Abstract
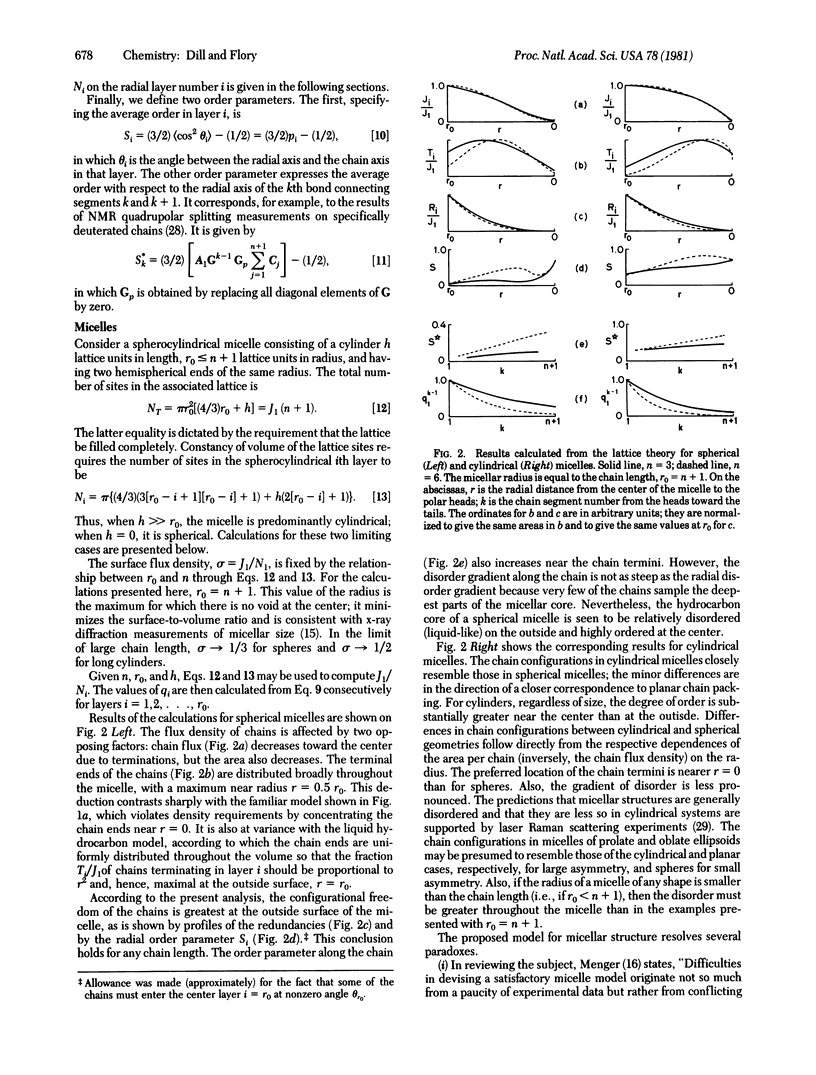
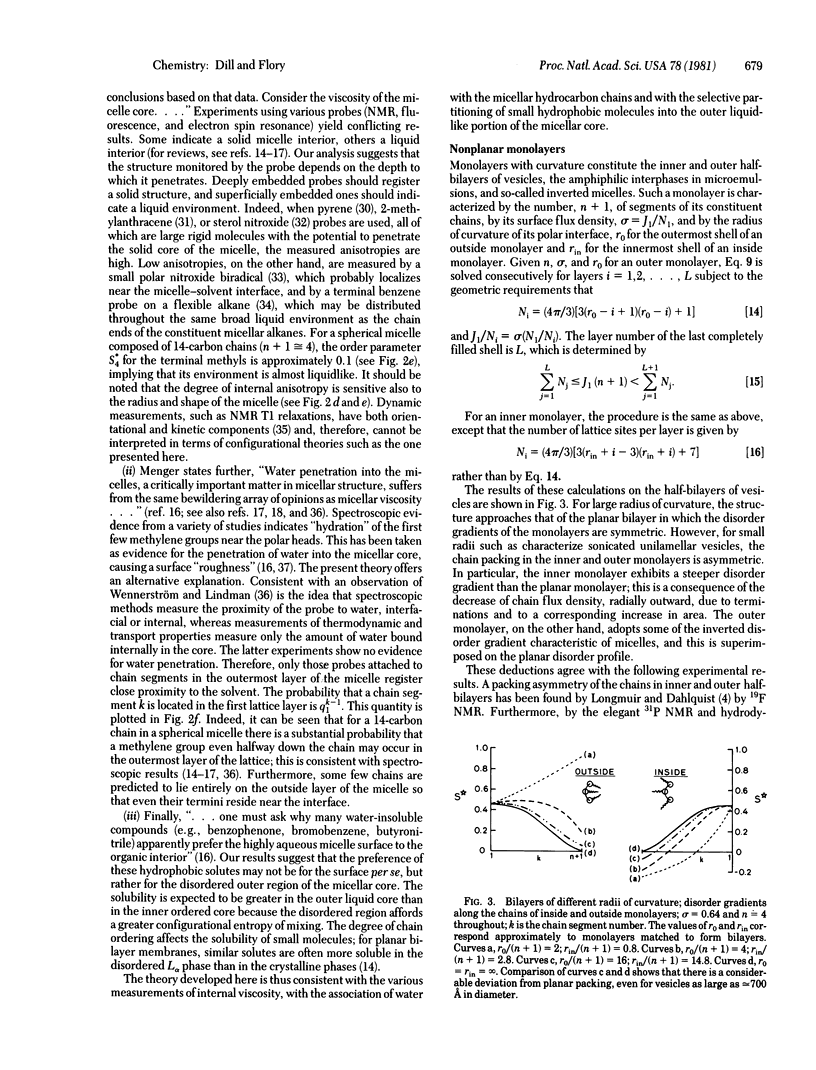
The configurations of the hydrocarbon chains in micelles are severely constrained by the space-filling requirements of the chain segments, by the continuity of the chains, and by the micellar geometry. A statistical theory that takes full account of these constraints is developed by using a lattice model. The chain ends are deduced to be nonuniformly distributed, with maximal incidence approximately midway between the center of the micelle and the outer surface. Whereas the chain disorder near the outside of the hydrophobic core may approach that of a liquid, crowding of the chains near the core center imposes a degree of order approaching that in a crystal. These results are at variance with the prevailing view that the micellar interior resembles a “liquid hydrocarbon droplet.” Also discussed are the effects of curvature on the chain configurations in monolayers and bilayers. It is found, for example, that the “disorder gradients” in inner and outer half-bilayers of small vesicles should be substantially different. Implications of these results are discussed.
Keywords: chain packing, disorder gradient, curved monolayers and bilayers, statistical lattice theory
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chrzeszczyk A., Wishnia A., Springer C. S., Jr The intrinsic structural asymmetry of highly curved phospholipid bilayer membranes. Biochim Biophys Acta. 1977 Oct 17;470(2):161–169. doi: 10.1016/0005-2736(77)90097-9. [DOI] [PubMed] [Google Scholar]
- Cornell B. A., Middlehurst J., Separovic F. The molecular packing and stability within highly curved phospholipid bilayers. Biochim Biophys Acta. 1980 May 23;598(2):405–410. doi: 10.1016/0005-2736(80)90018-8. [DOI] [PubMed] [Google Scholar]
- Dill K. A., Flory P. J. Interphases of chain molecules: Monolayers and lipid bilayer membranes. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3115–3119. doi: 10.1073/pnas.77.6.3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eytan G. D., Broza R. Selective incorporation of cytochrome oxidase into small liposomes. FEBS Lett. 1978 Jan 1;85(1):175–178. doi: 10.1016/0014-5793(78)81274-5. [DOI] [PubMed] [Google Scholar]
- Gaber B. P., Peticolas W. L. On the quantitative interpretation of biomembrane structure by Raman spectroscopy. Biochim Biophys Acta. 1977 Mar 1;465(2):260–274. doi: 10.1016/0005-2736(77)90078-5. [DOI] [PubMed] [Google Scholar]
- Huang C. H., Sipe J. P., Chow S. T., Martin R. B. Differential interaction of cholesterol with phosphatidylcholine on the inner and outer surfaces of lipid bilayer vesicles. Proc Natl Acad Sci U S A. 1974 Feb;71(2):359–362. doi: 10.1073/pnas.71.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Mason J. T. Geometric packing constraints in egg phosphatidylcholine vesicles. Proc Natl Acad Sci U S A. 1978 Jan;75(1):308–310. doi: 10.1073/pnas.75.1.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lentz B. R., Barenholz Y., Thompson T. E. Fluorescence depolarization studies of phase transitions and fluidity in phospholipid bilayers. 2 Two-component phosphatidylcholine liposomes. Biochemistry. 1976 Oct 5;15(20):4529–4537. doi: 10.1021/bi00665a030. [DOI] [PubMed] [Google Scholar]
- Longmuir K. J., Dahlquist F. W. Direct spectroscopic observation of inner and outer hydrocarbon chains of lipid bilayer vesicles. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2716–2719. doi: 10.1073/pnas.73.8.2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelsohn R., Sunder S., Bernstein H. J. The effect of sonication on the hydrocarbon chain conformation in model membrane systems: a Raman spectroscopic study. Biochim Biophys Acta. 1976 Feb 6;419(3):563–569. doi: 10.1016/0005-2736(76)90270-4. [DOI] [PubMed] [Google Scholar]
- Nagle J. F., Wilkinson D. A. Lecithin bilayers. Density measurement and molecular interactions. Biophys J. 1978 Aug;23(2):159–175. doi: 10.1016/S0006-3495(78)85441-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott H. L., Cherng S. L. Monte Carlo studies of phospholipid lamellae: effects of proteins, cholesterol, bilayer curvature, and lateral mobility on order parameters. Biochim Biophys Acta. 1978 Jul 4;510(2):209–215. doi: 10.1016/0005-2736(78)90021-4. [DOI] [PubMed] [Google Scholar]
- Seelig J. Deuterium magnetic resonance: theory and application to lipid membranes. Q Rev Biophys. 1977 Aug;10(3):353–418. doi: 10.1017/s0033583500002948. [DOI] [PubMed] [Google Scholar]
- Sheetz M. P., Chan S. I. Effect of sonication on the structure of lecithin bilayers. Biochemistry. 1972 Nov 21;11(24):4573–4581. doi: 10.1021/bi00774a024. [DOI] [PubMed] [Google Scholar]
- Sheetz M. P., Singer S. J. Biological membranes as bilayer couples. A molecular mechanism of drug-erythrocyte interactions. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4457–4461. doi: 10.1073/pnas.71.11.4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinitzky M., Dianoux A. C., Gitler C., Weber G. Microviscosity and order in the hydrocarbon region of micelles and membranes determined with fluorescent probes. I. Synthetic micelles. Biochemistry. 1971 May 25;10(11):2106–2113. doi: 10.1021/bi00787a023. [DOI] [PubMed] [Google Scholar]
- Stockton G. W., Polnaszek C. F., Tulloch A. P., Hasan F., Smith I. C. Molecular motion and order in single-bilayer vesicles and multilamellar dispersions of egg lecithin and lecithin-cholesterol mixtures. A deuterium nuclear magnetic resonance study of specifically labeled lipids. Biochemistry. 1976 Mar 9;15(5):954–966. doi: 10.1021/bi00650a003. [DOI] [PubMed] [Google Scholar]
- Suurkuusk J., Lentz B. R., Barenholz Y., Biltonen R. L., Thompson T. E. A calorimetric and fluorescent probe study of the gel-liquid crystalline phase transition in small, single-lamellar dipalmitoylphosphatidylcholine vesicles. Biochemistry. 1976 Apr 6;15(7):1393–1401. doi: 10.1021/bi00652a007. [DOI] [PubMed] [Google Scholar]
- Wilschut J. C., Regts J., Westenberg H., Scherphof G. Action of phospholipases A2 on phosphatidylcholine bilayers. Effects of the phase transition, bilayer curvature and structural defects. Biochim Biophys Acta. 1978 Apr 4;508(2):185–196. doi: 10.1016/0005-2736(78)90324-3. [DOI] [PubMed] [Google Scholar]





