Abstract
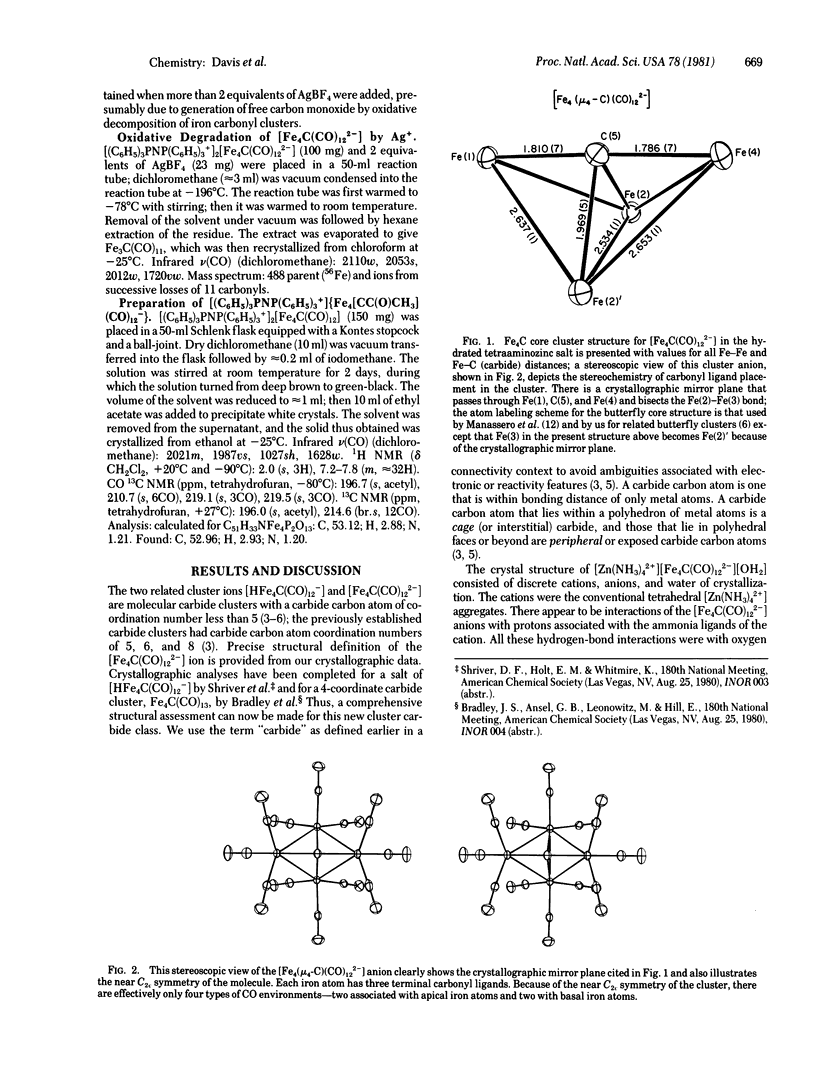
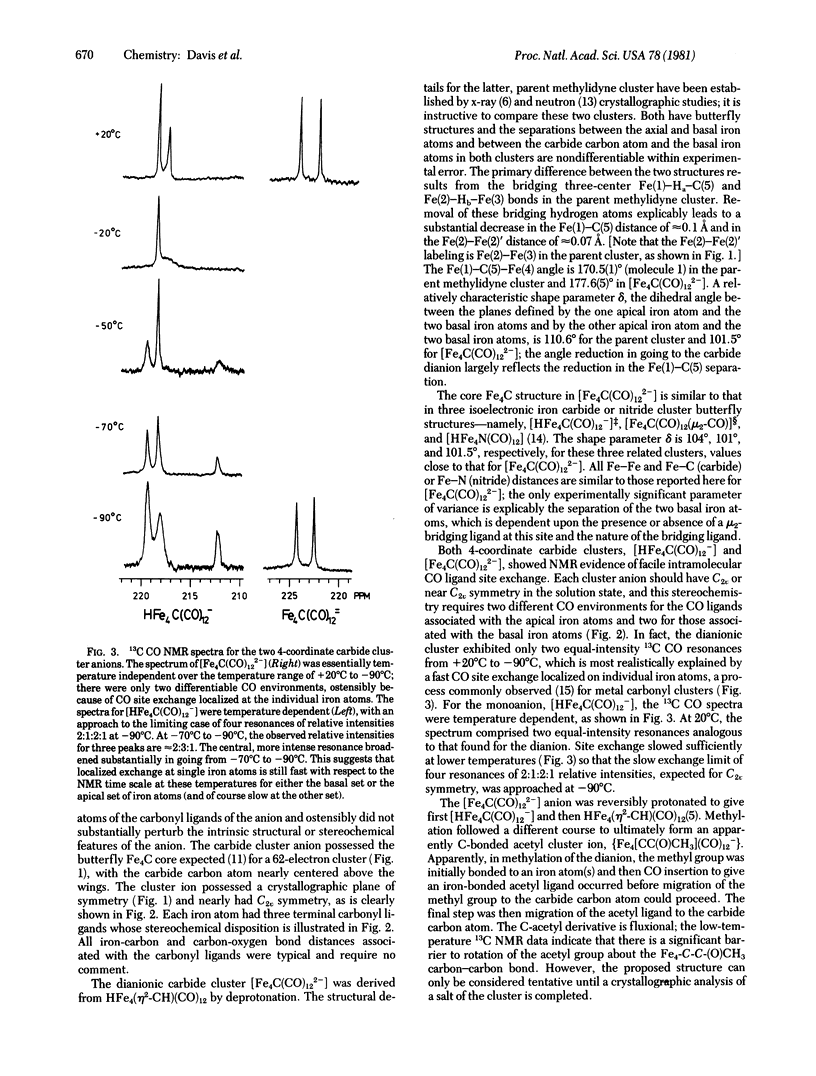
Molecular metal clusters with carbide carbon atoms of low coordination number have been prepared; they are the anionic [HFe4C(CO)12-] and [Fe4C(CO)122-] clusters. An x-ray crystallographic analysis of a tetraaminozinc salt of the latter has established a butterfly array of iron atoms with the carbide carbon atom centered above the wings of the Fe4 core. Each iron atom was bonded to three peripheral carbonyl ligands. The distances from the carbide carbon to iron were relatively short, particularly those to the apical iron atoms (1.80 Å average). Protonation of the anionic carbide clusters reversibly yielded HFe4(CH)(CO)12, and methylation of the dianion gave {Fe4[CC(O)CH3](CO)12-}. Oxidation of [Fe4C(CO)122-] yielded the coordinately unsaturated Fe4C(CO)12 cluster, which was extremely reactive. Hydrogen addition to this iron cluster was rapid below 0°C, and a C—H bond was formed in this transformation.
Keywords: metal cluster metal surface analogy, x-ray crystallographic study, reactivity of metal carbide carbon atoms
Full text
PDF