Abstract
Objective:
The aim of this study was to comparatively investigate the neutralizing effect of antioxidant treatment and delayed bonding after bleaching with hydrogen peroxide on the shear bond strength of a composite resin (CR) and resin-modified glass ionomer (RmGI) to enamel.
Materials and Methods:
Ninety-six freshly extracted human 3rd molars with flat enamel surfaces were divided into six experimental groups (n=12/group) and two control groups (n=12/group). After initial preparation, specimens in Groups 1 and 5 (control groups) were not bleached and the buccal enamel surface of specimens were bonded immediately with CR and RmGI. The samples of the remaining groups were all bleached six hours a day for seven days consecutively. Immediately after bleaching, groups two and six specimens were bonded with CR and RmGI. Groups 3 and 7 specimens were immersed in distilled water at 37°C for 7 days and the specimens in Groups 4 and 8 were treated with 10% sodium ascorbate as an antioxidant agent after bleaching. Specimens in Groups 3 and 4 were bonded with CR and Groups 7 and 8 specimens were bonded with RmGI immediately. After specimens were bonded, the shear bond strength (SBS) was measured. The SBS data analyses were subjected to one-way analysis of variance (ANOVA) followed by Tukey test for comparison of specific mean values.
Results:
The mean SBS value in Group 2 (immediately bonded with CR after bleaching) was significantly lower than other CR groups (P=0.045). RmGI did not bond to buccal enamel surface of specimens in group 6. There was no significant difference between other groups bonded with RmGI (P>0.05).
Conclusions:
Applying 10% sodium ascorbate hydrogel and one week delay before bonding resulted in reversal of reduced bond strength of CR and RmGI to bleached enamel.
Keywords: Bleaching, delayed bonding, shear bond strength, sodium ascorbate
INTRODUCTION
Vital tooth bleaching is a safe and well-accepted therapeutics for surface and intrinsic staining of teeth.[1]
Two methods of tooth bleaching are available: at home bleaching with bleaching agents that contain low concentrations of carbamide peroxide or hydrogen peroxide and in-office bleaching with whitener agents that consist of high concentrations of both peroxides.[2,3]
As vital tooth bleaching has become increasingly popular, clinicians should be aware of the consequences of the bleaching treatment and their interactions with additional esthetic interventions such as application of laminate veneers and replacement of old restorations and adhesive restorations.[4]
Bond strength of composite resin (CR) on bleached tooth surface has been reported in many studies.[5–8] Shear bond strength (SBS) of CRs that were bonded to tooth surfaces immediately after bleaching was significantly lower than those of non-bleached tooth surfaces due to presence of residual peroxide, which interfered with the resin attachment and inhibited the resin monomers polymerization.[9–12]
If the bleached surfaces were restored after a period of time, CRs are able to achieve their inherent and intended bond strength.[13–16]
The waiting period after bleaching for bonding procedure has been reported to vary from one day to four weeks.[13,14,17,18]
Some techniques have been suggested to reverse the post-bleach reduced bond strength of CR. Removal of superficial layer of enamel was proposed by Cvitko et al.[19]
Application of adhesive containing organic solvents was suggested by Khalili et al,[20] and Sung et al.[9] Barghi and Godwin used alcohol before restoration to treat bleached enamel.[21]
Some studies have demonstrated the effect of applying 10% sodium ascorbate solution as an antioxidant before resin bonding in reversing reduced bonding to the bleached enamel.
A recent study conducted by Lai et al, has shown that applying sodium ascorbate reversed the effect of sodium-hypochlorite-induced or hydrogen peroxide in reducing the bond strength of resin to enamel.[22]
Türkün and Kaya demonstrated that even a 10 min application of sodium ascorbate was enough for reversing the reduced bond strength.[23]
Most recent studies on bleached teeth and adhesive restorations focused on restorative composite materials.[12,19,21,22,24–26]
The aim of this study was to investigate the neutralizing effect of 10% sodium ascorbate hydrogel and delayed bonding after bleaching with 9.5% hydrogen peroxide on the SBS of CR and resin-modified glass ionomer (RmGI) to enamel.
MATERIALS AND METHODS
Ninety six recently extracted, intact, non-carious human third molars (maxillary and mandibular) were collected and stored in a solution of 0.1% thymol. The criteria for tooth selection were no pretreatment of chemical agents, intact buccal enamel, no cracks from forceps, no restorations and no caries. All tooth extractions were performed at the Department of Oral and Maxillofacial Surgery, having patients signed the appropriate informed consent form approved by the university ethics committee.
Before the experiment, the teeth were debrided from any residual tissue tags, washed under running tap water and pumiced. Thereafter, the teeth were vertically embedded in standardized, 18×20×32 mm3 polyethylene moulds containing self-curing resin with the crowns exposed; subsequently, they were preserved in distilled water at 5°C till required. Applied as restorative materials, CR was used in first four groups and RmGI was used in the other four groups.
As summarized in Table 1, the specimens were randomly divided into two control groups (n=12 each) and six experimental groups (n=12 each).
Table 1.
Treatment regimens before bonding
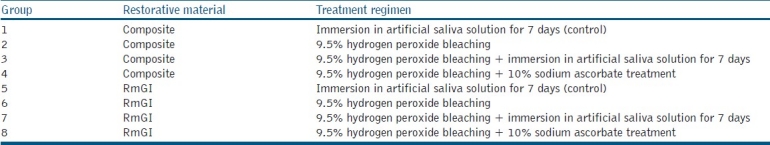
Groups 2 and 6 consisted of specimens bonded immediately after bleaching. In Groups 4 and 8, following bleaching, sodium ascorbate was applied to the enamel surface of the embedded teeth as an irrigation solution and then specimens bonded with CR and RmGI. Groups 3 and 7 specimens were immersed in artificial saliva at 37°C for 1 week after bleaching and then bonded with restorative materials. Specimens in the control groups (one and five) were not bleached, but immersed in artificial saliva for 1 week before bonding. As summarized in Table 1, all specimens were prepared according to the procedures described below.
Bleaching procedure
Enamel the surfaces of in all bleaching groups, bleached with 9.5% hydrogen peroxide bleaching gel (Rembrandt Xtra-Comfort; Den-Mat, Santa Maria, Calif., USA) at 100% relative humidity for 6 hours in a day according to manufacturer's instructions.
The enamel surface of the specimens coated with the bleaching gel did not contact with the artificial saliva by partially immersing specimens in the glass beaker containing saliva at 37°C. After daily bleaching procedure, specimens were thoroughly rinsed with water and air-dried with compressed air for 30 s. For the rest of the day, they were immersed and preserved in 250 mL of artificial saliva. This procedure was continued for seven days. Effective bleaching was obvious by color changes of the specimens.
Application of antioxidant
The specimens in Groups 4 and 8 were treated as follows: 10 ml of 10% sodium ascorbate hydrogel (pH 7.4) was applied under continuous agitation on the enamel surfaces of the embedded teeth following bleaching process. After 10 min, the enamel surfaces were rinsed with distilled water for 40 s and dried. Ten milliliter solution was dropped onto the enamel surface with a syringe with a flow rate of 1 mL min-1 in order to keep the surface wet.
Immersion in artificial saliva
The specimens in Groups 3 and 7 were immersed in 250 ml of artificial saliva solution at 37°C for one week immediately after bleaching process whereas those in control groups were only immersed in the artificial saliva for 7 days without prior bleaching.
The artificial saliva which has an electrolyte composition similar to that of human saliva was formed from 1 g sodium carboxymethylcellulose, 5 mg calcium chloride, 0.1 g potassium chloride, 1 mg potassium thiocyanate, 40 mg potassium phosphate, and 100 g distilled deionized water. The artificial saliva was changed twice daily during the 1-week time period. After removal of the specimens from the artificial saliva, the enamel surfaces were rinsed with an air-water syringe for 30 s.
Bonding procedure
The bonding area was delimited by placing a piece of adhesive tape with a 2-mm diameter opening which was carefully adapted to the centre of the labial enamel. The surface of delimited area was etched with one drop of 37% phosphoric acid (3M Dental Products, St. Paul, Minnesota, USA) for 15 s and gently air-dried. Then, a layer of bonding resin (3M, ESPE, St. Paul, Minnesota, USA) was applied with a brush according to the manufacturer's instructions and then cured for 20 s using a light-curing unit (Coltolux 50, Colten, Whaldent, USA) with intensity of 480 mW/cm-2. A translucent cylindrical plastic mould with a circular hole, 2 mm in diameter and 4 mm in high, was positioned over the hole of the adhesive tape and was affixed with a special bonding alignment apparatus into place. A CR Z100 (Z100, 3M, ESPE, St. Paul, MN, USA) was incrementally applied and light cured in the mould, making cylindrical posts perpendicular to the dentin surface. Each specimen was totally cured for 80 s circumferentially. After removal from the bonding alignment apparatus and the plastic mould, the specimens were stored in distilled water at 37°C for 24 h.
The RmGI bonding procedure was done according to manufacturer's instructions. Before bonding, the surface of delimited area was dried with oil-free air and primer was applied with a brush for 30 s, gently air-dried for 10 s and then cured for 10 s using a light-curing unit (Coltolux 50, Colten, Whaldent, USA), with intensity of 600 mW/cm-2; the light cured surface appeared shiny.
The RmGI was mixed by agglutination of powder to liquid (Vitremer, 3M, ESPE, St. Paul, MN, USA) for 20 s until a uniform color was achieved. Then the mixture of powder and liquid was placed in a plastic mould (2 mm in diameter and 4 mm in high) incrementally in a depth of 2 mm and was exposed to a light source.
Each specimen was totally cured for 80 s circumferentially. Afterwards, all the specimens were stored in distilled water at 37°C for 24 h.
In order to measure SBS, specimens were mounted in a jig of the universal testing machine (Dartec, HC10, U.K) and a knife-edge shearing rod was applied to the CR/tooth and RmGI/tooth interface until fracture occurred. The crosshead speed was set at 1 mm per minute, and the plunger direction was gingivo-occlusal. The data of applied load to specimens were expressed in MPa.
Statistical analysis was carried out with the SPSS 15.0 software system (SPSS Inc., Chicago, USA). In Group 6, cylinders were separated from surfaces after immersing in water. Therefore, all Group 6 related data recorded zero. The SBS data of groups were subjected to one-way analysis of variance (ANOVA) followed by Tukey test for comparison of specific mean values at 95% confidence level.
RESULTS
SBSs in MPa (mean±SD) for the groups are shown in Tables 2 and 3. The CR samples that were immediately bonded after bleaching (Group 2) demonstrated significantly lower SBS but there was no significant difference between Groups 1, 3 and 4. No bonding was observed in Group 6, samples that were immediately bonded after bleaching with RmGI, and there was no significant difference between other groups bonded with RmGI. (P<0.05)
Table 2.
Shear bond strength of composite resin groups (MPa)
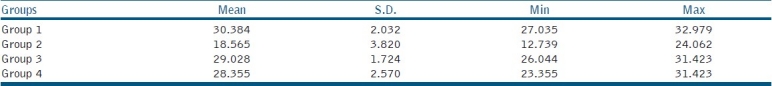
Table 3.
Shear bond strength of resin-modified glass ionomer groups (MPa)

DISCUSSION
The initial decrease in enamel bond strength after bleaching is clinically important because patients often need additional aesthetic restorations following bleaching procedures.[4]
Bleaching agents have been investigated at varying concentrations in order to evaluate the effects of bleaching on the SBS of adhesive restorations. Carbamide peroxide was applied more than other bleaching agents at different concentrations (10–16%) in previous studies.[23,25,27,28]
Results of this study have shown that SBS of CR to bleached enamel was significantly reduced while no bonding occurred between RmGI and bleached enamel immediately after bleaching. Present findings in the context of bleaching effects on SBS of resin-containing materials support these results.[10,29–32]
Several studies have focused on the physical alterations that occurred after bleaching treatments in search of a possible explanation for the decreased SBS after bleaching with carbamide peroxide. Some authors have suggested poor bonding surfaces because of modifications in enamel structure, a result of increased porosity as manifested by loss of prismatic form and an overetched appearance. Loss of calcium, decrease in microhardness, and alterations in the organic substance are other important factors that could contribute to the decreased bond strength.[33–35]
Many investigations showed that decreased bond strength might be due to residual oxygen from the bleaching material being developed at the enamel surface, which inhibits resin polymerization or interferes with the resin infiltration into the etched enamel.[23,30,31,36]
Titley et al, reported that in the SEM evaluation of bleached specimens, large areas of the enamel surface were resin free and tags were poorly defined, fragmented and penetrated to a lesser depth when compared with the unbleached control groups.[12]
In another study, Titley et al, displayed a porous and granular view with a bubbly appearance by SEM examination of resin and bleached enamel interfaces.[37]
Retained peroxide in the subsurface layer of the enamel developed gaseous bubbling from oxidizing reactions, which resulted in porous appearance of the interfaces.[38]
Dishman et al, have also shown that the composite bond quality is compromised through a decreased number of resin tags present.[30]
In the present study, 9.5% hydrogen peroxide gel was applied as a bleaching agent according to manufacturer's instructions to investigate the effect of antioxidant treatment and delayed bonding after bleaching on the SBS of CRs and RmGI to bleached enamel.
Bleaching with 9.5% hydrogen peroxide bleaching gel might affect the enamel surface structure less than higher concentrations of bleaching gel due to lower release of residual oxygen, which interferes with resin infiltration into the etched enamel. In fact, more studies are needed to recommend application of 9.5% hydrogen peroxide as a bleaching gel in clinical practice.
Although in different studies, there are remarkable variations among the recommended post-bleaching time periods; most researchers advised one week delaying in bonding procedure after bleaching, which is supported by the present study.[10,39,40]
The results of the current study support the previous investigations showing that immersion of specimens in artificial saliva or distilled water for one week resulted in a complete reversal of the enamel bond reduction.[13,35,37,39]
This may be due to removal of residual oxygen from the bleaching material by the immersion process; it has been expected that oral cavity saliva may have similar action after bleaching.
Although many methods were used to decrease the compromised bond strength, emphasis was placed on neutralizing the oxygen by application of ascorbic acid as an antioxidant. Ascorbic acid and its salts are famous for capability of reducing many oxidative compounds, especially free radicals.[41,42]
Protective effects of ascorbic acid in vivo against hydrogen-peroxide-induced damage in biological systems have been demonstrated by different studies.[43,44]
In the current study, the reduced SBS of CR and RmGI to enamel appeared to be restored by treatment of the bleached enamel with 10% sodium ascorbate hydrogel before bonding.
Ascorbic acid has also been shown to increase the bond strength of a chemically cured resin to tooth,[45] which might be attributed to the etching potential of ascorbic acid.
In more recent studies, 10 min of antioxidant treatment was tested, which is the same as the current study, and seemed to be reasonable time period in clinical conditions.[23,46] Lai et al, immersed the bleached specimens in 10% sodium ascorbate solution for 3 h.[47]
They hypothesized that by using an antioxidant the incorporation process of peroxide ions may also be reversed. Furthermore, they suggested that sodium ascorbate allows free-radical polymerization of the adhesive resin and avoids premature termination by restoring the altered redox potential of the oxidized bonding substrate, and therefore neutrilizes the compromised bonding.[22]
Zhao et al, have shown that under certain conditions, peroxide ions produced the peroxide-apatite by substituting hydroxyl radicals in the apatite lattice.[48]
Nagpal et al, demonstrated that 10% sodium ascorbate on hypochlorite treated dentin, reduced micro leakage and increased resin intra-tubular penetration.[49]
Kimyai et al, have shown that hydrogel and solution of sodium ascorbate reversed the compromised bond strength in bleached enamel.[36]
In Lai and Kimyai studies, specimens’ bleaching time was 8 h.[36,47] In the present study, bleaching agent was applied for 6 h. In this study, there were no significant differences between unbleached and delayed groups. This might be explained by the possible structural or morphological changes on enamel that were repaired during waiting period between bonding and bleaching while teeth were preserved in artificial saliva.[13,37,39]
Attin et al, showed that bleaching with 10% carbamide peroxide did not affect the RmGI bonding which is not supported by the current study in which bleaching time was longer.[6]
In the present study, bleaching with 9.5% hydrogen peroxide reduced SBS of RmGI and CR. Applying 10% sodium ascorbate hydrogel as an antioxidant and delaying bonding procedure for one week reversed the compromised bond strength.
The RmGI bonds to enamel by two mechanisms: 1) ionic bond of carboxylic groups of polyalkenoic acid to hydroxy apatite and 2) micro-mechanical bonding of polymer.[8,12,24,29] Since both CR and RmGI are resin containing materials, application of an antioxidant and all the other methods employed to adverse compromised CR bond strength apply for RmGI, too.[9,10,19–22,41]
In Group 6, no bonding occurred between RmGI and bleached teeth; there seemed to be ionic bonding of RmGI inhibited by bleaching procedure.
In Groups 7 and 8, bonding has occurred to bleached teeth which might be due to delayed bleaching procedure and the preparation of tooth surface by 10% sodium ascorbate hydrogel.
The following conclusions can be drawn from the results of the present study:
A 1-week delayed bonding procedure after bleaching reduced compromised SBS of CR and RmGI.
Applying 10% sodium ascorbate hydrogel in the treatment of bleached enamel surfaces reversed the compromised SBS and may be an alternative to delayed bonding, especially when restoration should be completed immediately after bleaching.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Matis BA, Cochran MA, Eckert G, Carlson TJ. The efficiacy and safety of a 10% carbamide peroxide bleaching gel. Quintessence International. 1998;29:555–63. [PubMed] [Google Scholar]
- 2.Haywood VB, Robinson FG. Vital tooth bleaching with Nightguard vital bleaching. Current Opinions in Cosmetic Dentistry. 1997;4:45–52. [PubMed] [Google Scholar]
- 3.Li Y. Biological properties of peroxide-containing tooth whiteners. Food and Chemical Toxicology. 1996;34:887–904. doi: 10.1016/s0278-6915(96)00044-0. [DOI] [PubMed] [Google Scholar]
- 4.Kihn PW. Vital tooth whitening. Dent Clin North Am. 2007;51:319–31. doi: 10.1016/j.cden.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 5.Miyazaki M, Sato H, Sato T, Moore BK, Platt JA. Effect of a whitening agent application on enamel bond strength of self etching primer systems. Am J Dent. 2004;17:151–5. [PubMed] [Google Scholar]
- 6.Attin T, Hannig C, Wiegand A, Attin R. Effect of bleaching on restorative mateials and restorations- a systematic review. Dent Mater. 2004;20:852–61. doi: 10.1016/j.dental.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 7.Shinohara MS, Peris AR, Pimenta LA, Ambrosano GM. Shear bond strength evaluation of composite resin on enamel and dentin after nonvital bleaching. J Esthet Restore Dent. 2005;17:22–9. doi: 10.1111/j.1708-8240.2005.tb00078.x. [DOI] [PubMed] [Google Scholar]
- 8.Cavalli V, de Carvalho RM, Giannini M. Influence of carbamide peroxide-based bleaching agents on the bond strength of resin-enamel/dentin interfaces. Pesqui Odontol Bras. 2005;19:23–9. doi: 10.1590/s1806-83242005000100005. [DOI] [PubMed] [Google Scholar]
- 9.Sung EC, Chan SM, Mito R, Caputo AA. Effect of carbamide peroxide bleaching on the shear bond strength of composite to dental bonding agent enhanced enamel. J Prosthet Dent. 1999;82:595–9. doi: 10.1016/s0022-3913(99)70060-0. [DOI] [PubMed] [Google Scholar]
- 10.Spyrides GM, Perdigão J, Pagani C, Araújo MA, Spyrides SM. Effect of whitening agents on dentin bonding. J Esthet Dent. 2000;12:264–70. doi: 10.1111/j.1708-8240.2000.tb00233.x. [DOI] [PubMed] [Google Scholar]
- 11.Teixeira EC, Hara AT, Turssi CP, Serra MC. Effect of non-vital tooth bleaching on resin/enamel shear bond strength. J Adhes Dent. 2002;4:317–22. [PubMed] [Google Scholar]
- 12.Titley KC, Torneck CD, Smith DC, Chernecky R, Adibfar A. Scanning electron microscopy observations on the penetration and structure of resin tags in bleached and unbleached bovine enamel. Journal of Endodontics. 1991;17:72–5. doi: 10.1016/S0099-2399(06)81611-0. [DOI] [PubMed] [Google Scholar]
- 13.Cavalli V, Reis AF, Giannini M, Ambrosano GM. The effect of elapsed time following bleaching on enamel bond strength of resin composite. Oper Dent. 2001;26:597–602. [PubMed] [Google Scholar]
- 14.Basting RT, Freitas PM, Pimenta LA, Serra MC. Shear bond strength after dentin bleaching with 10% carbamide peroxide agents. Pesqui Odontol Bras. 2004;18:162–7. doi: 10.1590/s1806-83242004000200013. [DOI] [PubMed] [Google Scholar]
- 15.Basting RT, Rodrigues JA, Serra MC, Pimenta LA. Shear bond strength of enamel treated with seven carbamide peroxide bleaching agents. J Esthet Restor Dent. 2004;16:250–9. doi: 10.1111/j.1708-8240.2004.tb00046.x. [DOI] [PubMed] [Google Scholar]
- 16.Bulut H, Turkun M, Kaya AD. Effect of an antioxidizing agent on the shear bond strength of brackets bonded to bleached human enamel. Am J Orthod Dentofacial Orthop. 2006;129:266–72. doi: 10.1016/j.ajodo.2004.03.043. [DOI] [PubMed] [Google Scholar]
- 17.Timpawat S, Nipattamanon C, Kijsamanmith K, Messer HH. Effect of bleaching agents on bonding to pulp chamber dentine. Int Endod J. 2005;38:211–7. doi: 10.1111/j.1365-2591.2004.00931.x. [DOI] [PubMed] [Google Scholar]
- 18.Shinohara MS, Peris AR, Rodrigues JA, Pimenta LA, Ambrosano GM. The effect of nonvital bleaching on the shear bond strength of composite resin using three adhesive systems. J Adhes Dent. 2004;6:205–9. [PubMed] [Google Scholar]
- 19.Cvitko E, Denehy GE, Swift EJ, Jr, Pires JA. Bond strength of composite resin to enamel bleached with carbamide peroxide. Journal of Esthetic Dentistry. 1991;3:100–2. doi: 10.1111/j.1708-8240.1991.tb00976.x. [DOI] [PubMed] [Google Scholar]
- 20.Kalili T, Caputo AA, Mito R, Sperbeck G, Matyas J. In vitro toothbrush abrasion and bond strength of bleached enamel. Practical Peroidontics and Aesthetic Dentistry. 1991;3:22–4. [PubMed] [Google Scholar]
- 21.Barghi N, Godwin JM. Reducing the adverse effect of bleaching on composite-enamel bond. Journal of Esthetic Dentistry. 1994;6:157–61. doi: 10.1111/j.1708-8240.1994.tb00852.x. [DOI] [PubMed] [Google Scholar]
- 22.Lai SC, Mak YF, Cheung GS, Osorio R, Toledano M, Carvalho RM, et al. Reversal of compromised bonding to oxidized etched dentin. Journal of Dental Research. 2001;80:1919–24. doi: 10.1177/00220345010800101101. [DOI] [PubMed] [Google Scholar]
- 23.Turkun M, Kaya AD. Effect of 10% sodium ascorbate on the shear bond strength of composite resin to bleached bovine enamel. Journal of Oral Rehabilitation. 2004;31:1184–91. doi: 10.1111/j.1365-2842.2004.01369.x. [DOI] [PubMed] [Google Scholar]
- 24.García-Godoy F, Dodge WW, Donohue M, O’Quinn JA. Composite resin bond strength after enamel bleaching. Operative Dentistry. 1993;18:144–7. [PubMed] [Google Scholar]
- 25.Stokes AN, Hood JA, Dhariwal D, Patel K. Effect of peroxide bleaches on resin-enamel bonds. Quintessence International. 1992;23:769–71. [PubMed] [Google Scholar]
- 26.Toko T, Hisamitsu H. Shear bond strength of composite resin to unbleached and bleached human dentin. Asian Journal of Aesthetic Dentistry. 1993;1:33–6. [PubMed] [Google Scholar]
- 27.Kihn PW, Barnes DM, Romberg E, Peterson K. A clinical evaluation of 10 percent vs.15 percent carbamide peroxide tooth-whitening agents. J Am Dent Assoc. 2000;131:1478–84. doi: 10.14219/jada.archive.2000.0061. [DOI] [PubMed] [Google Scholar]
- 28.Oltu U, Gürgan S. Effects of three concentrations of carbamide peroxide on the structure of enamel. J Oral Rehabil. 2000;27:332–40. doi: 10.1046/j.1365-2842.2000.00510.x. [DOI] [PubMed] [Google Scholar]
- 29.Titley KC, Torneck CD, Ruse ND, Krmec D. Adhesion of a resin composite to bleached and unbleached human enamel. J Endod. 1993;19:112–5. doi: 10.1016/S0099-2399(06)80504-2. [DOI] [PubMed] [Google Scholar]
- 30.Dishman MV, Covey DA, Baughan LW. The effects of peroxide bleaching on composite to enamel bond strength. Dent Mater. 1994;10:33–6. doi: 10.1016/0109-5641(94)90019-1. [DOI] [PubMed] [Google Scholar]
- 31.Metz MJ, Cochran MA, Matis BA, Gonzalez C, Platt JA, Pund MR. Clinical evaluation of 15% carbamide peroxide on the surface microhardness and shear bond strength of human enamel. Oper Dent. 2007;32:427–36. doi: 10.2341/06-142. [DOI] [PubMed] [Google Scholar]
- 32.Gurgan S, Alpaslan T, Kiremitci A, Cakir FY, Yazici E, Gorucu J. Effect of different adhesive systems and laser treatment on shear bond stress of bleached enamel. J Dent. 2009;37:527–34. doi: 10.1016/j.jdent.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 33.Ben-amar A, Liberman R, Gorfil C, Bernstein Y. Effect of mouthguard bleaching on enamel surface. Am J Dent. 1995;8:29–32. [PubMed] [Google Scholar]
- 34.McCracken MS, Haywood VB. Demineralization effect of 10 percent carbamide peroxide. J Dent. 1996;24:395–8. doi: 10.1016/0300-5712(95)00113-1. [DOI] [PubMed] [Google Scholar]
- 35.Josey AL, Meyers IA, Romaniuk K, Symons AL. The effect of a vital beaching technique on enamel surface morphology and the bonding of composite resin to enamel. J Oral Rehabil. 1996;23:244–50. doi: 10.1111/j.1365-2842.1996.tb00848.x. [DOI] [PubMed] [Google Scholar]
- 36.Kimyai S, Valizadeh H. The effect of hydrogel and solution of sodium ascorbate on bond strength in bleached enamel. Oper Dent. 2006;31:496–9. doi: 10.2341/05-85. [DOI] [PubMed] [Google Scholar]
- 37.Titley KC, Torneck CD, Ruse ND. The effect of carbamideperoxide gel on the shear bond strength of a microfilm resin to bovine enamel. Journal of Dental Research. 1992;71:20–4. doi: 10.1177/00220345920710010301. [DOI] [PubMed] [Google Scholar]
- 38.Usumez A, Aykent F. Bond strengths of porcelain laminate veneers to tooth surfaces prepared with acid and Er, Cr:YSGG laser etching. Journal of Prosthetic Dentistry. 2003;90:24–30. doi: 10.1016/s0022-3913(03)00235-x. [DOI] [PubMed] [Google Scholar]
- 39.Torneck CD, Titley KC, Smith DC, Adibfar A. Effect of water leaching on the adhesion of composite resin to bleached and unbleached bovine enamel. J Endod. 1991;17:156–60. doi: 10.1016/s0099-2399(06)82008-x. [DOI] [PubMed] [Google Scholar]
- 40.McGuckin RS, Thurmond BA, Osovitz S. Enamel shear bond strengths after vital bleaching. Am J Dent. 1992;5:216–22. [PubMed] [Google Scholar]
- 41.Buettner GR. The pecking order of free radicals and antioxidant: lipid peroxidation, alpha-tocopherol, and ascorbate. Arch Biochem Biophys. 1993;300:535–43. doi: 10.1006/abbi.1993.1074. [DOI] [PubMed] [Google Scholar]
- 42.Rose RC, Bode AM. Biology of free radical scavengers: an evaluation of ascorbate. FASEB J. 1993;7:1135–42. [PubMed] [Google Scholar]
- 43.Smit MJ, Anderson R. Biochemical mechanisms of hydrogen peroxide and hypochlorous acid-mediated inhibition of human mononuclear leukocyte functions in vitro: protection and reversal by anti-oxidants. Agents Actions. 1992;36:58–65. doi: 10.1007/BF01991229. [DOI] [PubMed] [Google Scholar]
- 44.Brennan LA, Morris GM, Wasson GR, Hannigan BM, Barnett YA. The effect of vitamin C or vitamin E supplementation on basal and H2O2-induced DNA damage in human lymphocytes. Br J Nutr. 2000;84:195–202. doi: 10.1017/s0007114500001422. [DOI] [PubMed] [Google Scholar]
- 45.Asmussen E, Peutzfeldt A. Bonding of dual-curing resin cements to dentin. Journal of Adhesive Dentistry. 2006;8:299–304. [PubMed] [Google Scholar]
- 46.Kaya AD, Türkün M. Reversal of dentin bonding to bleached teeth. Operative Dentistry. 2003;28:825–9. [PubMed] [Google Scholar]
- 47.Lai SC, Tay FR, Cheung GS, Mak YF, Carvalho RM, Wei SH, et al. Reversal of compromised bonding in bleached enamel. Journal of Dental Research. 2002;81:477–81. doi: 10.1177/154405910208100709. [DOI] [PubMed] [Google Scholar]
- 48.Zhao H, Li X, Wang J, Qu S, Weng J, Zhang X. Characterization of peroxide ions in hydroxyapatite lattice. J Biomed Mater Res. 2000;52:157–63. doi: 10.1002/1097-4636(200010)52:1<157::aid-jbm20>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 49.Nagpal R, Tewari S, Gupta R. Effect of various surface treatments on the microleakage and utra structure of resin-tooth interface. Oper Dent. 2007;32:16–23. doi: 10.2341/06-1. [DOI] [PubMed] [Google Scholar]


