973-3930.53124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.The Expert Panel. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III): Final report. Circulation. 2002;106:3143–421. [PubMed] [Google Scholar]
- 25.Buse JB, Ginsberg HN, Bakris GL, Clark NG, Costa F, Eckel R. Primary prevention of cardiovascular diseases in people with diabetes mellitus. Diabetes Care. 2007;30:162–72. doi: 10.2337/dc07-9917. [DOI] [PubMed] [Google Scholar]
- 26.Krentz AJ. Lipoprotein abnormalities and their consequences for patients with type 2 diabetes. Diabetes Obes Metab. 2003;5(Suppl 1):S19–27. doi: 10.1046/j.1462-8902.2003.0310.x. [DOI] [PubMed] [Google Scholar]
- 27.Goldberg IJ. Diabetic dyslipidemia: Causes and consequences. J Clin Endocrinol Metab. 2001;86:965–71. doi: 10.1210/jcem.86.3.7304. [DOI] [PubMed] [Google Scholar]
- 28.Marcovina S, Packard CJ. Measurement and meaning of apolipoprotein AI and apolipoprotein B plasma levels. J Intern Med. 2006;259:437–46. doi: 10.1111/j.1365-2796.2006.01648.x. [DOI] [PubMed] [Google Scholar]
- 29.Kathiresan S, Otvos JD, Sullivan LM, Keyes MJ, Schaefer EJ, Wilson PW. Increased small low-density lipoprotein particle number: A prominent feature of the metabolic syndrome in the Framingham Heart Study. Circulation. 2006;113:20–9. doi: 10.1161/CIRCULATIONAHA.105.567107. [DOI] [PubMed] [Google Scholar]


