Abstract
Objective:
This in vitro study investigated the effect of a desensitizer on the degree of conversion of two bonding resins using Fourier transform infrared (FTIR) spectroscopy.
Materials and Methods:
An etch-and-rise bonding resin and a self-etching adhesive resin were selected for the study. Vivasens (Ivoclar Vivadent) was used as a desensitizing agent. Grouping was done as follows: Group I: Adper Single Bond (n=10), Group II: Adper Single Bond + Vivasens (n=10), Group III: AdheSE One (n=10), Group IV: AdheSE One + Vivasens (n=10). The bonding resin alone was light cured for 20 seconds in groups I and III. For groups II and IV, 1 ml each of the bonding resin and the desensitizer was mixed in a vial and light cured for 20 seconds. The specimens were analysed using FTIR spectroscopy.
Results:
Group II (Adper Single Bond + Vivasens) showed a significantly higher degree of conversion compared to Group I (Adper Single Bond). Comparing Groups III and IV, Group IV (AdheSE One + Vivasens) showed a significantly higher degree of conversion compared to Group III (AdheSE One).
Conclusions:
The degree of conversion is increased when a dentin bonding agent is used along with a desensitizer. Hence, this combination can be recommended to effectively control postoperative sensitivity.
Keywords: Degree of conversion, dentin bonding resin, desensitizing agent, FTIR spectroscopy
INTRODUCTION
Research and development in dental adhesives have been focussed upon simplifying their application techniques. The most currently used adhesive system classification is based on the number of steps necessary for clinical application and their interaction with dental hard tissues. Total etch adhesive systems, which remove the smear layer with phosphoric acid and combine the functions of primer and adhesive in one bottle, have been widely used.[1] Although long term clinical success has been achieved with total-etch systems, the demand for simplified application has increased, resulting in the development of self-etching adhesive systems.[2,3] The bonding mechanism of self-etching adhesive systems is based on the simultaneous etching and priming of enamel and dentin without rinsing forming a continuum in the substrate and incorporating smear plugs into the resin tags.[4,5]
Effective adhesives are expected to seal the etched dentin surfaces by intertubular and peritubular hybridization and by resin tag formation in the opened dentinal tubules.[6] This seal prevents fluid shift across the tubules occurring in response to mechanical, thermal, or osmotic stimuli. However, abiding by the hydrodynamic theory, if the tags formed within the dentinal tubules were too long, it could cause post-operative sensitivity.[7,8] But combining a resin adhesive with a previous application of desensitizing agent seems to be contradictory at first sight, considering its effect on resin tag formation and the bond strength of composite resin to the tooth structure.[7]
Even though the use of a desensitizing agent prior to application of dentin bonding resin seems to be promising,[7,8] we are not aware of its effect on the degree of conversion of the dentin bonding resin. The degree of conversion (DC) of the resin material is related to many properties, including mechanical properties, solubility, dimensional stability, colour change and biocompatibility.[9] Hence, the aim of this in vitro study was to determine the degree of conversion of an etch-and-rinse dentin bonding resin and a self-etching adhesive resin used with and without a desensitizing agent using Fourier Transform Infrared (FTIR) spectroscopy.
MATERIALS AND METHODS
Adper Single Bond (3M ESPE), an etch-and-rinse bonding resin and Adhse One (Ivoclar Vivadent), a self-etching adhesive resin were chosen for the study. The materials used in this study and their composition are shown in Table 1. The four study groups are as follows:
Table 1.
Materials used, their manufacturer, and composition

Group I: Adper Single Bond (n=10)
Group II: Adper Single Bond + Vivasens (n=10)
Group III: AdheSE One (n=10)
Group IV: AdheSE One + Vivasens (n=10)
Group I and Group III specimens were prepared by curing 1 ml of the bonding resin in a vial for 20 seconds. Group II and Group IV specimens were prepared by mixing 1 ml each of the respective bonding agent and Vivasens in a vial, which was then immersed in an ultrasonic bath and mixed for 1 minute. The specimens were then light cured for 20 seconds. Ten such specimens were prepared for each group. The specimens were then sent for FTIR spectroscopy (Spectrum One, Perkin Elmer, USA) analysis.
Assessment of degree of conversion
Degree of monomer conversion (DC%) was assessed using FTIR Spectroscopy. First, the spectrum of the unpolymerized resin was measured and then the infrared spectrum was recorded. During spectra recording, the resin was continuously in contact with the sensor. DC% was calculated from the aliphatic C=C peak at 1638 cm-1 and normalized against the aromatic C=C peak at 1608 cm-1 according to the following formula
DC% = {1- [Caliphatic/ Caromatic] / [Ualiphatic/ Uaromatic]} × 100
Where Caliphatic = absorption peak at 1638cm-1 of the polymerized specimen,
Caromatic = absorption peak at 1608cm-1 of the polymerized specimen
Ualiphatic = absorption peak at 1638cm-1 of the unpolymerized specimen and
Uaromatic = absorption peak at 1608cm-1 of the unpolymerized specimen
Statistical analysis was based on ANOVA and Post-Hoc tests with a level of significance of P<0.05
RESULTS
Table 2 shows the mean degree of conversion (DC%) values and standard deviations of the study groups. The FTIR spectra of the study groups are shown in Figures 1–4.
Table 2.
Mean degree of conversion (DC%) values and standard deviations of the study groups
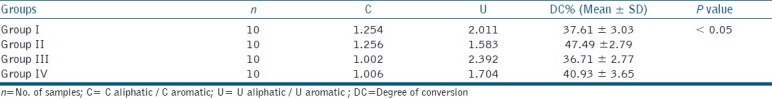
Figure 1.
FTIR spectra of group I (Adper Single Bond)
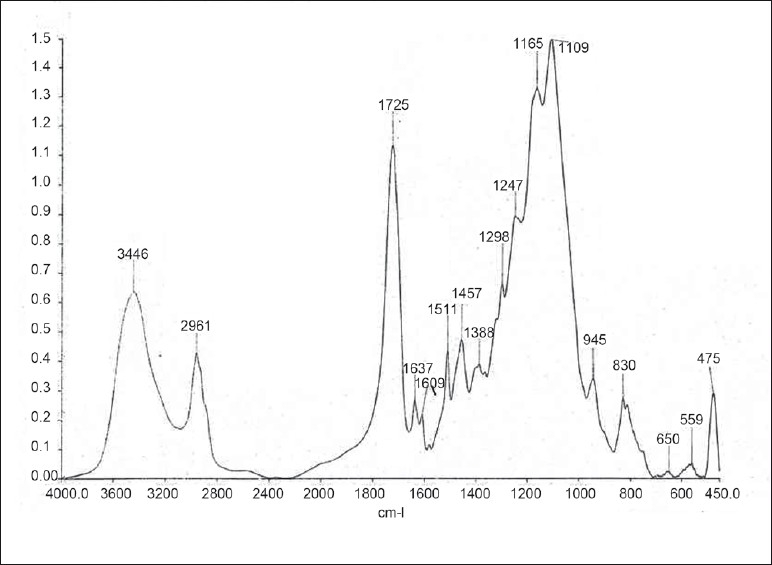
Figure 4.
FTIR spectra of group IV (AdheSE One + Desensitizer)
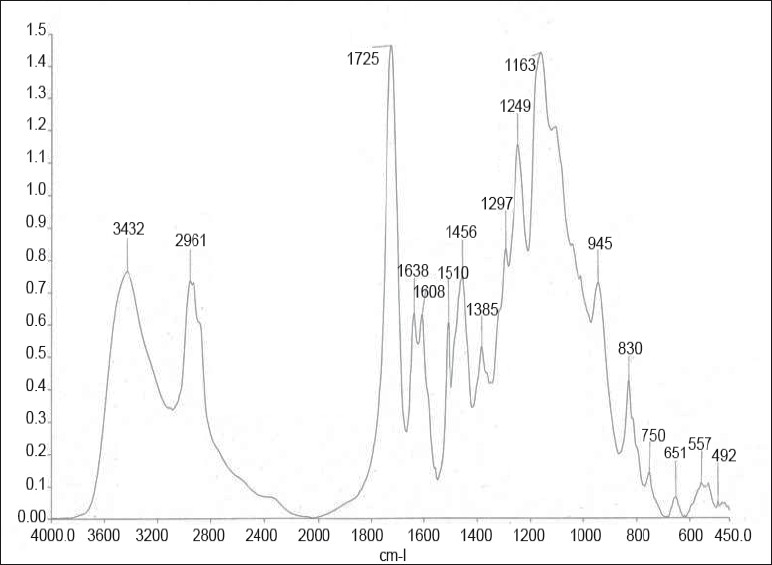
Figure 2.
FTIR spectra of group II (Adper Single Bond + Desensitizer)
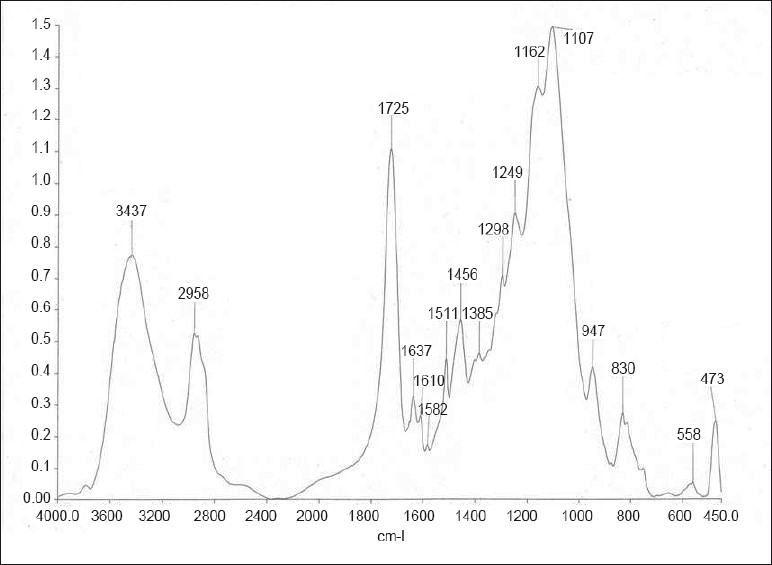
Figure 3.
FTIR spectra of group III (AdheSE One)
Statistical analysis showed a significantly higher DC% values for Group II (Adper Single Bond + desensitizer-47.49±2.79) compared to Group I (Adper Single Bond - 37.61±3.03). Comparing Groups III and IV, Group IV (AdheSE One+desensitizer - 40.93±3.65) showed a significantly higher DC% values compared to Group III (AdheSE One-36.71±2.77). Comparing the groups without desensitizer, no statistically significant difference was found between group I (37.61±3.03) and group III (36.71±2.77). Comparing the groups with desensitizer, group II (47.49±2.79) showed a significantly higher DC% compared to group IV (40.93±3.65).
DISCUSSION
The most commonly used esthetic restorative materials are the resin composites that are bonded to the tooth structure via an adhesive material. Adhesion of resins to tooth structure has been a goal for dental researchers for many years. The interest of adhesion focussed on the factors and techniques for enhancement of monomer penetration. Dentin-bonding agents have evolved over the years, with the introduction of a new system of bonding in every generation.[1]
The “Total-etch” adhesives or “Etch-and-rinse” adhesives combine the primer and the bonding agent into a single solution.[10] A separate etching step is still required. As this system requires several time consuming steps, manufacturers attempted to reduce the number of steps and corresponding application time, making more user-friendly adhesive systems. In recent years, self etching resins containing acidic methacrylates have been introduced in an attempt to eliminate the steps of etching and rinsing.
The application of dentin adhesive can result in the formation of long resin tags within the dentinal tubules, which may lead to postoperative sensitivity.[7,11] A method of reducing postoperative sensitivity is by using a desensitizer along with a dentin bonding agent. Studies have been done to check the effect of application of a layer of desensitizing agent on the bond strength of the bonding agent, but no studies have been done to check the degree of conversion of the bonding agent when a desensitizing agent is incorporated into it.
Hence this study was done to assess the degree of conversion of dentin bonding agents on addition of a desensitizing agent using FTIR spectroscopy. The extent of polymerization of a material is expressed as degree of conversion of monomeric C=C bonds into polymeric C-C bond, which affects both physical and mechanical properties.[12] Incomplete conversion results in residual unreacted monomer which may cause a reduction in material properties, a decrease in the long term stability of the restoration and pulpal irritation.[13–15] FTIR was used in this study as it provides a direct measure of unreacted methacrylate groups.[16] It is a reliable and fast technique used for detecting C=C bond stretching vibrations directly before and after curing of resins.[16]
According to this study, the combination of a dentin adhesive with a desensitizer showed an increase in the degree of conversion (Group III and Group IV) compared to when dentin adhesive was used alone (Group I and Group II). Dentin adhesive/desensitizer combination can be used to mechanically block patent dentinal tubules or physiologically decrease the excitability of the intradental nerves to relieve hypersensitivity. Vivasens, the desensitizing agent contains organic acids such as phosphoric acid methacrylate and solvents such as ethanol which induce the precipitation of proteins in the dentin liquid. Moreover, the sealing effect is enhanced by co-precipitating polyethylene glycol dimethacrylate, which is present in Vivasens.
Polyethylene glycol dimethacrylate is a condensation polymer, which acts as an organic filler and cross links molecular chains of polymers.[17] Therefore an increase in the degree of conversion in Group II and Group IV can probably be attributed to the presence of polyethylene glycol dimethacrylate.
CONCLUSIONS
Within the limitations of this in-vitro study, it can be concluded that the use of a desensitizing agent along with a dentin bonding resin does not affect the polymerization of the bonding resin and can be recommended to effectively prevent postoperative sensitivity associated with composite restorations.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Yazici AR, Celik C, Ozgunaltay G, Dayangac B. Bond strength of different adhesive systems to dental hard tissues. Oper Dent. 2007;32:166–72. doi: 10.2341/06-49. [DOI] [PubMed] [Google Scholar]
- 2.Aw TC, Lepe X, Johnson GH, Manel LA. A three year clinical evaluation of two-bottle versus one-bottle dentin adhesives. J Am Dent Assoc. 2005;136:311–22. doi: 10.14219/jada.archive.2005.0171. [DOI] [PubMed] [Google Scholar]
- 3.Swift EJ, Jr, Perdigao J, Wilder AD, Jr, Heymann HO, Sturdevant JR, Bayne SC. Clinical evaluation of two one-bottle dentin adhesives at three years. J Am Dent Assoc. 2001;132:1117–23. doi: 10.14219/jada.archive.2001.0337. [DOI] [PubMed] [Google Scholar]
- 4.Watanabe I, Nakabayashi N, Pashley DH. Bonding to ground dentin by a phenyl-P self-etching primer. J Dent Res. 1994;73:1212–20. doi: 10.1177/00220345940730061301. [DOI] [PubMed] [Google Scholar]
- 5.Gordan VV, Vargas MA, Cobb DS, Denehy GE. Evaluation of adhesive systems using acidic primers. Am J Dent. 1997;10:219–23. [PubMed] [Google Scholar]
- 6.Dondi dall’Orologio G, Lone A, Finger WJ. Clinical evaluation of the role of glutardialdehyde in a one-bottle adhesive. Am J Dent. 2002;15:330–4. [PubMed] [Google Scholar]
- 7.Soares CJ, Santos Filho PC, Barreto BC, Mota AS. Effect of previous desensitizer and rewetting agent application on shear bond strength of bonding systems to dentin. Cienc Odontol Bras. 2006;9:6–11. [Google Scholar]
- 8.Fu B, Shen Y, Wang H, Hannig M. Sealing ability of dentin adhesives/desensitizer. Oper Dent. 2007;32:496–503. doi: 10.2341/06-143. [DOI] [PubMed] [Google Scholar]
- 9.Cekic-Nagas I, Ergun G, Vallittu PK, Lassila LV. Influence of polymerization mode on degree of conversion and micropush-out bond strength of resin core systems using different adhesive systems. Dent Mater J. 2008;27:376–85. doi: 10.4012/dmj.27.376. [DOI] [PubMed] [Google Scholar]
- 10.Swift EJ, Jr, Perdigão J, Heymann HO, Wilder AD, Jr, Bayne SC, May KN., Jr Eighteen-month clinical evaluation of a filled and unfilled dentin adhesive. J Dent. 2001;29:1–6. doi: 10.1016/s0300-5712(00)00050-6. [DOI] [PubMed] [Google Scholar]
- 11.Schupbach P, Lutz F, Finger WJ. Closing of dentinal tubules by Gluma Desensitzer. Eur J Oral Sci. 1997;105:414–21. doi: 10.1111/j.1600-0722.1997.tb02138.x. [DOI] [PubMed] [Google Scholar]
- 12.Ferracane JL. Correlation between hardness and degree of conversion during the setting reaction of unfilled dental restorative resins. Dent Mater. 1985;1:11–4. doi: 10.1016/S0109-5641(85)80058-0. [DOI] [PubMed] [Google Scholar]
- 13.Carmichael AJ, Gibson JJ, Walls AW. Allergic contact dermatitis to bisphenol-A- glycigyldimethacrylate (BIS-GMA) dental resin associated with sensitivity to epoxy resin. Br Dent J. 1997;183:297–8. doi: 10.1038/sj.bdj.4809499. [DOI] [PubMed] [Google Scholar]
- 14.Nie J, Linden LA, Rabek JF, Fouassier JP, Morlet-Savary F, Scigalski F, et al. A reappraisal of the photopolymerisation kinetics of triethyleneglycol dimethacrylate initiated by camphoroquinone-N, N-dimethyl-p-toluidine for dental purposes. Acta Polymer. 1998;49:145–61. [Google Scholar]
- 15.Reichl FX, Durner J, Hickel R, Spahl W, Kehe K, Walther U, et al. Uptake, clearance and metabolism of TEGDMA in guinea pigs. Dent Mater. 2002;18:581–9. doi: 10.1016/s0109-5641(01)00094-x. [DOI] [PubMed] [Google Scholar]
- 16.Vinoth Kumar TS, Shyamala PV, Kavitha S, Laksminarayanan L. Invitro evaluation of degree of conversion of various luting resins at different levels of post space using FTIR spectroscopy. Endodontology. 2008;20:37–43. [Google Scholar]
- 17. [Last accessed on 2010 Jan 5]. Available from: http://www.chemicalland21.com/industrialchem/functional%20Monomer/POLYETHYLENE%20GLYCOL%20DIMETHACRYLATE.htm .


