Abstract
This review will cover the current strategies that are being adopted to efficiently deliver small interfering RNA using nonviral vectors, including the use of polymers such as polyethylenimine, poly(lactic-co-glycolic acid), polypeptides, chitosan, cyclodextrin, dendrimers, and polymers-containing different nanoparticles. The article will provide a brief and concise account of underlying principle of these polymeric vectors and their structural and functional modifications which were intended to serve different purposes to affect efficient therapeutic outcome of small-interfering RNA delivery. The modifications of these polymeric vectors will be discussed with reference to stimuli-responsiveness, target specific delivery, and incorporation of nanoconstructs such as carbon nanotubes, gold nanoparticles, and silica nanoparticles. The emergence of small-interfering RNA as the potential therapeutic agent and its mode of action will also be mentioned in a nutshell.
Introduction
A revolutionary discovery of the unique property of double-stranded RNA (dsRNA) to silence gene expression in the nematode worm Caenorhabditis elegans (Fire et al., 1998) cast its spell on the scientific community in biotechnology field. The pioneer work on RNA interference (RNAi) by Fire, Mello, and their colleagues was soon followed by the proof-of-principle experiments conducted by Tuschl group to establish the successful application of synthetic small-interfering RNA (siRNA) in sequence-specific suppression of endogenous and heterologous genes in mammalian cell lines (Elbashir et al., 2001). The emergence of RNAi as a unique endogenous powerful cellular mechanism which triggered downregulation of specific gene expression, propelled a flurry of research activities in the fields of biotechnology. Since then, the impetus to unravel the plethora of opportunities in the siRNA-mediated therapy triggers significant advancement in the treatment of various disease targets, including viral infections (Morrissey et al., 2005, Okumura et al., 2008) and cancers (Ptasznik et al., 2004; Xia et al., 2007; Kim et al., 2008).
In eukaryotic cells, RNAi constitutes a fundamental pathway which enables sequence-specific siRNA to target and cleave complementary mRNA. Normally RNAi is activated when long pieces of dsRNA is cleaved to siRNA (21–23 nucleotides long) by the enzyme Dicer (Bernstein et al., 2001) However, in practice, siRNA can be synthetically produced and directly introduced into the cell, thus involvement of Dicer mechanism can be bypassed. Inside the cell, siRNA associates itself with the multiprotein complex known as RNA induced silencing complex (RISC) (Rand et al., 2004). RISC contains a multifunctional protein, Argonaute 2, which unwinds the siRNA and subsequently the sense strand (or passenger strand) of the siRNA is cleaved (Matranga et al., 2005). The activated RISC then undergoes antisense strand (or guide strand)-mediated hybridization with the target mRNA which is complementary to the antisense strand (Ameres et al., 2007) and thereby elicits selective degradation of the mRNA. Though antisense strand-loaded RISC could undergo multiple catalytic cycles of gene regulation, repeated administration of siRNA is required due to its degradation within the cell.
RNAi machinery may enrich the therapeutic arsenal immensely by providing a platform to combat a wide spectrum of diseases which seem hard to cure through the administration of conventional therapies. Appropriately designed synthetic siRNA could silence almost any gene in the body. The initial but important steps toward the realization of such hopes have been witnessed in the successful application of synthetic siRNA in knocking down targets in various diseases in vivo, including hypercholesterolemia (Frank-Kamenetsky et al., 2008), livercirrhosis (Sato et al., 2008), hepatitis B virus (Song et al., 2003), human papillomavirus (Niu et al., 2006), ovarian cancer (Halder et al., 2006), and bone cancer (Takeshita et al., 2005). However, safe and successful clinical implementation indispensably requires further advancement in developing efficient delivery systems. Therefore, the development of safe and effective delivery system is one of the most crucial factors for the advancement in clinical implementations of this therapy. There are some target organs such as brain (DiFiglia et al., 2007) and lung (de Fougerolles and Novobrantseva, 2008) where efficient transfection can be achieved using naked, chemically modified siRNA. Localized delivery of siRNA can bypass several hurdles associated with their accessibility to the target organs or tissues though it is not feasible in most of the cases. Naked siRNA undergoes degradation by endogenous enzymes and is unable to penetrate cellular membranes due to its large size (∼13 kDa) and high negative charge. Therefore, a sophisticated delivery system becomes an indispensable component of siRNA-mediated therapy targeted at various body parts normally not fit for localized delivery of therapeutic cargos. To deliver siRNA via the systemic route and achieve gene silencing in disease-relevant tissues, the delivery system need to fulfill the following functions: (1) protect siRNA from nuclease degradation in circulation; (2) exhibit a proper pharmacokinetic and tissue distribution profile to deliver siRNA to disease-relevant organs; and (3) facilitate efficient uptake of siRNA into target cells and release siRNA into cytoplasm to knockdown the target (Kim and KIM, 2009).
Various delivery strategies have been employed, both viral and nonviral, to address these barriers with diverse outcomes. The use of viral vectors, despite their high efficiency, has been impaired greatly due to the associated mutagenicity or oncogenesis, several host immune responses, and high cost of production. Therefore, nonviral vectors draw significant attention in spite of their low efficacy. There is a continuous effort to modify and refine these siRNA delivery systems either by adopting different strategies or by installing various functional attributes to the existing systems. The challenge to integrate all the crucial functional attributes such as nuclease resistance, encapsulation, prolonged circulation, effective cell penetration, cytoplasmic delivery, pharmacokinetic, and tissue distribution, into one single system makes this field very exciting. Further, the clinical success of such nonviral vector mediated therapy is pivoted upon the development of optimal delivery system having a delicate balance between the cellular activity and the in vivo pharmacokinetic and tissue distribution properties.
This review will focus on the summarization of the polymeric siRNA carriers such as polyethylenimine (PEI), poly(lactic-co-glycolic acid) (PLGA), polypeptides, chitosan, cyclodextrin, dendrimers, and polymers-containing different nanoparticles which are currently used in vitro and in vivo, with respect to their structural and physicochemical properties and delivery efficiency as a siRNA delivery vehicle.
Polyethylenimine
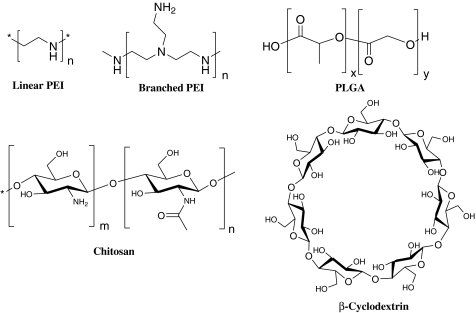
Though PEI is known as the gold standard for plasmid DNA (pDNA) delivery its effectiveness in siRNA delivery is considered to be far less pronounced (Hassani et al., 2005; Grayson et al., 2006). The reduced efficacy of PEI in the later case arises due to the short length of siRNA. The weak electrostatic cohesion between the negatively charged siRNA and the cationic PEI polymer plays crucial role in dissociation of PEI/siRNA complexes at the anionic cell surface (Spagnou et al., 2004; Bolcato-Bellemin et al., 2007). Additionally the associated toxicity of PEI becomes one of the major concerns for all kinds of nucleic acid delivery (Hunter and Moghimi, 2002; Moghimi et al., 2005). However, the availability of linear (LPEI) and branched PEI (BPEI) (Fig. 1) in a wide range of molecular weights (Godbey et al., 1999) and their extensive research establishes the optimal requirements such as structural and functional features. Judicious construction of cationic vector having PEI not only caters the cytotoxicity concern, but could also provide sufficient protection against serum or RNase through efficient complexation with siRNA. These cationic complexes usually undergo efficient endocytosis-mediated cellular uptake. Moreover, PEI owing to its high content of protonable amino groups can exert “proton sponge effect” to induce the release of siRNA to the cytoplasm (Boussif et al., 1995). siRNA must be released at the cytoplasm before being destroyed inside the endosome. It should be noted, however, that by far not all PEIs are suitable for the transport of nucleic acids like siRNAs (Werth et al., 2006). The most prevalent strategy that has been employed so far is the utilization of high complexation ability and efficient endosomal release of PEI. The associated toxicity of PEI has been regulated by incorporating low molecular weight (LMW) PEI into other polymeric construct fabricated as per various needs (Namgung et al., 2009a, 2010). The fabrication may impart several attributes to the systems such as target specificity (Schiffelers et al., 2004), prolonged circulation (Mao et al., 2006), enhanced serum stability, biocompatibility, and stimuli-responsiveness (de Las Heras Alarcon et al., 2005; Park et al., 2010).
FIG. 1.
Chemical structure of linear polyethylenimine (PEI), branched PEI, poly(lactic-co-glycolic acid) (PLGA), chitosan, and β-cyclodextrin.
Introduction of hydrophobic character into the PEI-based polymeric vector becomes a feasible approach to address the cytotoxicity concern. However, reduction of the positive charge density of the polymers should be moderate and the resulting surface charge must be positive enough to allow the formation of stable polyplexes with siRNA and to exert proton sponge effect to facilitate endosomal release of polyplexes. Hence, there should be a balance between the induced hydrophobic character and the reduced positive charge. Oskuee et al. incorporated alkylcarboxyl groups to branched PEI (25 kDa) to impart hydrophobicity as well as to neutralize the positive charge (Oskuee et al., 2010). Investigation on various formulations having varied degree of alkylcarboxylation revealed that at low degree of carboxylation (<20%) endosomal escape operated efficiently. Interestingly increase of the hydrophobic alkyl chain length generally resulted in improved complex stability with siRNA (Philipp et al., 2009). The toxicity of modified PEI derivatives was, as expected, greatly reduced in comparison to PEI 25 kDa, which is caused by the introduction of the additional negative charges lowering the strong interaction potential of PEI with cell surfaces and associated membrane damage (Hunter and Moghimi, 2002).
In contrast to high molecular weight (HMW) PEI, LMW PEI displays not only negligible cytotoxicity, but also demonstrates low transfection efficiency. The common strategy employed to manage such tradeoff between cytotoxicity and delivery efficiency involves cross-linked PEI containing disulfide bonds (Kim et al., 2010a; Son et al., 2010a, 2010b). Considerable gene delivery efficiency has been achieved utilizing bioreducible cross-linked PEI-SS. Goepferich's group (Breunig et al., 2008) cross-linked LMW LPEI (Mw = 2.6–4.6 kDa) using dithiodipropionic acid or cysteine linkages. The work indicated that the cellular uptake of siRNA was more efficient with increased branching of the polymer, that is, LPEI 5 kDa < LPEI cross-linked via disulfide bonds (ssPEI) < BPEI 25 kDa and thereby higher gene silencing were achieved. The combination of a high branching density and reductively cleavable disulfide bonds within the PEI polymer could be the governing factors in accomplishing improved siRNA delivery through effective complexation and facile release of siRNA in the cytoplasm in the presence of glutathione.
To integrate low cytotoxicity and higher transfection efficiency together Kim group developed water soluble lipopolymer (WSLP) by conjugating the cationic head group of LMW BPEI (1.8 kDa) with a hydrophobic lipid anchor, cholesterol chloroformate (Kim et al., 2007). The corresponding complex with siRNA was designed to inhibit human vascular endothelial growth factor (VEGF) expression and was investigated as a potential siRNA delivery vehicle. It readily formed nano-sized complexes (∼100 nm) with siRNA and protected siRNAs from enzymatic degradation in serum conditioned media. WSLP/siRNA complexes transfected in human prostate cancer (PC-3) cells derived from human prostate adenocarcinomas and inhibited the VEGF production significantly, while complexes of WSLP with control siRNA did not show this inhibitory effect. WSLP/siRNA complexes reduced the VEGF production by 40% when compared with unmodified BPEI. Moreover, WSLP/siRNA complexes reduced tumor volume by 55% at 21 days, and by 65% at 28 days when compared with controls. These results indicate that WSLP has potential as a siRNA delivering agent and can be applied for antiangiogenic tumor therapy.
In an attempt to fabricate multifunctional delivery system which could facilitate target specific treatment of tumor, Verma group studied the folate receptor (FR)-mediated delivery of dihydrofolate reductase (DHFR) siRNA to silence the DHFR gene in FR positive KB cells (Biswal et al., 2010). A DHFR siRNA sequence was cloned into a pSUPER-RNAi vector and complexed with the folate–polyethylene glycol–PEI (FOL–PEG–PEI) conjugate. They have carried out the complexation of DHFR siRNA expressing pDNA and FOL–PEG–PEI conjugate, and characterized the FOL–PEG–PEI/pSUPER-siDHFR complex by particle size analyzer, gel retardation, and DNase protection assay. The complex was transfected to FR overexpressing human epidermal carcinoma (KB) and FR negative human lung carcinoma (A549) cells. The transfection studies by fluorescence microscopy and RT-PCR showed that the complex delivered the siRNA vector and inhibited DHFR gene in KB cells; however, it remained unaffected when applied to A549 cells as control. Therefore, FR-mediated delivery of siDHFR complexed with FOL–PEG–PEI conjugate inhibits the DHFR expression in FR positive cells alone. The target specific delivery was further substantiated by the fact that lipofectamine-mediated transfection of pSUPER-siDHFR, delivered the vector and inhibited the DHFR gene in both KB and A549 cells. Target specificity was also confirmed by receptor blocking studies using free folic acid (FA). This strategy can be extended to a wide range of FR-targeted drug delivery and gene silencing therapeutics by siRNA expression pDNA.
There are several molecules such as galactose (Park et al., 2003; Kim et al., 2010b) and pullulan which can be utilized in liver targeted gene delivery owing to their preferential and high binding ability with asialoglycoprotein receptor. Yamaoka group has introduced pullulan into PEI for liver targeting (Kang et al., 2010). Pullulan is a water-soluble polysaccharide consisting of 3 α-1,4-linked glucose polymers with different α-1,6-glucosidic linkages. It is used for liver targeting because of its high affinity for the asialoglycoprotein receptor in the liver (Yamaoka et al., 1993; MEHVAR, 2003). They have developed a delivery system of pullulan-containing PEI/siRNA complexes for delivery into mice through the tail vein either by a hydrodynamics or nonhydrodynamics-based injection. During systemic injection, the PEI/fluorescein-labeled siRNA complex increased the level of fluorescence in the lung whereas PEI-pullulan/siRNA complex led to an increased fluorescence level in the liver. Further, an increase in N/P [the ratio of concentrations of total nitrogen atoms (N) of the polycation to the phosphate groups (P) of siRNA] ratio of PEI/siRNA complexes resulted in higher mice mortality but introduction of pullulan into PEI dramatically reduced mouse death after systemic injection. Therefore, PEI-pullulan polymeric conjugate provides a useful, low toxic approach to efficient delivery of siRNA into the liver.
Magnetic nanoparticles embedded within PEI derivatized polymeric material also gaining considerable interest in gene silencing. Park group developed pluronic/PEI shell cross-linked nanocapsules with embedded magnetite nanocrystals (PPMCs) to accomplish magnetically triggered delivery of siRNA (Lee et al., 2010). The PPMCs were positively charged and upon complexation formed stable nanosized polyelectrolyte complexes which were favorable for cell internalization. These complexes displayed enhanced intracellular uptake and silencing effect of siRNA in cancer cells under applied magnetic force. Pluronic, an amphiphilic tri-block copolymer consisting of poly-(ethylene oxide)–poly(propylene oxide)–poly(ethylene oxide) (PEO–PPO–PEO), is well-known for its biocompatibility (Kwon et al., 2009; Namgung et al., 2009b; Garripelli et al., 2010). Pluronic derivatives and oleic acid-coated iron oxide nanocrystals in an organic solvent were ultrasonically emulsified in an aqueous PEI solution. The cross-linking at emulsion interfaces resulted in the formation of a Pluronic/PEI composite shell layer encapsulating iron oxide nanocrystals in their hollow interior space. The cellular uptake of these polyelectrolyte complexes was remarkably increased upon exposure to an external magnetic field with a short incubation time. Further, GFP expression in cancer cells was effectively suppressed without encountering any severe cytotoxicity, as compared with BPEI (25 kDa).
Poly(dl-lactide-co-glycolide)
PLGA (Fig. 1) is a copolymer of glycolic acid (GA) and lactic acid (LA) which are linked together through ester linkages. PLGA undergoes bulk erosion through hydrolysis of the ester bonds to produce the original monomers, lactic acid and glycolic acid under normal physiological conditions and the rate of degradation depends on a variety of parameters including the LA/GA ratio, MW, and the shape and structure of the polymer matrix. PLGAs having different compositions of LA and GA have been commercially developed and are being investigated for a broad spectrum of biomedical applications. PLGA-based nanoparticles (NPs) have garnered tremendous attention as nonviral polymeric vectors in investigating delivery of nucleic acid owing to their small particle size, favorable safety profile, and sustained-release characteristics (Son and Kim, 2010). siRNA-loaded PLGA NPs have shown great promise in in vitro gene silencing (Yuan et al., 2006). Generally, 2 different approaches are used to load nucleic acids into PLGA NPs: (1) encapsulation into the core of NPs and (2) adsorption onto the surface of modified, cationic PLGA NPs via electrostatic interactions. Optimization of the encapsulation procedures has been continually developing the nucleic acid delivery systems endowed with sufficient loading, protection and controlled release profile. High stability, facile cellular uptake by endocytosis, targeting ability to specific tissues or organs by adsorption or binding with ligand at the surface of the PLGA NP, and biodegradability are the features that make polymeric PLGA NP an enticing entity in therapeutic fields. PLGA matrix can entrap siRNA to provide physical shielding against RNase activity and as it imparts favorable colloidal stability to the delivery formulation which facilitates safe, sustained, and tunable release profiles. The variation in molecular weight, the ratio of lactic acid to glycolic acid in the copolymers, and the structure of NPs enables the regulation of degradation time of PLGA which can be altered from days to years.
The susceptibility of RNA backbone to serum nuclease hydrolysis and transient silencing effects of siRNA makes protection and long-term sustain release the decisive features in siRNA delivery (Gary et al., 2007). Therefore, the initial challenge lies in accomplishing efficient encapsulation of hydrophilic siRNA into uniform nanosized PLGA particles which is hydrophobic in nature and further does not interact electrostatically with siRNA. Incorporation of cationic excipient to the PLGA matrix (Tahara et al., 2008) may elicit undue safety concerns in clinical application. Nielsen group fabricated siRNA-loaded PLGA NPs devoid of any cationic excipient and without compromising the siRNA integrity during the encapsulation (Cun et al., 2010). They carried out systematic investigation to establish the effects of important processes and formulation parameters on the encapsulation efficiency, particle size, and uniformity. They successfully incorporated siRNA into biodegradable PLGA NPs with high encapsulation efficiency using double emulsion solvent evaporation method. Generally siRNA is expected to be extremely vulnerable to the harsh conditions such as exposer to organic solvents, sonication and mechanical stress. However, the optimized process and formulation afforded siRNA-loaded PLGA NPs having spherical shape, smooth surface and a net negative surface charge and more importantly the intact siRNA preventing its degradation. By regulating the various formulations and processing parameters encapsulation efficiency up to 57% and particle diameter around 250 nm were obtained in the absence of any cationic excipients. However, the conventional PLGA formulation prepared by double emulsion solvent evaporation method is expected to have little practical applicability due to the low loading and encapsulation efficiency which could be attributed to the accumulative effects comprising of siRNA leakage owing to its small molecular weight, hydrophilic character, and the electrostatic repulsive forces developed between the phosphate backbone of siRNA and the anionic acid groups in PLGA polymers. Foged group prepared and evaluated various formulations and preparative processes of siRNA-loaded PLGA nanoparticles following design of experiments (Singh et al., 2005a, 2005b; Cun et al., 2011). The parameters under consideration for optimization were (1) the siRNA load, (2) the PLGA concentration, (3) the volume ratio between the inner water phase and the oil phase, (4) the sonication time for the primary emulsification and (5) the amount of acetylated bovine serum albumin (Ac-BSA) added to the inner water phase to stabilize the primary emulsion. They showed that PLGA concentration and the volume ratio played a crucial role in achieving encapsulation efficiency as high as 70%. High PLGA concentration increased the viscosity of the oil phase which induced enhanced encapsulation by stabilization of primary emulsion and arresting the siRNA leakage to the outer water phase. However, incorporation of Ac-BSA increased the encapsulation efficiency at low PLGA concentrations. The PLGA matrix protected siRNA against nuclease degradation, provided a burst release of surface-localized siRNA followed by a triphasic sustained release for 2 months.
Despite possessing excellent features like high stability, low toxicity and the controlled release of the cargo, PLGA NPs could not be applied efficiently in gene silencing due to the lower electrostatic interaction between PLGA and siRNA and lack of endosomal escape. The common strategy to circumvent the impediment involves the development of hybrid systems through incorporation of polycations into PLGA polymeric NP. In this context it could be worth-mentioning that another important advantage of using cationic excipients lies in enhancing the loading capacity by minimizing the siRNA leakage from the PLGA NP as mentioned earlier. Incorporation of cationic excipients certainly enhances the affinity between the siRNA and the PLGA matrix. To this end several cationic excipients such as DOTAP, PEI, or polyamine have been employed though not without the glitch of associated toxicity and undesired high retardation of siRNA release. Therefore, it is imperative to employ the optimal formulation and process to accomplish efficient encapsulation and sustained release of siRNA. The choice of polymers and the formulation should be such that the newly integrated attributes like better complexation and endosomal escape must not adversely affect the biocompatibility and stability of the delivery system.
Several research groups are engaged in improving the loading efficiency and cellular uptake of the siRNA by modifying the surface of the PLGA nanoparticles with cationic coating materials, which bind negatively charged therapeutic genes through electrostatic interactions (Schiffelers et al., 2004; HART, 2010; Xing et al., 2010). Wang et al. fabricated 4 types of biodegradable NPs as the vector for siRNA delivery system (Wang et al., 2010). The delivery systems were composed of PLGA and methoxy poly (ethylene glycol)–poly (lactide) (mPEG–PLA) and cationic materials such as chitosan and PEI for surface coating. PEI and chitosan served as the surfactants due to their cationic property and the permeability enhancing properties (Hombach and Bernkop-Schnürch, 2009). The comparative evaluation of the 4 NPs with respect to their interaction with siRNA and the induced siRNA transfection as measured by the inhibition effect of HBsAg (a surface antigen of the hepatitis B virus, which indicates current hepatitis B infection) expression, clearly demonstrated that the siRNA delivery followed a size and surface charge dependant manner and mPEG–PLA/PEI NPs with the smallest size showed highest transfection efficiency.
Recently, Howard group has optimized the delivery of siRNA by controlling the level and location of functional PEI in PLGA (Andersen et al., 2010). The work demonstrated rapid and efficient method to functionalize the surface of PLGA NPs during an emulsification-diffusion production method by using a cetyl derivative to anchor PEI into the matrix of PLGA particles. The binding of cetyl group to the matrix involved physical entrapment as well as hydrophobic interaction between the cetyl group and the PLGA. Such anchoring increased the zeta potential, siRNA loading, and 2-fold increase of the surface content of PEI in comparison with noncetylated-PEI. Specific knockdown of 53% and 46% was observed for oncogene BCL-w in osteosarcoma cells using cetylated-PEI/PLGA and noncetylated-PEI/PLGA particles, respectively. In addition cetylated-PEI/PLGA particles achieved 64% silencing of inflammatory cytokine TNFα in J774.1 cells.
Alshamsan et al. have demonstrated enhanced silencing potency of STAT3 siRNA and increased anticancer activity in B16 melanoma cells by siRNA and stearic acid (StA)-modified PEI complexes but not without inducing an antitumor immune response (Alshamsan et al., 2010). However, PLGA-based delivery system having StA molecules attached to PEI backbone (PEI-StA) can protect siRNA complexes against degradation in serum (Alshamsan et al., 2009). The incorporation of siRNA polyplexes into PLGA NPs improves the toxicity profile of PEI and enhances cellular uptake (Zhang et al., 2008). The physical encapsulation of siRNA/PEI and siRNA/PEI-StA polyplexes in PLGA nanoparticles (NPs) afforded encapsulation efficiency of 26% and 43%, respectively. Moreover, encapsulation in PLGA NPs significantly reduced PEI-associated toxicity on dendritic cells (DCs) (Alshamsan et al., 2010). Such strategy in formulating efficient vectors could lead to targeted siRNA delivery to DCs. The potential of this approach is not limited to STAT3 downregulation in DCs but can be used to target the expression of other proteins as well.
The common ploy to enhance the biocompatibility as well as the transfection of PEI (apart from hybrid gene delivery system consisting of a combination of cationic lipid or polymer with PEI) involves incorporation of PEI into the polymeric particles either by encapsulating it within the polymer or by coating the surface of the polymer-based carriers with PEI. Such delivery systems not only reduce the PEI toxicity, but also increase bioavailability and drug loading efficiency and further, the structural integrity of nucleic acids adsorbed on the preformed cationic particles could be preserved during the harsh preparative condition. PEI was incorporated into the PLGA particles by spontaneous modified emulsification diffusion method (Katas et al., 2009). Incorporation of PEI into PLGA particles with the PLGA to PEI weight ratio 29:1 gave rise to spherical and positively charged nanoparticles. Interestingly particle size of around 100 nm was obtained when 5% PVA (m/v) was used as a stabilizer. PLGA–PEI nanoparticles were shown to bind siRNA completely at N/P ratio 20:1 and thus provided protection for siRNA against nuclease degradation. The in vitro transfection studies showed that the ability of PLGA–PEI to transfect siRNA in cells was dependent on N/P ratio and cell line tested as cell lines differ widely with respect to their individual characteristics and cellular surface components which might affect cellular binding and uptake of these particles into cells.
Chitosan
For decades chitosan has been drawing immense application in diversified areas especially in pharmaceutics. The versatility of chitosan in its native or functionalized form stems from its high natural abundance, nonallergenicity, biocompatibility, and biodegradability. In addition to these favorable features the cationic property makes chitosan derivatives a potent vector for nucleic acids. Chitosan (Fig. 1) is a copolymer of N-acetyl-D-glucosamine (GlcNAc) and D-glucosamine (GlcN) and acts a weak base owing to the presence of the D-glucosamine residue having a pKa value of about 6.2–7.0. The protonation of the primary amines attached to the repeating glucosamine units imparts positive charge on the chitosan backbone at the pH below its pKa. The positively charged chitosan is exploited to bind and form polyplexes with pDNA or siRNA having negatively charged phosphate backbone.
Though chitosan-mediated pDNA delivery is prevalent in the literature, its application in siRNA delivery is yet to gain the due momentum (Mao et al., 2010). To improve the efficiency of RNA transfer with chitosan, several attempts on chitosan modification have been made over the past few years. Opanasopit group investigated in vitro siRNA delivery into stable and constitutive enhanced green fluorescent protein (EGFP)-expressing HeLa cells using various siRNA/chitosan (CS) salt complexes formulated with chitosan aspartate (CS-Asp), chitosan glutamate (CS-Glu), chitosan acetate (CS-Ac), and chitosan hydrochloride (CS-HCl). The thorough investigation revealed that the formation of chitosan/siRNA complexes was mainly affected by the weight ratio. The MW and the nature of salt did not influence the complexation significantly. The high gene-silencing efficiency was observed with LMW chitosan (20 kDa) and high weight ratio of 32. Over 80% average cell viabilities were observed for chitosan/siRNA complexes in all weight ratios in comparison to untreated cells. The same group used hydroxybenzotriazole as the acid and the resulting chitosan hydroxybenzotriazole salt (CS-HOBT) was evaluated (Opanasopit et al., 2010) for the in vitro delivery of siRNA. The study investigated the transfection efficiency of CS-HOBT for in vitro nucleic acid delivery and its dependency on the weight ratio and MW of CS. Agarose gel electrophoresis demonstrated complete complexation between CS-HOBT and siRNA, were formed at a weight ratio of above 4 and 2 (MW of chitosans are 45 kDa and 460 kDa). The particle sizes of CS-HOBT/siRNA complexes were in nanosize range. Maximum 60% silencing of the enhanced green fluorescent protein gene in human hepatocarcinoma cell lines (HepG2 cells) was observed for CS-HOBT/siRNA polyplex with the lowest CS MW of 20 kDa at a weight ratio of 80.
Similarly thiamine pyrophosphate (TPP), a water soluble vitamin, can also form salt with the amine group of chitosan through its phosphate groups which improves the water solubility of chitosan. Further, the amine groups of TPP, particularly the thaizolium moiety which remains protonated even at physiological pH, facilitate the binding with negatively charged siRNA. The enhanced solubility and condensation with siRNA play an important role in enhancing the overall transfection efficiency. Chitosan–thiamine pyrophosphate (CS-TPP) demonstrated higher gene silencing effect (70–73%) than that exerted by Lipofectamine 2000™ (61%) on the endogenous enhanced green fluorescent protein (EGFP) gene (Rojanarata et al., 2008).
In an another approach to circumvent the inherent difficulty of low solubility and inefficient endosomal buffering in chitosan mediated siRNA, Ghogn et al. (2008) modified chitosan with imidazole acetic acid (IAA) to introduce secondary and tertiary amines which facilitated the endosomal escape and enhanced solubility. The polymeric vector chitosan-IAA registered a 100-fold transfection for pDNA in comparison to native chitosan without compromising the cyto-compatibility of the polymer. Likewise the vector displayed efficient in vitro siRNA delivery as evidenced by efficient gene knockdown comparable to that obtained by commercially available siPORT amines (Ghosn et al., 2010). In their continued effort to develop more refined delivery system the group had extended the investigation to address dose dependency of the therapeutic load during systemic delivery. At high concentration owing to the increased viscosity the formulations tend to undergo aggregation which severely limits the application of such delivery system in in vivo application The developed PEGylated imidazole-modified chitosan (chitosan-imidazole-4-acetic acid [IAA])-siRNA nanoparticles showed efficient gene silencing after administration via either intravenous or intranasal routes. The delivery systems during intravenous delivery demonstrated significant knockdown of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) enzyme in both lung and liver at as low as 1 mg/kg siRNA dose. The delivery system was also efficient in dose-dependent silencing of apolipoprotein B in the liver. However, further optimization of such formulations is necessary with respect to its biodistribution, and safety profile for organ-specific in vivo delivery of siRNA.
Howard et al. have demonstrated the applicability of chitosan in overcoming the poor cellular uptake and rapid degradation of naked siRNA or miRNA both in vitro and in vivo. They were able to significantly reduce the number of enhanced green fluorescence protein (EGFP)-expressing epithelial cells in the bronchiole (43% and 37% reduction compared with the untreated and mismatch control, respectively) of mice via daily nasal administration of interpolyelectrolyte siRNA/chitosan complexes (Howard et al., 2006). The observations also corroborated the earlier report which enumerated that chitosan polyplexes could adhere to the epithelial cells lining the trachea and bronchioles of the mid-airway, and have high efficiency of transfection in those regions after their intratracheal administration to mice in vivo (Koping-Hoggard et al., 2001).
In aqueous medium amphiphilic α-tocopherol oligochitosan, obtained by conjugating α-tocopherol succinate to water soluble oligochitosans (having various molecular weights), underwent self-assembly to form single layered tocopherol-oligochitosan-based oligomersomes (TCOsomes) (Noh et al., 2011). Cryo-TEM images showed clear thickening in the unilamellar layer of TCOsomes upon complexation with siRNAs. TCOsomes self-assembled from tocopherol-oligochitosan 4K (TCOsome4K) significantly enhanced the cellular uptake of siRNAs (>98%), and reduced the expression of target proteins more effectively than Lipofectamine 2000. In tumor xenografted mice, the intratumoral administration of siMcl-1 using TCOsomes substantially silenced the expression of Mcl-1 and prevented the growth of tumor.
Delivery of chitosan/siRNA nanoparticle aerosols into the lungs of mice offered a simple noninvasive method for improved deposition of exact siRNA dosage (Nielsen et al., 2010). The ability of the aerosolized chitosan/siRNA nanoparticles to silence genes at pulmonary sites could encourage its application in pulmonary RNAi-based therapies.
Utilizing the improved and selective binding of cyclic Arg-Gly-Asp (RGD) peptide with ανβ3 integrin, which is overexpressed in a wide range of tumors, RGD containing polymeric formulation are widely used in tumor targeted gene delivery (Kim et al., 2005, 2006a). RGD labeled chitosan nanoparticles was fabricated for targeted silencing of multiple growth-promoting genes (Han et al., 2010). The RGD and chitosan were conjugated by thiolation reaction using N-succinimidyl 3-(2-pyridyldithio)-propionate. siRNA loaded RGD-chitosan polyplexes were efficiently and selectively delivered to ovarian cancer cells in orthotopic animal models.
Cyclodextrin
Cyclodextrins (CD) are naturally occurring cyclic oligosaccharides composed of 6 (α-CD), 7 (β-CD), or 8 (γ-CD) D(+)-glucose units linked by α-1,4-linkages. The reported CD derivatives in this review are β-CDs (Fig. 1) and hence for convenience denoted as CD only. CD containing polymers (CDP) have drawn considerable attention in targeted delivery of therapeutic loads due to its lack of immunogenicity. Moreover CD possesses unique geometric and structural features. The hydrophilic outer surface and hydrophobic inner cavity enable them to fit insoluble molecules to form inclusion complexes. Though the use of CDP is prevalent in pDNA delivery (Gonzalez et al., 1999), it has not been explored extensive in siRNA delivery. Utilizing the special structural characteristics of CD Davis and his coworkers (Bartlett and Davis, 2007) have developed synthetic delivery system based on a CDP that has demonstrated some success in delivering nucleic acid payloads that include pDNA, siRNA, and DNAzymes. Their strategy was evolved around the formation of inclusion complexes between adamantane (AD)-containing molecules and the β-CD molecules. The modular attachment of AD-PEG conjugates provides steric stabilization and further installation of targeting ligands (AD-PEG-transferrin) imparts cell-specific targeting. Such formulation displayed high efficiency in delivering the siRNA against oncogene to inactivate the tumor growth in a murine model of metastatic Ewing's sarcoma (Hu-Lieskovan et al., 2005).
The CD molecule can potentially be functionalized directly with various functional entities such as polycationic, amphiphilic pendants, PEG chains, and targeting ligands (Sallas and Darcy, 2008). Physicochemical and biological characterization of CDP and their nanoparticles formed with siRNA were investigated elaborately (Bartlett and Davis, 2007). The particles protect the nucleic acid payload from nuclease degradation and they do not aggregate at physiological salt concentrations, and cause minimal erythrocyte aggregation and complement fixation at the concentrations typically used for in vivo application. Therefore, in addition to low immunogenicity such approaches provide multitude of opportunities to incorporate various functionalities to address variable requirements in a range of therapeutic applications. However, one of the major obstacles encountered by CDP is their inability to escape endosomal pathway (Gonzalez et al., 1999; Kulkarni et al., 2005). CDPs modified with imidazole groups demonstrated enhanced intracellular delivery overcoming the degradative endosomal entrapments. The imidazole-containing CDP (CDPim) is known to buffer the pH inside endosomal vesicles, giving rise to increased intracellular delivery compared with unmodified CDPs (Kulkarni et al., 2005).
Another very interesting strategy which does not require any endosomolytic agent but is endowed with excellent potential in directed delivery of siRNA, involves the utilization of photochemical internalization (PCI) technology. PCI technology is pivoted around the preferential localization of photosensitizers (PSs) at the endosomal and lysosomal membranes. Illumination of these PSs triggers the photochemical damage of the membrane followed by release of endosomal and lysosomal content. Importantly it is also capable of producing light-directed delivery of siRNA into the tissue of interest. Bøe et al. (2010) demonstrated the utilization of PCI-mediated light-directed delivery of CDP–siRNA complex. Optimization of carrier/cargo ratio and illumination dose provided 80% and 90% silencing in the siRNA samples treated with PCI compared with untreated control. Further, time-lapse studies revealed maximum gene silencing at 5 hours after endosomal release substantiating rapid carrier decondensation for the CDP.
Polypeptides
Polypeptides are very common nucleic acid delivery components due to their less cytotoxic nature. However, their condensation ability is found to be less pronounced and release of nucleic acid payload at the cytosol becomes a major obstacle. To counter these shortcomings several functional and structural modifications are generally carried out. A cationic poly aspartamide derivative, poly{N-[N-(2-aminoethyl)-2-aminoethyl] aspartamide} (PAsp(DET)), possesses pH-sensitive endosome destabilizing activity (Miyata et al., 2008). PAsp(DET) can destabilize the cellular membrane only at the endosomal pH, probably due to the formation of the di-protonated form of 1,2-diaminoethane moiety in the PAsp(DET) side chain at endosomal pH and therefore PAsp(DET)-based polyplexes are able to demonstrate high transfection for pDNA without eliciting significant cytotoxicity. However, such polyplexes fails to show similar efficiency during siRNA delivery due to the instability of the polymer/siRNA complexes under physiological conditions. The lower complexation between siRNA and cationic polymers compared with pDNA/polycation arises due to the short and rigid structure of siRNA (De Smedt et al., 2000). Therefore, to negotiate the instability an additional associative force namely favorable hydrophobic interaction has usually been induced.
Kataoka group (Kim et al., 2010c) introduced stearoyl groups as a hydrophobic moiety to PAsp(DET) side chain and was able to achieve significantly enhanced cellular uptake of siRNA and improved in vitro RNAi activity. The developed stearoyl PAsp(DET)/PAsp(DET)–ST19/siRNA complexes formed at N/P of 7.5–10 displayed the optimal RNAi activity with low cytotoxicity. The complexes exhibited significantly higher efficiency in knocking down endogenous gene (BCL-2 and VEGF) against the human pancreatic adenocarcinoma (Panc-1) cells in vitro in comparison to unmodified PAsp(DET) complex and commercially available reagent. This finding suggests that the hydrophobic PAsp(DET)-mediated siRNA delivery is a promising platform for in vivo siRNA delivery.
Cell penetrating (or membrane translocational) peptides (CPP) could constitute a very promising and potent candidate for systemic siRNA delivery considering their efficacy in delivering various therapeutic cargos like pDNA and other biomolecules (Johansson et al., 2011). Kim group conjugated the cholesterol moiety and cationic cell-penetrating peptides oligo-D-arginine to develop hydrophobically modified protein transduction domain, cholesteryl oligo-D-arginine (Chol-R9) (Kim et al., 2006b). The efficient regression of a subcutaneous tumor in a mouse model upon local administration of complexed VEGF-targeting siRNA could be attributed to the enhanced cellular uptake and stability of Chol-R9/siRNA complexes as induced by the cationic cell-penetrating peptides and the cholesterol moiety, respectively.
In an interesting strategy to deliver siRNA, Harashima group (Sato et al., 2010) employed a multifunctional envelope-type nano device (MEND) which consists of a core containing complex of siRNA and cationic polymer stearyl octaarginine (STR-R8). Though no silencing effect has been observed for condensed siRNA particles without liposome, the MEND-encapsulated siRNA core showed a significant silencing effect which could be attributed to macropinocytosis-mediated internalization and the protection against lysosomal degradation. Harashima group also studied the comparative gene silencing activity of STR-R8 along with various polypeptides containing random sequence of basic amino acids polycations (Nakamura et al., 2007). The MEND was transfected into HeLa cells stably expressing luciferase and the silencing activity of the polycations was compared. The polypeptide containing ornithine and tryptophan (Orn/Trp) induced a higher knockdown than STR-R8. In addition, Orn/Trp induced a silencing effect at lower doses than STR-R8, as evidenced by dose–response data. The difference in silencing activities could arise due to the difference in the abilities of the polycations to release siRNA from the core particles. It is possible that a minor difference in structure around the amine group which generates the cationic charge, alters the rate of siRNA release. The investigation suggested that Orn/Trp was a superior polycation to STR-R8 for siRNA delivery.
Generally polypeptides lack endosomolytic property and the effective strategy to achieve release of the siRNA at the cytosol is to incorporate the histidine residue in the polypeptide backbone. Polylysine partially substituted with histidyl residues is known to demonstrate significant enhancement in pDNA transfection compared with polylysin alone (Midoux and Monsigny, 1999). At pH below 6.0 the imidazole groups of histidyl residues become protonated, thus confer a PEI-like proton sponge activity to trigger endosomal escape.
Leng et al. designed branched histidine-lysine (HK) peptides for the delivery of siRNAs and showed that H3K8b/siRNA polyplex inhibited beta-galactosidase expression by more than 80% in an endothelial cell line (SVR-bag4) (Leng et al., 2005). The work demonstrated that the histidine-rich domain and the terminal arms of H3K8 were crucial for siRNA delivery.
Bérangère Langlet-Bertin et al. (2010) developed short linear cationic amphipathic histidine-rich peptide LAH4 (KKALLALALHHLAHLALHLALALKKA) and its derivatives possessing high endosomal disruption ability and investigated their efficiency in delivering siRNA into mammalian cells. All the peptides efficiently delivered small dsRNA duplexes into human embryonic retinoblasts (911 cell line) and displayed efficient knock-down of gene expression. The operation of endosomolytic activity in regulating the transfection efficiency was confirmed by bafilomycin A1-mediated inhibition assay. Further, the increased efficiency may be related to the higher capacity of the peptide to favor association of the siRNA with the cells.
Dendrimers
Dendrimers are regular and highly branched monodisperse and usually highly symmetric, spherical synthetic macromolecules having tunable structure, molecular size, and surface charge. The unique structural features such as high chemical and structural homogeneity, high ligand, and functionality density enable them to load therapeutics by interior encapsulation, surface adsorption, or chemical conjugation. Polycationic dendrimers such as poly(amidoamine) (PAMAM) and poly(propylenimine) (PPI) dendrimers have been extensively studied as efficient vehicles for the delivery of genes and therapeutic drugs; however, there are only few reports describing their potential in siRNA delivery.
Polycationic PAMAM dendrimers bear primary amine groups on their surface, while having tertiary amine groups inside. The primary amine groups facilitates nucleic acid binding, their compaction into nanoscale particles and subsequent cellular uptake, while the tertiary amino groups trigger proton sponge in endosomes and enhance the release of nucleic acids into the cytoplasm. PAMAM dendrimers or their conjugates are more efficient in delivering antisense oligonucleotides or pDNA than delivering siRNA. Peng group developed flexible polycationic PAMAM dendrimers for siRNA delivery (Zhou et al., 2006). Triethanolamine was used as the core of the dendrimers and the branching units started 10 successive bonds away from the central nitrogen resulting in less densely packed branching units and end groups than that of the commercially available PAMAM dendrimers. Higher-generation dendrimers having structural features efficiently delivered siRNA and thus induced gene silencing in cell culture. The complexation ability of the dendrimer with siRNA increased with the increase in generation number, due to the stronger interactions occurring between dendrimer and siRNA. The formation of stronger siRNA/dendrimer complexes at neutral pH and the efficient internalization of the siRNA molecules into the cytoplasm at higher N/P ratio led to greater gene silencing efficiency presumably due to the dendrimer G7 (7: dendrimer generation number) mediated buffering of the endosomal cavity. The best gene silencing (ca. 80%) were observed with G7 at an N/P ratio of 10–20 and siRNA concentration of 100 nM.
Structurally flexible PAMAM dendrimers G7 having triethanolamine core were also effective in delivering heat-shock protein 27 (Hsp27) siRNA into human prostate cancer (PC-3) cells, leading to specific silencing of the hsp27 gene and a pronounced caspase-dependent apoptosis-induced anticancer effect (Liu et al., 2009).
To encounter the cytotoxicity of PAMAM-NH2 dendrimers Minko group modified the surface functionality and synthesized PAMAM-OH and PAMAM-NHAc dendrimers (Patil et al., 2008). They further installed quaternary cationic charges. The internally quaternized and surface-neutral dendrimers QPAMAM-OH and QPAMAM-NHAc demonstrated efficient complexation with siRNA and enhanced siRNA protection due to the formation of highly organized compact nanoparticles. Surface modified QPAMAM-OH and QPAMAM-NHAc dendrimers displayed significantly reduced cytotoxicity as nonspecific nanocarrier-cell interactions could be prevented in the absence of surface amine groups. However, only QPAMAM-NHAc dendrimers displayed significant cellular uptake in A2780 ovarian cancer cells. Therefore, it was proposed that apart from the reduced nonspecific interactions between dendrimer and cell membrane, the amide groups on the surface of the dendrimer might also play a crucial role by facilitating hydrogen bonding with cell membranes to enhance the cellular uptake of the complex.
However, to improve the siRNA delivery efficiency using the QPAMAM-OH dendrimer, the same group adopted 2 independent strategies (Patil et al., 2009). They evaluated the impact of introduction of a synthetic analog of Luteinizing hormone-releasing hormone (LHRH) targeting peptide to the QPAMAMOH dendrimer which was expected to enhance the intracellular delivery of siRNA by a receptor mediated endocytosis pathway. The other approach stemmed from the logical argument that almost complete quaternization of tertiary amine groups in a QPAMAM-OH dendrimer could hamper the proton sponge effect resulting in low siRNA uptake. However, despite the decrease in the degree of quaternization (75%) no significant enhancement in cellular uptake of the QPAMAMOH-siRNA complex had been achieved in terms of gene silencing.
However, targeted QPAMAM-OH-LHRH-siRNA complex significantly suppressed the expression of the BCL2 gene in cancer cells. The observations imply that degree of quaternization to some extent is important for the cellular uptake but not sufficient to achieve a gene silencing effect.
There are also few reports which illustrates poly(propylenimine) (PPI) dendrimers as cationic dendrimer in siRNA delivery (Taratula et al., 2011). Taratula et al. demonstrated that the higher generations of PPI dendrimers (G4 and G5) were more effective in forming discrete nanoparticles upon complexes with siRNA in comparison to lower generations of dendrimers (G2 and G3). Larger size and higher positive charge density of G5 dendrimer elicited higher toxicity whereas G4 dendrimer displayed maximum efficacy with respect to siRNA nanoparticles formation, intracellular siRNA internalization, and sequence specific gene silencing. The formulated siRNA–PPI dendrimer complexes exhibited dramatic enhancement in siRNA cellular internalization and the marked knockdown of targeted mRNA expression in A549 human lung cancer cells.
The same group also formulated PPI dendrimer based siRNA delivery systems through layer-by-layer modification approach included caging of siRNA-PPI (generation 5) dendriplex with a dithiol containing cross-linker molecules followed by coating with PEG polymer (Taratula et al., 2009). A synthetic analog of Luteinizing Hormone-Releasing Hormone (LHRH) peptide was conjugated to the distal end of PEG polymer to achieve targeted delivery of siRNA nanoparticles specifically to the cancer cells. The integrated attributes were effective in imparting serum resistance, increased stability in biological liquids, tumor-specific targeting, effective penetration into cancer cells, and accumulation of delivered siRNA in the cytoplasm while preserving the gene silencing ability.
In an interesting and novel approach Chen et al. efficiently packaged and deliver siRNA with low generation (G3) PPI dendrimers by using gold nanoparticles (Au NPs) as a “labile catalytic” packaging agent (Chen et al., 2010). The Au NPs facilitated low generation dendrimers to package siRNA into discrete nanoparticles but more importantly, the Au NPs could be selectively removed from the siRNA complex solution without influencing the integrity of the siRNA complexes. Moreover, G3 PPI modified with Au NPs could be internalized into cancer cells, and the delivered siRNAs can efficiently silence their target mRNA. The efficiency of mRNA silencing by this approach was even superior to higher generation dendrimers (G5 PPI).
Nanogel
Nanogels are nanosized, colloidally stable hydrogel particles composed of chemically or physically cross-linked polymer networks. High water contents, high loading capacity, high stability, biocompatibilibty, and protection of loaded biomolecule drugs including siRNA make nanogels potential carrier systems for the delivery of therapeutic cargos than marcroscopic hydrogels. Further, nanogels can be internalized by cells and transfer siRNA into the cytosol where siRNA-mediated gene silencing takes place. Therefore, nanogels are promising as siRNA delivery carriers (Vinogradov et al., 2002; Kabanov and Vinogradov, 2009; Oh et al., 2009). Vinofradov et al. developed the cationic nanogels consisting of cross-linked PEG-PEI chains, PEG-cl-PEI (Vinogradov et al., 1999) which bound and encapsulated negatively charged siRNA spontaneously through ionic interactions. Despite the stability of the siRNA-loaded PEG-cl-PEI nanogels in aqueous dispersion imparted by the PEG chains, this first generation nanogel exhibited insufficient efficacy in siRNA delivery to breast microvascular endothelial cells. Tamura et al. developed PEGylated polyamine nanogels which contained a cross-linked poly[2-(N,N-diethylaminoethyl) methacrylate] (PDEAMA) gel core and PEGylated shell (Tamura et al., 2009). The PDEAMA core triggered endosomal escape of nanogel through its buffering capacity. This intelligent PEGylated polyamine nanogel/siRNA complex exhibited higher stability against polyanion exchange reactions compared with noncross-linked counterpart. Further, the nanogel/siRNA complex showed remarkable enhancement of sequence-specific gene silencing activities in human hepatocarcinoma cells (HuH-7 cells) at low N/P ratio (N/P = 2). However, to improve the binding ability of nanogel with siRNA and the stability of the complex quaternary ammonium groups were introduced in the polyamine gel core (Tamura et al., 2010). The quaternized polyamine nanogel (degree of quaternization = 10%) enhanced the endogenous gene silencing activity against the survivin gene in HuH-7 cells.
Biodegradability remained an important factor in promoting cationic nanogel based systems for siRNA delivery. Raemdonck et al. reported the development of biodegradable cationic dextran nanogels (dex-HEMA-co-TMAEMA) through UV light-mediated radical polymerization process within the internal aqueous dextran-hydroxyethyl methacrylate (dex-HEMA) phase, supplemented with [2-(methacryloyloxy)ethyl]-trimethyl-ammonium chloride (TMAEMA) and photoinitiator (Raemdonck et al., 2009). The nanogels could load siRNA based on electrostatic interaction and the loading capacity of the gel was significantly high. The siRNA-loaded nanogels were internalized in HuH-7 cells without relevant cytotoxicity due to the degradation of the network by hydrolysis of carbonate ester linkage in HEMA group. Incorporation of a photosensitizer (PS, mesotetraphenylporphine disulfonate, TPPS2a) and an influenza-derived fusogenic peptide (diINF-7) into nanogel facilitated the enhancement of silencing efficacy of siRNA-loaded nanogels. The accumulation of nanogel degradation products in the endosomal lumen induced the disruption of the vesicular membrane, possibly through an osmotic effect, thereby released active siRNA into the cytosol. Dex-HEMA-co-TMAEMA nanogel-mediated sustained gene silencing was also achieved by internalization of PS in the nanogel (Raemdonck et al., 2010). For prolonging gene silencing, PS activation was applied at different time-points after transfecting with siRNA-nanogel on cells. The siRNA-loaded dex-nanogel acted as a depot of siRNA and remained inside intracellular vesicles. The irradiation of light for 6 days post-transfection substantially prolonged the gene silencing in cells transfected with the dex-HEMA nanogels, maintaining 50% gene knockdown up to 2 weeks in cancer cells.
The large surface area of nanogels is conducive to functionalization to achieve targeted siRNA delivery. Blackburn et al. prepared a peptide-functionalized core/shell nanogel for cancer cell specific siRNA delivery (Blackburn et al., 2009). This core/shell nanogel was composed of cross-linked poly(N-isopropylmethacrylamide) (pNIPMAm), an amphiphilic, thermoresponsive polymer that was strongly hydrated at physiological temperature. The loaded siRNA was coated with a porous hydrogel shell which possessed ligation sites for attaching the appropriate targeting moieties. To achieve targeted siRNA delivery for cancer, the core/shell pNIPMAm nanogel was chemically conjugated with 12-amino acid peptides (YSAYPDSVPMMS or YSA), which binds to the erythropoietin-producing hepatocelluar A2 receptor (EphA2) that specifically expressed in a number of tumors (eg, ovarian, prostate, breast, and colon cancers). Such observations suggested that targeted delivery of siRNAs by nanogels might be a promising strategy to enhance the efficacy of chemotherapy drugs and simultaneously to reduce the related side effect for the treatment of ovarian and other cancers.
Nanomaterial-Mediated siRNA Delivery Vectors
Nanomaterials such as gold nanoparticles, carbon nanotube, and silica nanoparticles attracted considerable attention in their pharmaceutical applications due to their biocompatibility and ability to facilitate the delivery of therapeutic cargos. So the fusion of nanomaterials and polymer has the potential and was applied to gene and siRNA delivery.
Gold nanoparticles
Gold nanoparticles (AuNPs) have emerged as a promising siRNA delivery carrier due to easy synthesis, well-defined surface chemistry for facile modification, and good biocompatibility (Ghosh et al., 2008). The conventional strategy employs immobilization of siRNA on the AuNPs surface to develop (Giljohann et al., 2009) siRNA–AuNPs conjugates possessing greater half-life in serum condition and prolonged gene knockdown ability. Lee et al. have conjugated AuNPs to siRNA via biodegradable disulfide linkages and coated these particle with poly(β-amino ester)s (PBAEs) (Lee et al., 2009) and further incorporation of cationic polymer in AuNPs improved the gene knockdown efficacy. Self-assembled layer-by-layer (LbL) system namely PEI/siRNA/PEI-AuNPs was developed utilizing mercaptoundecanoic acid (MUA). The delivery system reduced the EGFP production to about 28% in CHO-K1 cells (Elbakry et al., 2009). The high stability of LbL-coated AuNPs retarded the release of siRNA in cytoplasm. To overcome the impediment a charge-reversal copolymers such as cis-aconitic anhydride-functionalized poly(allylamine) (PAH-Cit) was integrated into the polymer-coated AuNPs by LbL technique. The copolymers could undergo charge conversion at endosomal or lysosomal (pH 5–6) compartments triggering endosomal disruption and subsequent siRNA release (Lee et al., 2008; Miyata et al., 2008). However, Song et al. resorted to a simpler approach to fabricate a siRNA delivery system using PEI-capped AuNPs (Song et al., 2010) and achieved efficient gene silencing in MDA-MB-435s cells, targeting both exogenous GFP and endogenous PLK1 genes.
Mesoporous silica nanoparticles
Though mesoporous silica nanoparticles (MSNs) are relatively new entrant in the landscape of siRNA delivery some of their unique structural and functional properties such as chemically stable mesoporous structures, large surface areas, tunable pore sizes, encapsulation of small molecules, and well-defined surface chemistry, have made them highly captivating delivery system. Moreover biocompatibility (Trewyn et al., 2007; Hom et al., 2009), facile synthesis maneuvering the sizes, shapes and surface functionalities enhance their potential immensely. The first report of MSNs-mediated siRNA delivery involved the incorporation of PEI to the surface of MSN through noncovalent adsorption (Xia et al., 2009). The MSN-PEI/siRNAs coated with the 10 kDa and 25 kDa PEI were capable of knocking down of GFP level by 55% and 60%, respectively, in GFP expressing HEPA-1 cells. Use of low molecular weight PEI (1.2 kDa) in PEI-MSN formulation can silence both exogenous and endogenous genes with reduced cytotoxicity (Perriman et al., 2010). Favorable binding of siRNA at the surface and facilitated encapsulation of small drugs within the porous channels having high loading capacity make MSN very useful carrier particularly for drug/siRNA combination delivery. PEI-functionalized MSN based Doxorubicin/siRNA codelivery system induces apoptosis of drug-resistant cancer cell line (KB-V1 cells) through synergistic method (Meng et al., 2010).
Carbon nanotubes
Another recent development in nanoparticle-mediated siRNA delivery is the incorporation of carbon nanotubes (CNTs) (Lacerda et al., 2006; Cheung et al., 2010) into the polymeric composite system due to chemical inertness, easy surface functionalization, and unique structure of CNTs. Inherent hydrophobicity of pristine CNT necessitates functionalization of CNT to impart water solubility and biocompatibility. The potential strategies for such functionalization could include either noncovalent coating by amphiphilic polymer on CNTs or introduction of cationic moieties through covalent reactions. Noncovalent adsorption of phospholipid conjugated PEG (PL-PEG), an amphiphilic polymer, to single walled nanotubes (SWNTs) imparts the desired solubility in water (Kam et al., 2005). The subsequent conjugation of polymer-coated SWNTs with siRNA through a cleavable disulfide bond using bifunctional cross-linker yielded an efficient delivery system as evidenced by the high gene silencing efficacy in mammalian cells. Similar SWNT-siRNA conjugates were efficiently transported to human T cells and primary cells, which were known to be inert to commercially available liposome-based nonviral vector and silenced specific gene in those cells (Liu et al., 2007). Same polymer coated SWNT conjugated to canonical transient receptor potential 3 (TRPC3) gene knockdown siRNA achieved efficient silencing of TRPC3 gene in extracted mouse skeletal muscle cells ex vivo (Lanner et al., 2009).
The capacity of polymer conjugated CNT to deliver siRNA into cells has not been explored till date. However, there are reports which demonstrate successful utilization of polyamidoamine (PAMAM) dendrons conjugated MWNTs in accomplishing efficient gene silencing (Herrero et al., 2009; Al-Jamal et al., 2010). Therefore, huge potential of polymer conjugated CNT has remained unexplored but expected to explode sooner or later.
Conclusion
Polymer-mediated siRNA delivery has several advantages such as facile chemical modifications, good biocompatibility, multifunctionality, fusion with inorganic materials which can address various impediments associated with efficient siRNA delivery. Integration of various functionalities into polymeric siRNA delivery systems could have profound impacts on biomedical research and promise to revolutionize the wider spectrum of therapeutic field in curing many debilitating afflictions of mankind.
Acknowledgment
This study was supported by the grants of the Korea Healthcare Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (A080919), Republic of Korea.
Author Disclosure Statement
No competing financial interests exist.
References
- AL-JAMAL K.T. TOMA F.M. YILMAZER A. ALI-BOUCETTA H. NUNES A. HERRERO M.A. TIAN B. EDDAOUDI A. AL-JAMAL W.T. BIANCO A. PRATO M. KOSTARELO K. Enhanced cellular internalization and gene silencing with a series of cationic dendron-multiwalled carbon nanotube:siRNA complexes. FASEB J. 2010;24:4354–4365. doi: 10.1096/fj.09-141036. [DOI] [PubMed] [Google Scholar]
- ALSHAMSAN A. HADDADI A. HAMDY S. SAMUEL J. EL-KADI A.O. ULUDAG H. LAVASANIFAR A. STAT3 silencing in dendritic cells by siRNA polyplexes encapsulated in PLGA nanoparticles for the modulation of anticancer immune response. Mol. Pharm. 2010;7:1643–1654. doi: 10.1021/mp100067u. [DOI] [PubMed] [Google Scholar]
- ALSHAMSAN A. HADDADI A. INCANI V. SAMUEL J. LAVASANIFAR A. ULUDAĞ H. Formulation and delivery of siRNA by oleic acid and stearic acid modified polyethylenimine. Mol. Pharm. 2009;6:121–133. doi: 10.1021/mp8000815. [DOI] [PubMed] [Google Scholar]
- ALSHAMSAN A. HAMDY S. SAMUEL J. EL-KADI A.O. LAVASANIFAR A. ULUDAĞ H. The induction of tumor apoptosis in B16 melanoma following STAT3 siRNA delivery with a lipid-substituted polyethylenimine. Biomaterials. 2010;31:1420–1428. doi: 10.1016/j.biomaterials.2009.11.003. [DOI] [PubMed] [Google Scholar]
- AMERES S.L. MARTINEZ J. SCHROEDER R. Molecular basis for target RNA recognition and cleavage by human RISC. Cell. 2007;130:101–112. doi: 10.1016/j.cell.2007.04.037. [DOI] [PubMed] [Google Scholar]
- ANDERSEN M.Ø. LICHAWSKA A. ARPANAEI A. RASK JENSEN S.M. KAUR H. OUPICKY D. BESENBACHER F. KINGSHOTT P. KJEMS J. HOWARD K.A. Surface functionalisation of PLGA nanoparticles for gene silencing. Biomaterials. 2010;31:5671–5677. doi: 10.1016/j.biomaterials.2010.03.069. [DOI] [PubMed] [Google Scholar]
- BARTLETT D.W. DAVIS M.E. Physicochemical and biological characterization of targeted, nucleic acid-containing nanoparticles. Bioconjug. Chem. 2007;18:456–468. doi: 10.1021/bc0603539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERNSTEIN E. CAUDY A.A. HAMMOND S.M. HANNON G.J. Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature. 2001;409:363–366. doi: 10.1038/35053110. [DOI] [PubMed] [Google Scholar]
- BISWAL B.K. DEBATA N.B. VERMA R.S. Development of a targeted siRNA delivery system using FOL–PEG–PEI conjugate. Mol. Biol. Rep. 2010;37:2919–2926. doi: 10.1007/s11033-009-9853-3. [DOI] [PubMed] [Google Scholar]
- BLACKBURN W.H. DICKERSON E.B. SMITH M.H. MCDONALD J.F. LYON L.A. Peptide-functionalized nanogels for targeted siRNA delivery. Bioconjug. Chem. 2009;20:960–968. doi: 10.1021/bc800547c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BØE S.L. LONGVA A.S. HOVIG E. Cyclodextrin-containing polymer delivery system for light-directed siRNA gene silencing. Oligonucleotides. 2010;20:175–182. doi: 10.1089/oli.2010.0230. [DOI] [PubMed] [Google Scholar]
- BOLCATO-BELLEMIN A.L. BONNET M.E. CREUSAT G. ERBACHER P. BEHR J.P. Sticky overhangs enhance siRNA-mediated gene silencing. Proc. Natl. Acad. Sci. U.S.A. 2007;104:16050–16055. doi: 10.1073/pnas.0707831104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOUSSIF O. LEZOUALC'H F. ZANTA M.A. MERGNY M.D. SCHERMAN D. DEMENEIX B. BEHR J.P. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc. Natl. Acad. Sci. U.S.A. 1995;92:7297–7301. doi: 10.1073/pnas.92.16.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BREUNIG M. HOZSA C. LUNGWITZ U. WATANABE K. UMEDA I. KATO H. GOEPFERICH A. Mechanistic investigation of poly(ethylene imine)-based siRNA delivery: disulfide bonds boost intracellular release of the cargo. J. Control. Release. 2008;130:57–63. doi: 10.1016/j.jconrel.2008.05.016. [DOI] [PubMed] [Google Scholar]
- CHEN A.M. TARATULA O. WEI D. YEN H.I. THOMAS T. THOMAS T.J. MINKO T. HE H. Labile catalytic packaging of DNA/siRNA: control of gold nanoparticles “out” of DNA/siRNA complexes. ACS Nano. 2010;4:3679–3688. doi: 10.1021/nn901796n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEUNG W. PONTORIERO F. TARATULA O. CHEN A.M. HE H. DNA and carbon nanotubes as medicine. Adv. Drug Deliv. Rev. 2010;62:633–649. doi: 10.1016/j.addr.2010.03.007. [DOI] [PubMed] [Google Scholar]
- CUN D. FOGED C. YANG M. FRØKJAER S. NIELSEN H.M. Preparation and characterization of poly(dl-lactide-co-glycolide) nanoparticles for siRNA delivery. Int. J. Pharm. 2010;390:70–75. doi: 10.1016/j.ijpharm.2009.10.023. [DOI] [PubMed] [Google Scholar]
- CUN D. JENSEN D.K. MALTESEN M.J. BUNKER M. WHITESIDE P. SCURR D. FOGED C. NIELSEN H.M. High loading efficiency and sustained release of siRNA encapsulated in PLGA nanoparticles – Quality by design optimization and characterization. Eur. J. Pharm. Biopharm. 2011;77:26–35. doi: 10.1016/j.ejpb.2010.11.008. [DOI] [PubMed] [Google Scholar]
- DE FOUGEROLLES A. NOVOBRANTSEVA T. siRNA and the lung: research tool or therapeutic drug? Curr. Opin. Pharmacol. 2008;8:280–285. doi: 10.1016/j.coph.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE LAS HERAS ALARCON C. PENNADAM S. ALEXANDER C. Stimuli responsive polymers for biomedical applications. Chem. Soc. Rev. 2005;34:276–285. doi: 10.1039/b406727d. [DOI] [PubMed] [Google Scholar]
- DE SMEDT S.C. DEMEESTER J. HENNINK W.E. Cationic polymer based gene delivery systems. Pharm. Res. 2000;17:113–126. doi: 10.1023/a:1007548826495. [DOI] [PubMed] [Google Scholar]
- DIFIGLIA M. SENA-ESTEVES M. CHASE K. SAPP E. PFISTER E. SASS M. YODER J. REEVES P. PANDEY R.K. RAJEEV K.G. MANOHARAN M. SAH D.W. ZAMORE P.D. ARONIN N. Therapeutic silencing of mutant huntingtin with siRNA attenuates striatal and cortical neuropathology and behavioral deficits. Proc. Natl. Acad. Sci. U.S.A. 2007;104:17204–17209. doi: 10.1073/pnas.0708285104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELBAKRY A. ZAKY A. LIEBL R. RACHEL R. GOEPFERICH A. BREUNIG M. Layer-by-Layer Assembled Gold Nanoparticles for siRNA Delivery. Nano Lett. 2009;9:2059–2064. doi: 10.1021/nl9003865. [DOI] [PubMed] [Google Scholar]
- ELBASHIR S.M. HARBORTH J. LENDECKEL W. YALCIN A. WEBER K. TUSCHL T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494–498. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- FIRE A. XU S. MONTGOMERY M.K. KOSTAS S.A. DRIVER S.E. MELLO C.C. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- FRANK-KAMENETSKY M. GREFHORST A. ANDERSON N.N. RACIE T.S. BRAMLAGE B. AKINC A. BUTLER D. CHARISSE K. DORKIN R. FAN Y. GAMBA-VITALO C. HADWIGER P. JAYARAMAN M. JOHN M. JAYAPRAKASH K.N. MAIER M. NECHEV L. RAJEEV K.G. READ T. RÖHL I. SOUTSCHEK J. TAN P. WONG J. WANG G. ZIMMERMANN T. DE FOUGEROLLES A. VORNLOCHER H.P. LANGER R. ANDERSON D.G. MANOHARAN M. KOTELIANSKY V. HORTON J.D. FITZGERALD K. Therapeutic RNAi targeting PCSK9 acutely lowers plasma cholesterol in rodents and LDL cholesterol in nonhuman primates. Proc. Natl. Acad. Sci. U.S.A. 2008;105:11915–11920. doi: 10.1073/pnas.0805434105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARRIPELLI V.K. KIM J.K. NAMGUNG R. KIM W.J. REPKA M.A. JO S. A novel thermosensitive polymer with pH-dependent degradation for drug delivery. Acta Biomater. 2010;6:477–485. doi: 10.1016/j.actbio.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARY D.J. PURI N. WON Y.Y. Polymer-based siRNA delivery: perspectives on the fundamental and phenomenological distinctions from polymer-based DNA delivery. J. Control. Release. 2007;121:64–73. doi: 10.1016/j.jconrel.2007.05.021. [DOI] [PubMed] [Google Scholar]
- GHOSH P. HAN G. DE M. KIM C.K. ROTELLO V.M. Gold nanoparticles in delivery applications. Adv. Drug Deliv. Rev. 2008;60:1307–1315. doi: 10.1016/j.addr.2008.03.016. [DOI] [PubMed] [Google Scholar]
- GHOSN B. KASTURI S.P. ROY K. Enhancing polysaccharide-mediated delivery of nucleic acids through functionalization with secondary and tertiary Amines. Curr. Top Med. Chem. 2008;8:331–340. doi: 10.2174/156802608783790947. [DOI] [PubMed] [Google Scholar]
- GHOSN B. SINGH A. LI M. VLASSOV A.V. BURNETT C. PURI N. ROY K. Efficient gene silencing in lungs and liver using imidazole-modified chitosan as a nanocarrier for small interfering RNA. Oligonucleotide. 2010;20:163–172. doi: 10.1089/oli.2010.0235. [DOI] [PubMed] [Google Scholar]
- GILJOHANN D.A. SEFEROS D.S. PRIGODICH A.E. PATEL P.C. MIRKIN C.A. Gene Regulation with Polyvalent siRNA-Nanoparticle Conjugates. J. Am. Chem. Soc. 2009;131:2072–2073. doi: 10.1021/ja808719p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GODBEY W.T. WU K.K. MIKOS A.G. Size matters: molecular weight affects the efficiency of poly(ethylenimine) as a gene delivery vehicle. J. Biomed. Mater. Res. 1999;45:268–275. doi: 10.1002/(sici)1097-4636(19990605)45:3<268::aid-jbm15>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- GONZALEZ H. HWANG S.J. DAVIS M.E. New class of polymers for the delivery of macromolecular therapeutics. Bioconjug. Chem. 1999;10:1068–1074. doi: 10.1021/bc990072j. [DOI] [PubMed] [Google Scholar]
- GRAYSON A.C. DOODY A.M. PUTNAM D. Biophysical and structural characterization of polyethylenimine-mediated siRNA delivery in vitro. Pharm. Res. 2006;23:1868–1876. doi: 10.1007/s11095-006-9009-2. [DOI] [PubMed] [Google Scholar]
- HALDER J. KAMAT A.A. LANDEN C.N., Jr. HAN L.Y. LUTGENDORF S.K. LIN Y.G. MERRITT W.M. JENNINGS N.B. CHAVEZ-REYES A. COLEMAN R.L. GERSHENSON D.M. SCHMANDT R. COLE S.W. LOPEZ-BERESTEIN G. SOOD A.K. Focal adhesion kinase targeting using in vivo short interfering RNA delivery in neutral liposomes for ovarian carcinoma therapy. Clin. Cancer Res. 2006;12:4916–4924. doi: 10.1158/1078-0432.CCR-06-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAN H.D. MANGALA L.S. LEE J.W. SHAHZAD M.M. KIM H.S. SHEN D. NAM E.J. MORA E.M. STONE R.L. LU C. LEE S.J. ROH J.W. NICK A.M. LOPEZ-BERESTEIN G. SOOD A.K. Targeted gene silencing using RGD labeled chitosan nanoparticles, Clin. Cancer Res. 2010;16:3910–3922. doi: 10.1158/1078-0432.CCR-10-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HART S.L. Multifunctional nanocomplexes for gene transfer and gene therapy. Cell Biol. Toxicol. 2010;26:69–81. doi: 10.1007/s10565-009-9141-y. [DOI] [PubMed] [Google Scholar]
- HASSANI Z. LEMKINE G.F. ERBACHER P. PALMIER K. ALFAMA G. GIOVANNANGELI C. BEHR J.P. DEMENEIX B.A. Lipid-mediated siRNA delivery down-regulates exogenous gene expression in the mouse brain at picomolar levels. J. Gene Med. 2005;7:198–207. doi: 10.1002/jgm.659. [DOI] [PubMed] [Google Scholar]
- HERRERO M.A. TOMA F.M. AL-JAMAL K.T. KOSTARELOS K. BIANCO A. DA ROS T. BANO F. CASALIS L. SCOLES G. PRATO M. Synthesis and characterization of a carbon nanotube-dendron series for efficient siRNA delivery. J. Am. Chem. Soc. 2009;131:9843–9848. doi: 10.1021/ja903316z. [DOI] [PubMed] [Google Scholar]
- HOM C. LU J. TAMANOI F. Silica nanoparticles as a delivery system for nucleic acid-based reagents. J. Mater. Chem. 2009;19:6308–6316. doi: 10.1039/b904197d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOMBACH J. BERNKOP-SCHNÜRCH A. Chitosan solutions and particles: evaluation of their permeation enhancing potential on MDCK cells used as blood brain barrier model. Int. J. Pharm. 2009;376:104–109. doi: 10.1016/j.ijpharm.2009.04.027. [DOI] [PubMed] [Google Scholar]
- HOWARD K.A. RAHBEK U.L. LIU X. DAMGAARD C.K. GLUD S.Z. ANDERSEN M.O. HOVGAARD M.B. SCHMITZ A. NYENGAARD J.R. BESENBACHER F. KJEMS J. RNA interference in vitro and in vivo using a novel chitosan/siRNA nanoparticle system. Mol. Ther. 2006;14:476–484. doi: 10.1016/j.ymthe.2006.04.010. [DOI] [PubMed] [Google Scholar]
- HU-LIESKOVAN S. HEIDEL J.D. BARTLETT D.W. DAVIS M.E. TRICHE T.J. Sequence-specific knockdown of EWS-FLI1 by targeted, nonviral delivery of small interfering RNA inhibits tumor growth in a murine model of metastatic Ewing's sarcoma. Cancer Res. 2005;65:8984–8992. doi: 10.1158/0008-5472.CAN-05-0565. [DOI] [PubMed] [Google Scholar]
- HUNTER A.C. MOGHIMI S.M. Therapeutic synthetic polymers: a game of Russian roulette? Drug Discov. Today. 2002;7:998–1001. doi: 10.1016/s1359-6446(02)02444-3. [DOI] [PubMed] [Google Scholar]
- JOHANSSON H.J. ANDALOUSSI S.E. LANGEL U. Mimicry of protein function with cell-penetrating peptides. Methods Mol. Biol. 2011;683:233–47. doi: 10.1007/978-1-60761-919-2_17. [DOI] [PubMed] [Google Scholar]
- KABANOV A.V. VINOGRADOV S.V. Nanogels as pharmaceutical carriers: finite networks of infinite capabilities. Angew. Chem. Int. Ed. Engl. 2009;48:5418–5429. doi: 10.1002/anie.200900441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAM N.W. LIU Z. DAI H. Functionalization of carbon nanotubes via cleavable disulfide bonds for efficient intracellular delivery of siRNA and potent gene silencing. J. Am. Chem. Soc. 2005;127:12492–12493. doi: 10.1021/ja053962k. [DOI] [PubMed] [Google Scholar]
- KANG J.H. TACHIBANA Y. KAMATA W. MAHARA A. HARADA-SHIBA M. YAMAOKA T. Liver-targeted siRNA delivery by polyethylenimine (PEI)-pullulan carrier. Bioorg. Med. Chem. 2010;18:3946–39. doi: 10.1016/j.bmc.2010.04.031. [DOI] [PubMed] [Google Scholar]
- KATAS H. CEVHER E. ALPAR H.O. Preparation of polyethylenimine incorporated poly(d,l-lactide-co-glycolide) nanoparticles by spontaneous emulsion diffusion method for small interfering RNA delivery. Int. J. Pharm. 2009;369:144–154. doi: 10.1016/j.ijpharm.2008.10.012. [DOI] [PubMed] [Google Scholar]
- KIM H.J. ISHII A. MIYATA K. LEE Y. WU S. OBA M. NISHIYAMA N. KATAOKA K. Introduction of stearoyl moieties into a biocompatible cationic polyaspartamide derivative, PAsp(DET), with endosomal escaping function for enhanced siRNA-mediated gene knockdown. J. Control. Release. 2010c;145:141–148. doi: 10.1016/j.jconrel.2010.03.019. [DOI] [PubMed] [Google Scholar]
- KIM S.K. PARK K.M. SINGHA K. KIM J. AHN Y. KIM K. KIM W.J. Galactosylated cucurbituril-inclusion polyplex for hepatocyte-targeted gene delivery. Chem. Commun. 2010b;46:692–694. doi: 10.1039/b920753h. [DOI] [PubMed] [Google Scholar]
- KIM T.I. LEE M. KIM S.W. A guanidinylated bioreducible polymer with high nuclear localization ability for gene delivery systems. Biomaterials. 2010a;31:1798–1804. doi: 10.1016/j.biomaterials.2009.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIM W.J. CHRISTENSEN L.V. JO S. YOCKMAN J.W. JEONG J.H. KIM Y.H. KIM S.W. Cholesteryl oligoarginine delivering vascular endothelial growth factor siRNA effectively inhibits tumor growth in colon adenocarcinoma. Mol. Ther. 2006b;14:343–350. doi: 10.1016/j.ymthe.2006.03.022. [DOI] [PubMed] [Google Scholar]
- KIM W.J. KIM S.W. Efficient siRNA delivery with non-viral polymeric vehicles. Pharm. Res. 2009;26:657–666. doi: 10.1007/s11095-008-9774-1. [DOI] [PubMed] [Google Scholar]
- KIM W.J. CHANG C.W. LEE M. KIM S.W. Efficient siRNA delivery using water soluble lipopolymer for anti-angiogenic gene therapy. J. Control. Release. 2007;118:357–363. doi: 10.1016/j.jconrel.2006.12.026. [DOI] [PubMed] [Google Scholar]
- KIM W.J. YOCKMAN J.W. JEONG J.H. CHRISTENSEN L.V. LEE M. KIM Y.H. KIM S.W. Anti-angiogenic inhibition of tumor growth by systemic delivery of PEI-g-PEG-RGD/pCMV-sFlt-1 complexes in tumor-bearing mice. J. Control. Release. 2006a;114:381–388. doi: 10.1016/j.jconrel.2006.05.029. [DOI] [PubMed] [Google Scholar]
- KIM W.J. YOCKMAN J.W. LEE M. JEONG J.H. KIM Y.H. KIM S.W. Soluble Flt-1 gene delivery using PEI-g-PEG-RGD conjugate for anti-angiogenesis. J. Control. Release. 2005;106:224–234. doi: 10.1016/j.jconrel.2005.04.016. [DOI] [PubMed] [Google Scholar]
- KIM S.H. JEONG J.H. LEE S.H. KIM S.W. PARK T.G. Local and systemic delivery of VEGF siRNA using polyelectrolyte complex micelles for effective treatment of cancer. J. Control. Release. 2008;129:107–116. doi: 10.1016/j.jconrel.2008.03.008. [DOI] [PubMed] [Google Scholar]
- KOPING-HOGGARD M. TUBULEKAS I. GUAN H. EDWARDS K. NILSSON M. VARUM K.M. ARTURSSON P. Chitosan as a nonviral gene delivery system. Structure–property relationships and characteristics compared with polyethylenimine in vitro and after lung administration in vivo. Gene Ther. 2001;8:1108–1121. doi: 10.1038/sj.gt.3301492. [DOI] [PubMed] [Google Scholar]
- KULKARNI R.P. MISHRA S. FRASER S.E. DAVIS M.E. Single cell kinetics of intracellular, nonviral, nucleic acid delivery vehicle acidification and trafficking. Bioconjug. Chem. 2005;16:986–994. doi: 10.1021/bc050081u. [DOI] [PubMed] [Google Scholar]
- KWON J.S. PARK I.K. CHO A.S. SHIN S.M. HONG M.H. JEONG S.Y. KIM Y.S. MIN J.J. JEONG M.H. KIM W.J. JO S. PUN S.H. CHO J.G. PARK J.C. KANG J.C. AHN Y. Enhanced angiogenesis mediated by VEGF plasmid-loaded thermo-responsive amphiphilic hydrogel in rat myocardial infarction model. J. Control. Release. 2009;138:168–176. doi: 10.1016/j.jconrel.2009.05.023. [DOI] [PubMed] [Google Scholar]
- LACERDA L. BIANCO A. PRATO M. KOSTARELOS K. Carbon nanotubes as nanomedicines: from toxicology to pharmacology. Adv. Drug Deliv. Rev. 2006;58:1460–1470. doi: 10.1016/j.addr.2006.09.015. [DOI] [PubMed] [Google Scholar]
- LANGLET-BERTIN B. LEBORGNE C. SCHERMAN D. BECHINGER B. MASON A.J. KICHLER A. Design and evaluation of histidine rich amphipathic peptides for siRNA delivery. Pharm. Res. 2010;27:1426–1436. doi: 10.1007/s11095-010-0138-2. [DOI] [PubMed] [Google Scholar]
- LANNER J.T. BRUTON J.D. ASSEFAW-REDDA Y. ANDRONACHE Z. ZHANG S.J. SEVERA D. ZHANG Z.-B. MELZER W. ZHANG S.-L. KATZ A. WESTERBLAD H. Knockdown of TRPC3 with siRNA coupled to carbon nanotubes results in decreased insulin-mediated glucose uptake in adult skeletal muscle cells. FASEB J. 2009;23:1728–1738. doi: 10.1096/fj.08-116814. [DOI] [PubMed] [Google Scholar]
- LEE J.S. GREEN J.J. LOVE K.T. SUNSHINE J. LANGER R. ANDERSON D.G. Gold, poly(β-amino ester) nanoparticles for small interfering RNA delivery. Nano Lett. 2009;9:2402–2406. doi: 10.1021/nl9009793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEE K. BAE K.H. LEE Y. LEE S.H. AHN C.H. PARK T.G. Pluronic/polyethylenimine shell crosslinked nanocapsules with embedded magnetite nanocrystals for magnetically triggered delivery of siRNA. Macromol. Biosci. 2010;10:239–245. doi: 10.1002/mabi.200900291. [DOI] [PubMed] [Google Scholar]
- LEE Y. MIYATA K. OBA M. ISHII T. FUKUSHIMA S. HAN M. KOYAMA H. NISHIYAMA N. KATAOKA K. Charge-conversion ternary polyplex with endosome disruption moiety: a technique for efficient and safe gene delivery. Angew. Chem., Int. Ed. Engl. 2008;47:5163–5166. doi: 10.1002/anie.200800963. [DOI] [PubMed] [Google Scholar]
- LENG Q. SCARIA P. ZHU J. AMBULOS N. CAMPBELL P. MIXSON A.J. Highly branched HK peptides are effective carriers of siRNA. J. Gene. Med. 2005;7:977–986. doi: 10.1002/jgm.748. [DOI] [PubMed] [Google Scholar]
- LIU X.X. ROCCHI P. QU F.Q. ZHENG S.Q. LIANG Z.C. GLEAVE M. IOVANNA J. PENG L. PAMAM dendrimers mediate sirna delivery to target hsp27 and produce potent antiproliferative effects on prostate cancer cells. ChemMedChem. 2009;4:1302–1310. doi: 10.1002/cmdc.200900076. [DOI] [PubMed] [Google Scholar]
- LIU Z. WINTERS M. HOLODNIY M. DAI H. siRNA delivery into human T cells and primary cells with carbon-nanotube transporters. Angew. Chem. Int. Ed. Engl. 2007;46:2023–2027. doi: 10.1002/anie.200604295. [DOI] [PubMed] [Google Scholar]
- MAO S. NEU M. GERMERSHAUS O. MERKEL O. SITTERBERG J. BAKOWSKY U. KISSEL T. Influence of polyethylene glycol chain length on the physicochemical and biological properties of poly(ethylene imine)-graft-poly(ethylene glycol) block copolymer/SiRNA polyplexes. Bioconjug. Chem. 2006;17:1209–1218. doi: 10.1021/bc060129j. [DOI] [PubMed] [Google Scholar]
- MAO S. SUN W. KISSEL T. Chitosan-based formulations for delivery of DNA and siRNA. Adv. Drug Deliv. Rev. 2010;62:12–27. doi: 10.1016/j.addr.2009.08.004. [DOI] [PubMed] [Google Scholar]
- MATRANGA C. TOMARI Y. SHIN C. BARTEL D.P. ZAMORE P.D. Passenger-strand cleavage facilitates assembly of siRNA into ago2-containing RNAi enzyme complexes. Cell. 2005;123:607–620. doi: 10.1016/j.cell.2005.08.044. [DOI] [PubMed] [Google Scholar]
- MEHVAR R. Recent trends in the use of polysaccharides for improved delivery of therapeutic agents: Pharmacokinetic and pharmacodynamic perspectives. Curr. Pharm. Biotechnol. 2003;4:283–302. doi: 10.2174/1389201033489685. [DOI] [PubMed] [Google Scholar]
- MENG H. LIONG M. XIA T. LI Z. JI Z. ZINK J.I. NEL A.E. Engineered design of mesoporous silica nanoparticles to deliver doxorubicin and p-glycoprotein siRNA to overcome drug resistance in a cancer cell line. ACS Nano. 2010;4:4539–4550. doi: 10.1021/nn100690m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIDOUX P. MONSIGNY M. Efficient gene transfer by histidylated polylysine/pDNA complexes. Bioconjug. Chem. 1999;10:406–411. doi: 10.1021/bc9801070. [DOI] [PubMed] [Google Scholar]
- MIYATA K. OBA M. NAKANISHI M. FUKUSHIMA S. YAMASAKI Y. KOYAMA H. NISHIYAMA N. KATAOKA K. Polyplexes from poly(aspartamide) bearing 1, 2-diaminoethane side chains induce pH-selective, endosomal membrane destabilization with amplified transfection and negligible cytotoxicity. J. Am. Chem. Soc. 2008;130:16287–16294. doi: 10.1021/ja804561g. [DOI] [PubMed] [Google Scholar]
- MOGHIMI S.M. SYMONDS P. MURRAY J.C. HUNTER A.C. DEBSKA G. SZEWCZYK A. A two-stage poly(ethylenimine)-mediated cytotoxicity: implications for gene transfer/therapy. Mol. Ther. 2005;11:990–995. doi: 10.1016/j.ymthe.2005.02.010. [DOI] [PubMed] [Google Scholar]
- MORRISSEY D.V. LOCKRIDGE J.A. SHAW L. BLANCHARD K. JENSEN K. BREEN W. HARTSOUGH K. MACHEMER L. RADKA S. JADHAV V. VAISH N. ZINNEN S. VARGEESE C. BOWMAN K. SHAFFER C.S. JEFFS L.B. JUDGE A. MACLACHLAN I. POLISKY B. Potent and persistent in vivo anti-HBV activity of chemically modified siRNAs. Nat. Biotech. 2005;23:1002–1007. doi: 10.1038/nbt1122. [DOI] [PubMed] [Google Scholar]
- NAKAMURA Y. KOGURE K. FUTAKI S. HARASHIMA H. Octaarginine-modified multifunctional envelope-type nano device for siRNA. J. Control. Release. 2007;119:360–367. doi: 10.1016/j.jconrel.2007.03.010. [DOI] [PubMed] [Google Scholar]
- NAMGUNG R. KIM J. SINGHA K. KIM C.H. KIM W.J. Synergistic effect of low cytotoxic linear polyethylenimine and multiarm polyethylene glycol: study of physicochemical properties and in vitro gene transfection. Mol. Pharm. 2009a;6:1826–1835. doi: 10.1021/mp900096u. [DOI] [PubMed] [Google Scholar]
- NAMGUNG R. NAM S. KIM S.K. SON S. SINGHA K. KWON J.S. AHN Y. JEONG M.H. PARK I.K. GARRIPELLI V.K. JO S. KIM W.J. An acid-labile temperature-responsive sol-gel reversible polymer for enhanced gene delivery to the myocardium and skeletal muscle cells. Biomaterials. 2009b;30:5225–5233. doi: 10.1016/j.biomaterials.2009.05.073. [DOI] [PubMed] [Google Scholar]
- NAMGUNG R. SINGHA K. YU M.K. JON S. KIM Y.S. AHN Y. PARK I.K. KIM W.J. Hybrid superparamagnetic iron oxide nanoparticle-branched polyethylenimine magnetoplexes for gene transfection of vascular endothelial cells. Biomaterials. 2010;31:4204–4213. doi: 10.1016/j.biomaterials.2010.01.123. [DOI] [PubMed] [Google Scholar]
- NIELSEN E.J. NIELSEN J.M. BECKER D. KARLAS A. PRAKASH H. GLUD S.Z. MERRISON J. BESENBACHER F. MEYER T.F. KJEMS J. HOWARD K.A. Pulmonary gene silencing in transgenic EGFP mice using aerosolised chitosan/siRNA nanoparticles. Pharm. Res. 2010;27:2520–2527. doi: 10.1007/s11095-010-0255-y. [DOI] [PubMed] [Google Scholar]
- NIU X.Y. PENG Z.L. DUAN W.Q. WANG H. WANG P. Inhibition of HPV 16 E6 oncogene expression by RNA interference in vitro and in vivo. Int. J. Gynecol. Cancer. 2006;16:743–751. doi: 10.1111/j.1525-1438.2006.00384.x. [DOI] [PubMed] [Google Scholar]
- NOH S.M. HAN S.E. SHIM G. LEE K.E. KIM C.W. HAN S.S. CHOI Y. KIM Y.K. KIM W.K. OH Y.K. Tocopheryl oligochitosan-based self assembling oligomersomes for siRNA delivery. Biomaterials. 2011;32:849–857. doi: 10.1016/j.biomaterials.2010.09.027. [DOI] [PubMed] [Google Scholar]
- OH J.K. LEE D.I. PARK J.M. Biopolymer-based microgels/nanogels for drug delivery applications. Prog. Polym. Sci. 2009;34:1261–1282. [Google Scholar]
- OKUMURA A. PITHA P.M. HARTY R.N. ISG15 inhibits Ebola VP40 VLP budding in an L-domain-dependent manner by blocking Nedd4ligase activity. Proc. Natl. Acad. Sci. U.S.A. 2008;105:3974–3979. doi: 10.1073/pnas.0710629105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OPANASOPIT P. TECHAARPORNKUL S. ROJANARATA T. NGAWHIRUNPAT T. RUKTANONCHAI U. Nucleic acid delivery with chitosan hydroxybenzotriazole. Oligonucleotides. 2010;20:127–136. doi: 10.1089/oli.2009.0227. [DOI] [PubMed] [Google Scholar]
- OSKUEE R.K. PHILIPP A. DEHSHAHRI A. WAGNER E. RAMEZANI M. The impact of carboxyalkylation of branched polyethylenimine on effectiveness in small interfering RNA delivery. J. Gene. Med. 2010;12:729–738. doi: 10.1002/jgm.1490. [DOI] [PubMed] [Google Scholar]
- PARK I.K. IHM J.E. PARK Y.H. CHOI Y.J. KIM S.I. KIM W.J. AKAIKE T. CHO C.S. Galactosylated chitosan (GC)-graft-poly(vinyl pyrrolidone) (PVP) as hepatocyte-targeting DNA carrier: preparation and physicochemical characterization of GC-graft-PVP/DNA complex (1) J. Control. Release. 2003;86:349–359. doi: 10.1016/s0168-3659(02)00365-6. [DOI] [PubMed] [Google Scholar]
- PARK I.K. SINGHA K. AROTE R.B. CHOI Y.-J. KIM W.J. CHO C.S. pH-Responsive polymers as gene carriers. Macromol. Rapid Commun. 2010;31:1122–1133. doi: 10.1002/marc.200900867. [DOI] [PubMed] [Google Scholar]
- PATIL M.L. ZHANG M. BETIGERI S. TARATULA O. HE H. MINKO T. Surface-modified and internally cationic polyamidoamine dendrimers for efficient siRNA delivery. Bioconjug. Chem. 2008;19:1396–1403. doi: 10.1021/bc8000722. [DOI] [PubMed] [Google Scholar]
- PATIL M.L. ZHANG M. TARATULA O. GARBUZENKO O.B. HE H. MINKO T. Internally cationic polyamidoamine pamam-oh dendrimers for sirna delivery: effect of the degree of quaternization and cancer targeting. Biomacromolecules. 2009;10:258–266. doi: 10.1021/bm8009973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERRIMAN A.W. WILLIAMS D.S. JACKSON A.J. GRILLO I. KOOMULLIL J.M. GHASPARIAN A. ROBINSON J.A. MANN S. Synthetic viruslike particles and hybrid constructs based on lipopeptide self-assembly. Small. 2010;6:1185–1190. doi: 10.1002/smll.200901186. [DOI] [PubMed] [Google Scholar]
- PHILIPP A. ZHAO X. TARCHA P. WAGNER E. ZINTCHENKO A. Hydrophobically modified oligoethylenimines as highly efficient transfection agents for siRNA delivery. Bioconjug. Chem. 2009;20:2055–2061. doi: 10.1021/bc9001536. [DOI] [PubMed] [Google Scholar]
- PTASZNIK A. NAKATA Y. KALOTA A. EMERSON S.G. GEWIRTZ A.M. Short interfering RNA (siRNA) targeting the Lyn kinase induces apoptosis in primary, and drugresistant, BCR-ABL1+ leukemia cells. Nat. Med. 2004;10:1187–1189. doi: 10.1038/nm1127. [DOI] [PubMed] [Google Scholar]
- RAEMDONCK K. NAEYE B. BUYENS K. VANDENBROUCKE R.E. HØGSET A. DEMEESTER J. SMEDT S.C.D. Biodegradable dextran nanogels for RNA interference: focusing on endosomal escape and intracellular siRNA delivery. Adv. Funct. Mater. 2009;19:1406–1415. [Google Scholar]
- RAEMDONCK K. NAEYE B. HØGSET A. DEMEESTER J. SMEDT S.C.D. Prolonged gene silencing by combining siRNA nanogels and photochemical internalization. J. Control. Release. 2010;145:281–288. doi: 10.1016/j.jconrel.2010.04.012. [DOI] [PubMed] [Google Scholar]
- RAND T.A. GINALSKI K. GRISHIN N.V. WANG X. Biochemical identification of Argonaute 2 as the sole protein required for RNA-induced silencing complex activity. Proc. Natl Acad. Sci. U.S.A. 2004;101:14385–14389. doi: 10.1073/pnas.0405913101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROJANARATA T. OPANASOPIT P. TECHAARPORNKUL S. NGAWHIRUNPAT T. RUKTANONCHAI U. Chitosan-thiamine pyrophosphate as a novel carrier for siRNA delivery. Pharm. Res. 2008;25:2807–2814. doi: 10.1007/s11095-008-9648-6. [DOI] [PubMed] [Google Scholar]
- SALLAS F. DARCY R. Amphiphilic cyclodextrins – advances in synthesis and supramolecular chemistry. Eur. J. Org. Chem. 2008;2008:957–969. [Google Scholar]
- SATO Y. HATAKEYAMA H. HARASHIMA H. Ornithine and tryptophan analogs as efficient polycations for short interference RNA delivery to tumor cells. Biol. Pharm. Bull. 2010;33:1246–1249. doi: 10.1248/bpb.33.1246. [DOI] [PubMed] [Google Scholar]
- SATO Y. MURASE K. KATO J. KOBUNE M. SATO T. KAWANO Y. TAKIMOTO R. TAKADA K. MIYANISHI K. MATSUNAGA T. TAKAYAMA T. NIITSU Y. Resolution of liver cirrhosis usingvitamin A-coupled liposomes to deliver siRNA against a collagen-specific chaperone. Nat. Biotech. 2008;26:431–442. doi: 10.1038/nbt1396. [DOI] [PubMed] [Google Scholar]
- SCHIFFELERS R.M. ANSARI A. XU J. ZHOU Q. TANG Q. STORM G. MOLEMA G. LU P.Y. SCARIA P.V. WOODLE M.C. Cancer siRNA therapy by tumor selective delivery with ligand-targeted sterically stabilized nanoparticle. Nucleic Acids Res. 2004;32:e149. doi: 10.1093/nar/gnh140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SINGH B. DAHIYA M. SAHARAN P. AHUJA N. Optimizing drug delivery systems using systematic “design of experiments.” Part II: retrospect and prospects. Crit. Rev. Ther. Drug Carrier Syst. 2005b;22:215–293. doi: 10.1615/critrevtherdrugcarriersyst.v22.i3.10. [DOI] [PubMed] [Google Scholar]
- SINGH B. KUMAR R. AHUJA N. Optimizing drug delivery systems using systematic “design of experiments.” Part I: fundamental aspects. Crit. Rev. Ther. Drug Carrier Syst. 2005a;22:27–105. doi: 10.1615/critrevtherdrugcarriersyst.v22.i1.20. [DOI] [PubMed] [Google Scholar]
- SON S. KIM W.J. Biodegradable nanoparticles modified by branched polyethylenimine for plasmid DNA delivery. Biomaterials. 2010;31:133–143. doi: 10.1016/j.biomaterials.2009.09.024. [DOI] [PubMed] [Google Scholar]
- SON S. HWANG D.W. SINGHA K. JEONG J.H. PARK T.G. LEE D.S. KIM W.J. RVG peptide tethered bioreducible polyethylenimine for gene delivery to brain. J. Control. Release. 2010b doi: 10.1016/j.jconrel.2010.08.011. [DOI] [PubMed] [Google Scholar]
- SON S. SINGHA K. KIM W.J. Bioreducible BPEI-SS-PEG-cNGR polymer as a tumor targeted nonviral gene carrier. Biomaterials. 2010a;31:6344–6354. doi: 10.1016/j.biomaterials.2010.04.047. [DOI] [PubMed] [Google Scholar]
- SONG E. LEE S.K. WANG J. INCE N. OUYANG N. MIN J. CHEN J. SHANKAR P. LIEBERMAN J. RNA interference targeting Fas protects mice from fulminant hepatitis. Nat. Med. 2003;9:347–351. doi: 10.1038/nm828. [DOI] [PubMed] [Google Scholar]
- SONG W.J. DU J.Z. SUN T.M. ZHANG P.Z. WANG J. Gold nanoparticles capped with polyethylenimine for enhanced siRNA delivery. Small. 2010;6:239–246. doi: 10.1002/smll.200901513. [DOI] [PubMed] [Google Scholar]
- SPAGNOU S. MILLER A.D. KELLER M. Lipidic carriers of siRNA: differences in the formulation, cellular uptake, and delivery with plasmid DNA. Biochemistry. 2004;43:13348–13356. doi: 10.1021/bi048950a. [DOI] [PubMed] [Google Scholar]
- TAHARA K. SAKAI T. YAMAMOTO H. TAKEUCHI H. KAWASHIMA Y. Establishing chitosan coated PLGA nanosphere platform loaded with wide variety of nucleic acid by complexation with cationic compound for gene delivery. Int. J. Pharm. 2008;354:210–216. doi: 10.1016/j.ijpharm.2007.11.002. [DOI] [PubMed] [Google Scholar]
- TAKESHITA F. MINAKUCHI Y. NAGAHARA S. HONMA K. SASAKI H. HIRAI K. TERATANI T. NAMATAME N. YAMAMOTO Y. HANAI K. KATO T. SANO A. OCHIYA T. Efficient delivery of small interfering RNA to bone-metastatic tumors by using atelocollagen in vivo. Proc. Natl Acad. Sci. U.S.A. 2005;102:12177–12182. doi: 10.1073/pnas.0501753102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAMURA A. OISHI M. NAGASAKI Y. Enhanced cytoplasmic delivery of siRNA using a stabilized polyion complex based on PEGylated nanogels with a cross-linked polyamine structure. Biomacromolecules. 2009;10:1818–1827. doi: 10.1021/bm900252d. [DOI] [PubMed] [Google Scholar]
- TAMURA A. OISHI M. NAGASAKI Y. Efficient siRNA delivery based on PEGylated and partially quaternized polyamine nanogels: enhanced gene silencing activity by the cooperative effect of tertiary and quaternary amino groups in the core. J. Control. Release. 2010;146:378–387. doi: 10.1016/j.jconrel.2010.05.031. [DOI] [PubMed] [Google Scholar]
- TARATULA O. GARBUZENKO O.B. KIRKPATRICK P. PANDYA I. SAVLA R. POZHAROV V.P. HE H. MINKO T. Surface-engineered targeted PPI dendrimer for efficient intracellular and intratumoral siRNA delivery. J. Control. Release. 2009;140:284–293. doi: 10.1016/j.jconrel.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TARATULA O. SAVLA R. HE H. MINKO T. Poly(propyleneimine) dendrimers as potential siRNA delivery nanocarrier: from structure to function. Int. J. Nanotechnol. 2011;8:36–52. [Google Scholar]
- TREWYN B.G. GIRI S. SLOWING I.I. LIN V.S.-Y. Mesoporous silica nanoparticle based controlled release, drug delivery, and biosensor systems. Chem. Commun. 2007:3236–3245. doi: 10.1039/b701744h. [DOI] [PubMed] [Google Scholar]
- VINOGRADOV S. BATRAKOVA E. KABANOV A. Poly(ethylene glycol)-polyethylenimine NanoGel™ particles: novel drug delivery systems for antisense oligonucleotides. Colloids Surfaces B: Biointerfaces. 1999;16:291–304. [Google Scholar]
- VINOGRADOV S.V. BRONICH T.K. KABANOV A.V. Nanosized cationic hydrogels for drug delivery: preparation, properties and interactions with cells. Adv. Drug Deliv. Rev. 2002;54:135–147. doi: 10.1016/s0169-409x(01)00245-9. [DOI] [PubMed] [Google Scholar]
- WANG J. FENG S.S. WANG S. CHEN Z.Y. Evaluation of cationic nanoparticles of biodegradable copolymers as siRNA delivery system for hepatitis B treatment. Int. J. Pharm. 2010;400:194–200. doi: 10.1016/j.ijpharm.2010.08.026. [DOI] [PubMed] [Google Scholar]
- WERTH S. URBAN-KLEIN B. DAI L. HOBEL S. GRZELINSKI M. BAKOWSKY U. CZUBAYKO F. AIGNER A. A low molecular weight fraction of polyethylenimine (PEI) displays increased transfection efficiency of DNA and siRNA in fresh or lyophilized complexes. J. Control. Release. 2006;112:257–270. doi: 10.1016/j.jconrel.2006.02.009. [DOI] [PubMed] [Google Scholar]
- XIA T. KOVOCHICH M. LIONG M. MENG H. KABEHIE S. GEORGE S. ZINK J.I. NEL A.E. Polyethylenimine coating enhances the cellular uptake of mesoporous silica nanoparticles and allows safe delivery of siRNA and DNA constructs. ACS Nano. 2009;3:3273–3286. doi: 10.1021/nn900918w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- XIA C.F. ZHANG Y. ZHANG Y. BOADO R. PARDRIDGE W. Intravenous siRNA of brain cancer with receptor targeting and avidin–biotin technology. Pharm. Res. 2007;24:2309–2316. doi: 10.1007/s11095-007-9460-8. [DOI] [PubMed] [Google Scholar]
- XING J. DENG L. GUO S. DONG A. LIANG X.J. Polycationic nanoparticles as nonviral vectors employed for gene therapy in vivo. Mini Rev. Med. Chem. 2010;10:126–137. doi: 10.2174/138955710791185127. [DOI] [PubMed] [Google Scholar]
- YAMAOKA T. TABATA Y. IKADA Y. Body distribution profile of polysaccharides after intravenous administration. Drug Delivery. 1993;1:75–82. [Google Scholar]
- YUAN X. LI L. RATHINAVELU A. HAO J. HE M. HEITLAGE V. TAM L. VIQAR S. SALEHI M. SiRNA drug delivery by biodegradable polymeric nanoparticles. J. Nanosci. Nanotechnol. 2006;6:2821–2828. doi: 10.1166/jnn.2006.436. [DOI] [PubMed] [Google Scholar]
- ZHANG X.Q. INTRA J. SALEM A.K. Comparative study of poly (lactic-co-glycolic acid)-poly ethyleneimine-plasmid DNA microparticles prepared using double emulsion methods. J. Microencapsul. 2008;25:1–12. doi: 10.1080/02652040701659347. [DOI] [PubMed] [Google Scholar]
- ZHOU J. WU J. HAFDI N. BEHR J.P. ERBACHER P. PENG L. PAMAM dendrimers for efficient siRNA delivery and potent gene silencing. Chem. Commun. 2006;22:2362–2364. doi: 10.1039/b601381c. [DOI] [PubMed] [Google Scholar]