Sir,
Emergence of multidrug resistance tuberculosis is now a health problem faced by most of the developing countries as well as developed countries across the globe. MDR-TB is defined as resistance to isoniazid and rifampicin with or without resistance to other anti-TB drugs.[1] The prevalence of MDR-TB is 1-3% in new cases and around 12-17% in retreatment cases or failure cases.[2,3] The treatment of these cases includes a regimen for 24 month duration including initial 6-9 months of intensive phase. Drugs used for treatment of MDR-tuberculosis have a number of adverse effects, the effects on thyroid functions are rare but well known adverse effects of para-aminosalicylic acid (PAS) or ethionamide (Eto) or combination of both.[1] The effects of these drugs especially ethionamide and para-aminosalycylic acid on thyroid functions are well documented, but relatively uncommon and often overlooked adverse effects. This study was planned to evaluate the effects of antitubercular drugs used in treatment of MDR-TB on thyroid functions.
This prospective, observational, longitudinal study was carried out in Departments of Pharmacology and Respiratory Medicine, J.L.N. Medical College, Ajmer. The study was conducted in between 2005 and February 2011.
The study was conducted in compliance with good clinical practice guidelines, declaration of Helsinki and standard operating procedures of Institutional Ethics Committee (IEC). The study protocol and informed consent forms were approved by IEC. A written informed consent was obtained from all patients included in the study. Fifty-four patients (38 females and 16 males) were included in the study and the study participants received isoniazid, pyrazinamide, and second-line antitubercular drugs, such as Eto, PAS, cycloserine, kanamycin, ofloxacin, and clarithromycin. The effect of first- and second-line antituberculosis agents on thyroid function was monitored. All the patients included in the study were confirmed cases of MDR-TB diagnosed by mycobacterial culture sensitivity. The study was conducted before the institution of DOTS PLUS in the state of Rajasthan and hence all the samples for culture sensitivity were sent to the New Delhi Tuberculosis Center New Delhi, New Delhi. Diagnosis was made using a conventional L. J. medium for culture method and the method recommended was proportion method. A standard technique (proportion method on a conventional L. J. medium) was used for performing drug sensitivity in every case. The species identification was done in every case based on colony characteristics and time of appearance of growth in culture media followed by biochemical tests such as the niacin test and para-nitro benzoic acid (PNB) inhibition test.
All these patients were taking treatment on their own. When this observational study was started, the Category IV regimen under Revised National Tuberculosis Control Programme (RNTCP) was not started in the state of Rajasthan. In this study, WHO recommended standard treatment regimen, which includes initial 6 months of intensive phase with kanamycin, ofloxacin, Eto, cycloserine, pyrazinamide, and ethambutol followed by 18 months continuation phase with ofloxacin, Eto, cycloserine, and ethambutol was used. PAS was used in 14 cases (25.92%) when the patient could not tolerate kanamycin or cycloserine.
Even definition of MDR-TB is resistance to isoniazid and rifampicin, but isoniazid was added to some patients as the formulation available for PAS was in the form of combination of PAS and isoniazid. Clarithromycin was given to only two patients (3.70%) who could not tolerate cycloserine (psychosis), Eto (severe gastritis), and kanamycin (ototoxicity) simultaneously. The mean age of patients included in the study was 38.57 ± 10.9 years. All those patients who were compliant with the treatment with a regular follow-up were included in the study and all the patients were taking treatment on their own, all these were buying medicines from the same manufacturer available at the government shop at subsidized rates. Patients having history of any thyroid disease/DM/HTN/any other co-morbid disease, pregnant woman, patient having h/o radiotherapy, and having +ve family history of hypothyroidism were excluded from the study. Baseline thyroid function tests (T3, T4, and TSH) were carried out before starting drug therapy and repeated at the end of 3 months, 6 months, 9 months, 1 year, 18 months, and at the end of treatment duration. Patients were specifically examined for any clinical evidence of enlarged thyroid gland; any symptoms of thyroid dysfunction, i.e. lethargy, fatigue, weight gain, constipation, cold intolerance, etc. Wherever in doubt, an ultrasound of thyroid gland was also done to rule out any increase in the size of thyroid gland. Five milliliter of venous blood sample was collected from a anticubital vein using antiseptic measures, after clotting of blood samples were centrifuged and the supernatant serum was collected for estimation of thyroid hormone levels by a radioimmunoassay (RIA) method.
Out of 54 patients (male 16; female 38), 6 patients (11%) developed hypothyroidism as evident by a rise in TSH levels and a decrease in T3 and T4 levels. Four patients (7.4%) developed goiter, out of which two patients (3.70%) were euthyroid and two patients were hypothyroid. The average duration of development of goiter after starting of antitubercular drugs was 8 months (range, 6–13 months). In all cases, hypothyroidism responded to thyroxine. We were able to reduce the dose of thyroxine while all patients were continued treatment with both PAS and ETO.
The most common symptoms related to hypothyroidism were fatigue and constipation present in all six patients, although they were responding well to the therapy with antitubercular drugs. Out of six patients who developed clinical hypothyroidism, 4 (7.40%) were on Eto and PAS simultaneously, while 2 (3.70%) were only on the PAS containing regimen, Eto being withheld due to severe gastritis in these patients. Other common adverse drug reactions developed in this study were: Gastritis and abdominal symptoms in 12 patients (22.22%), arthralgia and hyperuricemia in three patients (5.55%), depression and psychotic symptoms in four patients (7.40%), ototoxicity in four patients (7.40%), gynaecomastia in one patient (1.85%). All these patients were managed with symptomatic treatment. In only two (3.70%) patients, we had to withdraw kanamycin, cycloserine and Eto as these patients developed adverse drug reactions simultaneously [Figure 1].
Figure 1.
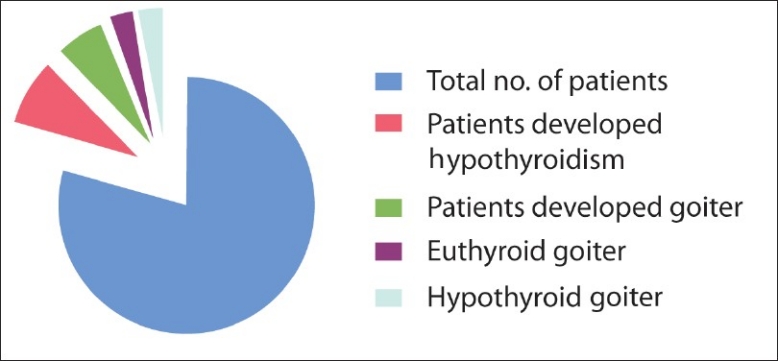
Number of patients developed thyroid function test abnormalities
Hypothyroism is a syndrome resulting from deficiency of thyroid hormones and is manifested largely by a reversible slowing down of all body functions. Hypothyroidism can occur with or without goiter formation. Drug-induced hypothyroidism is an under active thyroid gland due to a reaction from medication. “Drug-induced” means caused or brought by medication. Iodine deficiency remains the most common cause of hypothyroidism worldwide. In areas of iodine sufficiency, autoimmune disease (Hashimoto's thyroiditis) and iatrogenic causes (treatment of hyperthyroidism) are most common. Iatrogenic hypothyroidism can often be detected by screening before symptoms develop.[4–6] Drugs causing primary hypothyroidism are: Iodine excess (including iodine-containing contrast media and amiodarone), lithium, antithyroid drugs, PAS, interferon and other cytokines, aminoglutethimide,[6] phenylbutazone, Eto, bexarotene.[1,4] Subclinical hypothyroidism, a milder form of thyroid dysfunction, has been reported to occur in up to 23% of patients on lithium therapy, compared to rates of approximately 10% in the background population.[7] The incidence of hypothyroidism is about 13% in iodine replete countries and 6% in area of lower iodine intake in amiodarone-treated patients.[6] Drug-induced hypothyroidism can be satisfactorily managed with levothyroxine therapy if the offending agent cannot be stopped. In the case of amiodarone-induced hypothyroidism, levothyroxine therapy may be necessary even after discontinution of therapy because of amiodarone's very long half-life.[4] Among above-mentioned drugs causing hypothyroidism, PAS and Eto are used for treatment of MDR-TB. In our prospective, longitudinal, observational study, treatment with PAS and Eto was associated with a high rate of hypothyroidism with goiter or without goiter (all cases occurring after a median of 8-13 months from beginning of treatment), leading to initiation of thyroxine. All cases responded to treatment with thyroxine. All the patients improved clinically and biochemical parameters returned to normal after administration of thyroxine. Our study has been unique as we cannot find the incidence of deranged thyroid functions resultant from drugs used for treatment of MDR-TB in the literature. PAS and Eto are considered as incriminated in drug-induced hypothyroidism. Incidence of PAS/Eto-induced hypothyroidism in our study is 11.11% (6/54). The 7.4% (4/54 developed hypothyroidism without goiter and 3.7% (2/54) patients developed hypothyroidism with goiter formation and 3.7% (2/54) patients developed euthyroid goiter. Out of 6 patients who developed clinical hypothyroidism, four (7.40%) were on Eto and PAS simultaneously, while two (3.70%) were only on PAS containing regimen, Eto being withheld due to severe gastritis in these patients. No patient in our study developed hyperthyroidism/thyrotoxicosis. Post-treatment follow-up of all patients could not be done to evaluate further course of hypothyroidism, although we were able to maintain normal thyroid functions in all patients with thytoxine while treatment of MDR-TB was continued. Although any conclusion based on available evidence is preliminary, the frequency of drug-induced hypothyroidism in patients exposed to MDR-TB, and treated with PAS, Eto or combination of both is occupying. Drug-induced hypothyroidism can occur several months after the beginning of treatment (in our study average duration of development is 8 months ranging from 6 to 13 months). Furthermore, drug-induced hypothyroidism may or may not be associated with goiter and can be virtually asymptomatic. Systematic monitoring of patients and their biochemical tests monitoring are mandatory.
REFERENCES
- 1.New Delhi: Directorate General of Health Services, Ministry of Health & Family Welfare; 2010. [Last accessed on 2011 Mar 14]. DOTS-Plus Guidelines. Available from: http://www.tbcindia.org/pdfs/ DOTS_Plus_Guidelines_Jan2 010.pdf . [Google Scholar]
- 2.Paramasivan CS, Venkataraman P. Drug Resistance in tuberculosis in India. Indian J Med Res. 2004;120:377–86. [PubMed] [Google Scholar]
- 3.Mahadev B, Kumar P, Agarwal SP, Chauhan LS, Srikantaramu N. Surveillance of drug resistance to antituberculosis drugs in district of hoogli in West Bengal and Mayurbhanj in Orissa. Indian J Tuberc. 2005;52:5–10. [Google Scholar]
- 4.Chambers HF, Deck DH. Basic and Clinical Pharmacology. In: Katzung BG, Masters SB, Trever AJ, editors. 11th ed. Noida, UP, India: Tata acgraw- Hill; 2009. [Google Scholar]
- 5.Rang HP, Dale MM, Ritter JM, Flower RJ, editors. 6th Ed. USA: Churchill Livingstone Elsevier, Elsievier limited; 2007. Rang and Dale's Pharmacology. [Google Scholar]
- 6.Jameson JL, Weetman AP. Disorder of the Thyroid gland. Harrison's Principels of Internal Medicine. In: Fauci AS, Braunwald E, Kasper DL, Hauser SL, Longo DL, Jameson JL, Joseph L, editors. 17th ed. USA: The McGraw-Hill Companies, Inc; 2008. [Google Scholar]
- 7.Kleiner J, Altshuler L, Hendrick V, Hershman J. Lithium-Induced Subclinical Hypothyroidism: Review of the Literature and Guidelines for Treatment. J Clin Psychiatry. 1999;60:249–255. [PubMed] [Google Scholar]


