Sir,
The liver is involved in the maintenance of homeostasis within the body. Other functions of the liver include protein synthesis, storage and metabolism of fats and carbohydrates, detoxification and excretion of drug and other toxins.[1] Diabetes developed as a complication of cirrhosis is known as hepatogenous diabetes. Around 30–60% of cirrhotic patients suffer from this metabolic disorder.[2] There is a high prevalence of metabolic syndrome, obesity, and type 2 diabetes mellitus with cryptogenic cirrhosis.[3] Several reports have claimed a specific association between hepatitis C virus (HCV) infection and type 2 diabetes, but in most instances patients were a mixture of cases with cirrhosis, hepatitis and diabetes.[4] Silymarin is a hepatoprotective drug obtained from Silibum marium. Silymarin is reported to have different properties like hepatoprotective activity, anti-inflammatory activity, antioxidant activity, and anti-cancer activity.[5] Previous reports of both pre-clinical and clinical studies revealed that silymarin has got some anti-diabetic potential.[6–8] Considering all the above reports and keeping the evidences in mind, this study was undertaken with the objective to assess the effect of silymarin in diabetes mellitus patients with hepatic diseases.
The study was conducted on cirrhotic patients with diabetes mellitus admitted to Medical Trust Hospital, Cochin, Kerala, during the period from July 2009 to December 2009. Patients of both gender, aged between 20 and 70 years, were included in this study. Pregnant females and patients with chronic pancreatitis were excluded from the study. Hepatitis B virus and HCV infected patients were also excluded from the study. The study was conducted after obtaining approval from Institutional Ethical Committee. We selected 10 patients with silymarin + insulin therapy and another 10 patients with insulin + (L-ornithine + L-aspartate). The effectiveness was determined by monitoring the random sugar levels, total bilirubin, serum glutamic oxaloacetic transaminase (SGOT), serum glutamic pyruvic transaminase (SGPT), alkaline phosphatase (ALP), serum albumin before treatment, and after 3 and 5 months of treatment.
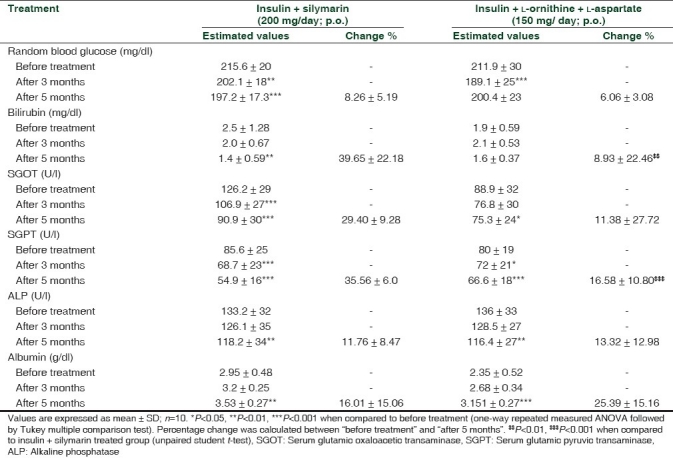
Both insulin + silymarin treated group and insulin + (L-ornithine + L-aspartate) treated group showed reduction in random blood sugar level after 3 and 5 months of treatment when compared to before treatment, but no significant reduction was observed between the groups at different times of treatment. The percentage reduction in random blood sugar levels after 5 months of treatment with silymarin was found to be 8.26 ± 5.19% and in those taking L-ornithine + L-aspartate, it was 6.06 ± 3.08%. Our study also revealed that the decrease in the bilirubin, SGOT, SGPT and ALP levels after 5 months of treatment with silymarin were 39.65 ± 22.18%, 29.40 ± 9.28%, 35.56 ± 6.0%, 11.76 ± 8.47%, respectively, whereas with L-ornithine + L-aspartate treatment, the decrease in levels were 8.93 ± 22.46%, 11.38 ± 27.72%, 16.58 ± 10.80%, 13.32 ± 12.98, respectively. Silymarin produced significant reduction in bilirubin (P<0.01) and SGPT (P<0.001) levels after 5 months of treatment when compared to L-ornithine + L-aspartate. The percentage increase in the albumin levels with silymarin treatment was 16.01 ± 15.06% and with L-ornithine + L-aspartate was 25.39 ± 15.16% [Table 1]. The reduction in random blood glucose, SGOT and SGPT level produced by silymarin was consistent with the results of previous authors.[8,9] The hypoglycemic potential of silymarin may be due its antioxidant activity by reducing insulin resistance. Our study revealed that silymarin has good effect in the restoration of liver function and also established efficacy in controlling blood glucose level in diabetes patients with liver diseases. Silymarin may make a breakthrough as a new approach to protect other organs in addition to liver.
Table 1.
Effect of silymarin and L-ornithine + L-aspartate on serum random blood glucose, bilirubin, SGOT, SGPT, ALP, albumin levels in patients with diabetes and hepatic disease
REFERENCES
- 1.Ward FM, Daly MJ. Liver disease. In: Roger W, Clive E, editors. Clinical pharmacy and therapeutics. 3rd ed. Edinburgh: Churchill Livingstone; 2003. pp. 209–25. [Google Scholar]
- 2.Garcia-Compean D, Jaquez-Quintana JO, Gonzalez JA, Maldonado-Garza H. Liver cirrhosis and diabetes: Risk factors, pathophysiology, clinical implications and management. World J Gastroentrol. 2009;15:280–8. doi: 10.3748/wjg.15.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu X, Ling Q, He ZL, Gao F, Zheng SS. Post-transplant diabetes mellitus in liver transplantation: Hangzhou experience. Hepatobiliary Pancreat Dis Int. 2008;7:465–70. [PubMed] [Google Scholar]
- 4.Hussnain RR, Koukab G, Qayyum M, Asim M, Khanum A. Association of diabetes with hepatitis C virus infected male and female patients along with different risk factors. Int J Agric Biol. 2007;9:736–40. [Google Scholar]
- 5.Dixit N, Baboota S, Kohli K, Ahmad S, Ali J. Silymarin: A review of Pharmalogical aspects and bioavilability enhancement approches. Indian J Pharamacol. 2007;39:172–9. [Google Scholar]
- 6.Maghrani M, Zeggwagh NA, Lemhadri A, Amraoui M, Michel J, Eddouks M. Study of the hypoglycaemic activity of silymarin in an animal model of type 1 diabetes mellitus. J Ethnopharmacol. 2004;91:309–16. doi: 10.1016/j.jep.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 7.Velussi M, Cernisoi AM, DeMonte A, Dapas F, Caffau C, Zilli M. Long term treatment with an anti-oxidant drug silymarin is effective on hyperinsulinemia exogenous insulin need and malondialdehyde levels in cirrhotic diabetic patients. Hepatology. 2005;26:871–9. doi: 10.1016/s0168-8278(97)80255-3. [DOI] [PubMed] [Google Scholar]
- 8.Huseini HF, Larijani B, Heshmat R, Radjabipour B, Toliat T, Raza M. The efficacy of Silybum marianum Gaertn.in the treatment of type 2 diabetes: A randomized double blind placebo controlled clinical trial. Phytother Res. 2006;20:1036–9. doi: 10.1002/ptr.1988. [DOI] [PubMed] [Google Scholar]
- 9.Pradhan SC, Girish C. Hepatoprotective herbal drug silymarin from experimental pharmacology to clinical medicine. Indian J Med Res. 2006;124:491–504. [PubMed] [Google Scholar]


