Abstract
Aim:
To study the adverse drug reaction (ADR) pattern in a pediatric population in a tertiary care hospital.
Materials and Methods:
An observational study was done in the department of pediatrics in a tertiary care hospital. The ADRs occurring in the inpatient wards and outpatient department of pediatrics were actively monitored. The collected reports were analyzed for ADR pattern, drug groups, demographic profile, causality, severity, and preventability of the ADR.
Results:
A total of 30 ADRs were documented during the mid period of 2009 among pediatric patients. Most of the ADRs (60%) occurred below the age of 1 year. Antibiotics comprised the major group of drugs causing ADRs (67%). Rashes and urticaria were the most common type of ADR (37%) followed by fever, anaphylactic shock, vomiting, chills, and rigors. A single case of death had been reported in the study period. There were more occurrences of ADRs with multiple drugs compared to single drug therapy. About 80% of the ADRs were of probable causality and 87% were of probable preventability. There were no mild reactions, with 77% of reactions being moderate and 23% of reactions being severe in the severity scale.
Conclusions:
ADRs occur more among infants and antibiotics were more commonly implicated. Most of the reactions were of moderate severity. This indicates the need for a rigid ADR monitoring among pediatric patients to ensure safety of drug therapy.
Keywords: Adverse drug reaction, causality, pediatric, pharmacovigilance
INTRODUCTION
The safety of drugs used in patients of an adult age group cannot be extrapolated to a pediatric age group. The pharmacokinetics and pharmacodynamics of many commonly used drugs vary significantly between these two age groups of patients.[1] Further, adverse drug reactions (ADRs) in children can have a relatively more severe effect when compared to adults. Thus, the ADRs can lead to significant morbidity among children.[2] It has been observed that ADRs in children not only result in hospital admissions or prolonged hospitalization but also may lead to permanent disability or even death.[3] The information regarding the frequency, severity and types of drugs most frequently involved in adverse reactions in the pediatric age group is of particular interest, since pre-marketing clinical trials are done mostly in adults.[1] They constitute a reported incidence of 9.5%, including 2.1% of hospital admissions, with 39.3% of them being life-threatening.[4] The safety profile of a drug thus marketed with its testing done on adults can vary significantly when used in children.[5] This aspect of drug therapy is often difficult to predict for newer drugs. An active drug surveillance system is needed to capture risk information in children.[6] Pharmacovigilance which deals with the detection, assessment, understanding, and prevention of ADRs can help in providing continuous information on safety of drug used. Hence, we investigated the ADR profile in pediatric age group in a tertiary care hospital.
MATERIALS AND METHODS
The study was an observational study conducted by the pharmacovigilance centre during the mid period of 2009. The Institute Human Ethics Committee approval for waiver of consent was obtained prior to initiation of the study. Patients in the wards and outpatient department of pediatrics during the study period were monitored actively for occurrences of any ADRs till their discharge from the hospital. All patients of the pediatric age group less than 12 years of age and of either gender were included in the study. Monitoring for adverse effects was based on regular questioning of the caretaker and the health care workers for occurrences of ADRs and laboratory investigations if indicated clinically. Laboratory indications included complete hemogram, peripheral smear, electrolytes, and liver and renal function tests. In view of under-reporting of ADRs, the pharmacovigilance center organizes pharmacovigilance awareness programs for the health care professionals of the institute which includes physicians, nurses, medical students, and pharmacists. Pediatric inpatient, outpatient, and intensive care units were provided ADR drop boxes[7] with notification forms. The notification form is a simplified version of the Central drug standard control organization (CDSCO) ADR reporting form[8] adopted by the centre to facilitate easy reporting by the physician. The physicians had been instructed to fill the notification forms about the ADR and put them in the drop boxes, which were then collected by the pharmacovigilance center. The direct reporting of ADR to the pharmacovigilance center through telephonic conversation was also encouraged among health care professionals. In the outpatient setting, the ADRs were collected from the patient during their visits and reported to the pharmacovigilance centre through ADR drop boxes or through telephonic conversation. The inpatients include those who were admitted because of an ADR or those who encountered an ADR during the treatment period. The collected reports were documented and analyzed for causality, severity, preventability, and demographic profile. ADRs were classified on the basis of Anatomical and Therapeutic Classification System (ATC 1999).[9,10] Causality of ADRs was assessed by Naranjo's algorithmic scale[11] which is a questionnaire-based classification of the suspected ADRs as definite, probable, possible, or doubtful by a scoring method. Severity of the ADRs was assessed by Modified Hartwig and Siegel Scale[12] which gives an overview of the severity of ADR whether it is mild, moderate, or severe in nature. Preventability of the ADRs was assessed by Modified Schumock and Thornton Scale.[13,14] This scale of preventability classifies the ADRs as definitely preventable, probably preventable, and not preventable.
RESULTS
At the end of the study period, a total of 30 pediatric patients with ADRs were observed from July to September, 2009. It was found that among those patients who had adverse drug reactions 63% were females and 37% were males. The infants less than one year of age (60%) were most susceptible to ADRs among pediatric patients. The age groups 1-3 years (20%) and 4-6 (20%) years were relatively less susceptible to ADRs compared to infants and were found to be similar in their incidences of ADRs. There were no ADRs observed in children more than 6 years of age. The distribution of ADRs in various age groups is given in Table 1. The classification of ADRs by Anatomical and Therapeutic Classification System (1999)[9] showed antibiotics (67%) as the most common drug group involved in ADRs. The antibiotics associated with ADR include vancomycin, cloxacillin, amoxicillin, ampicillin, meropenem, ciprofloxacin, and cefixime in order of significance. The other drug groups includes drugs used for treatment of disorders of nervous system (17%) which includes anti-epileptics like phenytoin and general anesthetics like thiopentone sodium. The drugs pertaining to cardiovascular system (10%) include digoxin and diuretics like furosemide. The alimentary system drugs (3%) include antiulcer drugs like famotidine, omeperazole, and domperidone. The drugs for dermatological systems (3%) were found to be least involved in ADRs. There were no ADRs involving the respiratory system, musculoskeletal system, or endocrine system. Assessment by modified Hartwig and Siegel scale for severity of ADRs showed that there were no mild reactions. Most of the reactions had a severity score of being moderate (77%) and the remainder being severe [Figure 1]. Rashes and urticaria were the most common type of ADRs, followed by fever, anaphylactic shock, vomiting, chills, and rigors. The ADRs with a low incidence include aphthous ulcer, diarrhea, seizures, burning sensation, bradycardia, and acute dystonia. A single case of death had been reported in the study period [Figure 2]. The number of ADRs was more with polypharmacy (60%) compared to monotherapy (40%). Assessment by Modified Schumock and Thornton Scale of ADR preventability showed that most of the ADRs had a score of probable preventability among the pediatric patients. A relatively lower number of ADRs were not preventable. Very few ADRs were definitely preventable (3%) among pediatric age group [Figure 1]. There were very few ADRs with a definite causality by Naranjo's Algorithm Scoring system. Most of the reactions had a probable causality score followed by possible causality score [Figure 1].
Table 1.
Distribution of ADRs in different pediatric age groups

Figure 1.
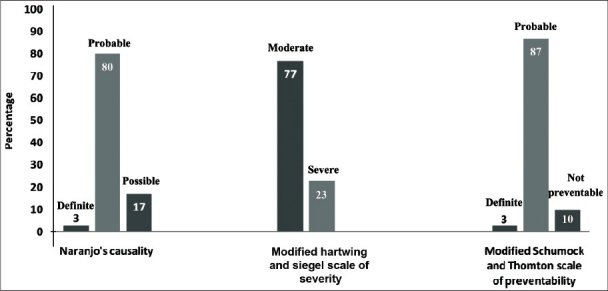
Causality, severity and preventability assessment of adverse drug reactions
Figure 2.
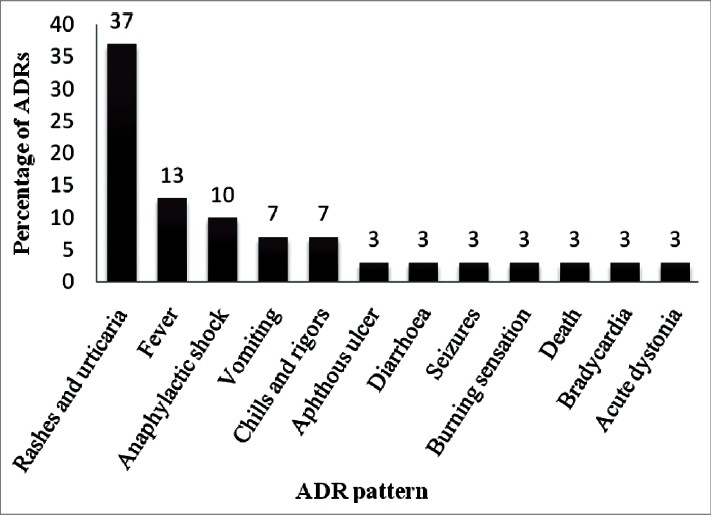
ADR pattern in a pediatric population
DISCUSSION
ADRs in a pediatric population are an important public health problem.[15] Despite efforts being made to reduce the incidence of medication related adverse events, the morbidity, and mortality especially in pediatric population due to drug-induced reactions continue to be unacceptably high.[16,17]
Studies have been done in different parts of the world on ADRs among pediatric patients. It has been found that ADRs were associated with 243 reported deaths among young children each year, in the age groups of newborn to 2 years of age.[18] Similarly, in our present study nearly 60% of the ADRs occurred in patients less than 1 year of age. A case of death had also been reported during the 2 month study period. This was a case in which a 4-year-old male child diagnosed to have status epilepticus was administered thiopentone sodium injection followed by which he had vomiting, skin rashes and death. The causality assessment was done for this case and it was found to be of “possible” category. Studies estimate that 2.5% of children who were treated with any drug, and 12% of children treated with an antibiotic, will experience a cutaneous ADR. However, they were rarely considered serious.[18,19] This is in concordance with our study where antibiotics were the major drug group associated with the ADRs (67%) and cutaneous ADRs were the most common manifestations of such reactions (37%). The antibiotics associated with ADR in the present study include vancomycin, cloxacillin, amoxicillin, ampicillin, meropenem, ciprofloxacin, and cefixime.
Studies on ADRs of nonsteroidal anti-inflammatory drugs (NSAIDs) and COX-2 inhibitors in a pediatric population have shown that NSAID exposures were a significant cause of morbidity in children. A cross-reactive hypersensitivity between NSAIDs and paracetamol has been proposed based on an autoimmune mechanism of drug reaction to NSAIDs.[20] But in the present study there were no reported ADRs caused by NSAIDs.
A study over a period of 13 years showed 166 adverse effects to influenza vaccine in children less than 2 years of age with the median age of 13 months.[21] But in our study there were no reactions to vaccines. In a study conducted in Nigeria in children, the two most frequently reported suspected ADRs were diarrhea (51%) and skin rashes (18%). In our study, skin rashes were the most common ADR. However, diarrhea constituted a small fraction of ADRs in children. In a metaanalysis conducted in Italy, it was found that the percentage of severe ADRs ranged between 2% and 30%. In these studies the ADRs with a cause assessed as definite/probable ranged between 56% and 91%.[22–24] Similarly, in our study 23% of reactions were severe and 80% of the ADRs were of “probable” causality. The severe reactions include dicyclomine and sodium valproate induced Steven Johnson syndrome, digoxin-induced bradycardia, vomiting, and anti-snake venom-induced anaphylaxis.
In a prospective study done in 347 Indian children, it was found that antibiotics especially sulphonamides were associated with the adverse reactions and that skin rashes were the most common reactions reported. A single case of death was also reported during the study period which shows a similar ADR pattern depicted in the present study.[25] In our study, we were not able to get information on total number of patients being treated during the study period due to logistic reasons. This we consider as a limitation of the study.
The methods for ADR detection, evaluation, and monitoring should be strengthened for a pediatric population. The role of pharmacovigilance in monitoring the safety of drugs in children should be evaluated in detection of newer and rarer ADRs. The awareness of spontaneous reporting of ADRs among health care professionals and general population should be given due considerations for preventing the morbidity and mortality among the pediatric population.
CONCLUSIONS
In this study ADRs occurred more among infants and antibiotics were more commonly implicated. Most of the reactions were of moderate severity. This indicates the need for a rigid ADR monitoring among pediatric patients to ensure safety of drug therapy. Various pharmacovigilance awareness programs should be conducted to increase the spontaneous reporting of ADRs.
ACKNOWLEDGEMENT
We gratefully acknowledge Indian Council of Medical Research, New Delhi for funding this project (Sanction number: 21/PO/JIPG-1/09-BMS).
Footnotes
Source of Support: Indian Council of Medical Research, BMS Division
Conflict of Interest: None declared.
REFERENCES
- 1.Chien JY, Ho RJ. Drug delivery trends in clinical trials and translational medicine: Evaluation of pharmacokinetic properties in special populations. J Pharm Sci. 2011;100:53–8. doi: 10.1002/jps.22253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aagaard L, Hansen EH. Adverse drug reactions reported for systemic antibacterials in Danish children over a decade. Br J Clin Pharmacol. 2010;70:765–8. doi: 10.1111/j.1365-2125.2010.03732.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Le J, Nguyen T, Law AV, Hodding J. Adverse drug reactions among children over a 10-year period. Pediatrics. 2006;118:555–62. doi: 10.1542/peds.2005-2429. [DOI] [PubMed] [Google Scholar]
- 4.Bavdekar SB, Karande S. National pharmacovigilance program. Indian Pediatr. 2006;43:27–32. [PubMed] [Google Scholar]
- 5.Clavenna A, Bonati M. Adverse drug reactions in childhood: A review of prospective studies and safety alerts. Arch Dis Child. 2009;94:724–8. doi: 10.1136/adc.2008.154377. [DOI] [PubMed] [Google Scholar]
- 6.Johann-Liang R, Wyeth J, Chen M, Cope JU. Pediatric drug surveillance and the Food and Drug Administration's adverse event reporting system: An overview of reports, 2003-2007. Pharmacoepidemiol Drug Saf. 2009;18:24–7. doi: 10.1002/pds.1679. [DOI] [PubMed] [Google Scholar]
- 7.Drop box. Uppsala Reports. [last cited on 2010 Dec 24]. Available from: http://www.who-umc.org/graphics/9628.pdf .
- 8.ADR form. [last cited on 2011 May 8]. Available from http://www.cdsco.nic.in/pharmacovigilance.htm .
- 9.Skrbo A, Zulic I, Hadzic S, Gaon ID. (Anatomic-therapeutic-chemical classification of drugs) Med Arh. 1999;53:57–60. [PubMed] [Google Scholar]
- 10.Skrbo A, Begovic B, Skrbo S. [Classification of drugs using the ATC system (Anatomic, Therapeutic, Chemical Classification) and the latest changes] Med Arh. 2004;58:138–41. [PubMed] [Google Scholar]
- 11.Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239–45. doi: 10.1038/clpt.1981.154. [DOI] [PubMed] [Google Scholar]
- 12.Hartwig SC, Siegel J, Schneider PJ. Preventability and severity assessment in reporting adverse drug reactions. Am J Hosp Pharm. 1992;49:2229–32. [PubMed] [Google Scholar]
- 13.Schumock GT, Seeger JD, Kong SX. Control charts to monitor rates of adverse drug reactions. (1091-2, 1095-6).Hosp Pharm. 1995;30:1088. [PubMed] [Google Scholar]
- 14.Schumock GT, Thornton JP. Focusing on the preventability of adverse drug reactions. Hosp Pharm. 1992;27:538. [PubMed] [Google Scholar]
- 15.Brunlof G, Tukukino C, Wallerstedt SM. Individual case safety reports in children in commonly used drug groups - signal detection. BMC Clin Pharmacol. 2008;8:1. doi: 10.1186/1472-6904-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berry MA, Shah PS, Brouillette RT, Hellmann J. Predictors of mortality and length of stay for neonates admitted to children's hospital neonatal intensive care units. J Perinatol. 2008;28:297–302. doi: 10.1038/sj.jp.7211904. [DOI] [PubMed] [Google Scholar]
- 17.Neubert A, Dormann H, Weiss J, Egger T, Criegee-Rieck M, Rascher W, et al. The impact of unlicensed and off-label drug use on adverse drug reactions in paediatric patients. Drug Saf. 2004;27:1059–67. doi: 10.2165/00002018-200427130-00006. [DOI] [PubMed] [Google Scholar]
- 18.Moore TJ, Weiss SR, Kaplan S, Blaisdell CJ. Reported adverse drug events in infants and children under 2 years of age. Pediatrics. 2002;110:e53. doi: 10.1542/peds.110.5.e53. [DOI] [PubMed] [Google Scholar]
- 19.Segal AR, Doherty KM, Leggott J, Zlotoff B. Cutaneous reactions to drugs in children. Pediatrics. 2007;120:e1082–96. doi: 10.1542/peds.2005-2321. [DOI] [PubMed] [Google Scholar]
- 20.Titchen T, Cranswick N, Beggs S. Adverse drug reactions to nonsteroidal anti-inflammatory drugs, COX-2 inhibitors and paracetamol in a paediatric hospital. Br J Clin Pharmacol. 2005;59:718–23. doi: 10.1111/j.1365-2125.2005.02444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McMahon AW, Iskander J, Haber P, Chang S, Woo EJ, Braun MM, et al. Adverse events after inactivated influenza vaccination among children less than 2 years of age: Analysis of reports from the vaccine adverse event reporting system, 1990-2003. Pediatrics. 2005;115:453–60. doi: 10.1542/peds.2004-1519. [DOI] [PubMed] [Google Scholar]
- 22.Buajordet I, Wesenberg F, Brors O, Langslet A. Adverse drug events in children during hospitalization and after discharge in a Norwegian university hospital. Acta Paediatr. 2002;91:88–94. doi: 10.1080/080352502753458021. [DOI] [PubMed] [Google Scholar]
- 23.dos Santos DB, Coelho HL. Adverse drug reactions in hospitalized children in Fortaleza, Brazil. Pharmacoepidemiol Drug Saf. 2006;15:635–40. doi: 10.1002/pds.1187. [DOI] [PubMed] [Google Scholar]
- 24.Oshikoya KA, Senbanjo IO, Njokanma OF. Parental reporting of suspected adverse drug reactions in children in Lagos, Nigeria. Arch Dis Child. 2009;94:469–73. doi: 10.1136/adc.2008.152629. [DOI] [PubMed] [Google Scholar]
- 25.Dharnidharka VR, Kandoth PN, Anand RK. Adverse drug reactions in pediatrics with a study of in-hospital intensive surveillance. Indian Pediatr. 1993;30:745–51. [PubMed] [Google Scholar]


