Abstract
Pulmonary hypertension (PH) is poorly characterized in the critically ill. No prior studies describe the burden of or outcomes associated with PH in a general medical intensive care unit population. We hypothesize that PH is an important comorbidity prevalent in the modern medical intensive care unit. We undertook a preliminary investigation to define the consequences of Doppler-defined PH in the critically ill. A single-center retrospective case–control study of medical intensive care patients admitted over a 1-year period was conducted. Eligible patients had an echocardiogram within 4 days of admission. PH was defined to include both pulmonary arterial and venous hypertension and required a tricuspid regurgitant jet velocity ≥3 m/sec. Cases and controls were compared for comorbidities, illness severity, diagnoses, and mortality. Multivariable regression was performed to identify clinical features associated with PH and mortality. 299 (21% of admissions) patients had an eligible echocardiogram. Patients with PH (N=126) had a higher unadjusted mortality than did controls (N=173) (37% vs. 25%, P=0.04) and PH remained significantly associated with mortality after controlling for other clinical factors (HR=1.59, 95% CI=1.03–2.44, P=0.036). Low ejection fraction (OR=2.21, 95% CI=1.19–4.11, P=0.012) and pulmonary embolism (OR=4.28, 95% CI=1.59–11.5, P=0.004) were independently associated with PH. Doppler-defined PH is associated with mortality in the critically ill. Prospective studies are needed to define the prevalence of pulmonary venous hypertension versus pulmonary arterial hypertension, and the clinical consequences of each, in a general medical intensive care unit population.
Keywords: Critical illness, echocardiography, pulmonary arterial hypertension, pulmonary venous hypertension
INTRODUCTION
Pulmonary hypertension (PH) in the medical intensive care unit patient population is not well characterized. PH in the intensive care unit may be due to pre-existing pulmonary vascular disease or may occur acutely as a response to de novo cardiac, lung, or vascular injury.[1] Prior investigation of PH in critically ill patients has been limited to the description of PH in association with certain disorders such as acute respiratory distress syndrome (ARDS), pulmonary embolism, or after cardiothoracic surgery.[2–9] Much less is known of the overall incidence of PH in an unselected critically ill population; moreover, the association of PH with clinical outcomes in a general medical intensive care unit population has not been previously reported.
In an effort to better define risk factors and clinical outcomes of PH in a general medical intensive care unit patient population, we retrospectively reviewed admissions to a tertiary care medical intensive care unit to examine the hypotheses that PH is common in the modern medical intensive care unit population and that the presence of PH adversely impacts mortality. The information gained from this study will provide the background necessary to plan further prospective epidemiologic and therapeutic trials of PH in a critically ill medical population. The results of this investigation were presented in abstract form at the 2010 American Thoracic Society meeting.
MATERIALS AND METHODS
Study design and study population
A retrospective case–control study of patients admitted to one tertiary academic medical center over a 1-year period (July 2008 through June 2009) was conducted. All patients over the age of 18 years, admitted to the medical intensive care unit, and who had an echocardiogram performed within the first 4 days of intensive care unit admission, were eligible. This time period was chosen to optimize enrollment while limiting the number of patients who develop PH as a result of prolonged critical illness, such as those who progress to end stage heart, liver, or renal disease. These patients likely represent a different cohort from those who develop PH early in their intensive care unit stay. This study was approved by the University's Institutional Review Board.
Data collection
Baseline demographics, intensive care unit admission diagnoses, and mortality were obtained from the electronic medical record. Echocardiograms were performed in the course of routine clinical care and were interpreted by academic cardiology attending physicians. Patients with echocardiogram quantification of tricuspid regurgitant (TR) jet velocity and ejection fraction had the following data extracted from the medical record: pre-existing comorbidities, laboratory data, use of mechanical ventilation, renal replacement therapy, systemic or pulmonary vasoactive medications, red blood cell transfusion, and right heart catheterization data (if available). Discharge diagnoses for the following disorders were also extracted via review of ICD-9 diagnostic codes: acute respiratory distress syndrome (518.5 and 518.82),[10] pulmonary embolus (415.1, 415.11, and 415.19), sepsis (995.91 and 995.92) and acute myocardial infarction (410.0–410.9).
Definitions
Doppler-defined PH was defined as a TR jet velocity ≥3 m/sec by echocardiography, a value that was selected based on accepted guidelines and population-based echocardiographic investigation.[11,12] A TR jet velocity <3 m/sec defined the control patients. Therefore, all the eligible patients of the study underwent echocardiography and our definitions of case and control can be considered as a comparison between patients with “high versus low” TR jet velocity. Comorbidities were classified as present if noted in an admission history or a consultation performed in the intensive care unit. Immunosuppression was defined as known infection with the human immunodeficiency virus or chronic use of any immunosuppressant medication, including prednisone ≥10 mg/day. Renal replacement therapy was defined as any intermittent or continuous renal replacement therapy. Pulmonary vasodilator use was defined via physician order for any pulmonary vasodilator medication while the patient was admitted to the intensive care unit.
Statistical analysis
Univariate analysis of baseline demographics and mortality between those who did and did not undergo echocardiography was performed via appropriate methods. Baseline characteristics and clinical outcomes were expressed as mean±standard deviation (continuous variables) or as percent (categorical variables). Univariate analysis of variables between groups with and without Doppler-defined PH were compared using Student's t-tests or Wilcoxon rank sum tests, as appropriate, for continuous variables, and using chi-square tests for categorical variables. Multivariable logistic regression analysis using Doppler-defined PH as the dependent outcome was performed in an effort to identify the relevant variables. Time of survival was calculated from date of intensive care unit admission until death. Data were censored at the date of hospital discharge. Survival was assessed using the Kaplan–Meier method and the log-rank test in univariate analysis, and with the Cox proportional hazard method for multivariable analysis. For all regression analyses, variables found to have a P value ≤0.20 in univariate analysis were included in a backward elimination process to arrive at the final model. Age was forced into all survival models. Logistic regression model discrimination was assessed via the Hosmer and Lemeshow test. The proportional hazard assumption was assessed using Schoenfeld residuals. A two-sided P value of ≤0.05 was considered statistically significant. All statistical analyses were performed with Stata, version 10 (StataCorp LP, College Station, TX, USA).
RESULTS
Patient characteristics
Exactly 1431 patients were admitted to our medical intensive care unit during the study period. The primary reasons for intensive care unit admission were sepsis (15%), respiratory failure (13%), pneumonia (7%), heart disease (6%), gastrointestinal disease (6%), renal failure (3%), malignancy-related (3%), neurologic disease (3%), complications of chronic liver disease (3%), complications of diabetes mellitus (2%), drug or alcohol related (<1%), or other miscellaneous disorders (38%). Of these patients, 449 (31%) had echocardiography performed within 4 days of admission to the intensive care unit and were significantly older (61±17 vs. 57±18 years, P<0.001), more likely females (52 vs. 43% P=0.002), and had a higher unadjusted mortality (28 vs. 19%, P=0.001) compared to those patients who did not undergo echocardiography. Of the 449 echocardiography studies, 299 (67%) reported both a TR jet velocity and left ventricular ejection fraction and composed the study sample [Figure 1]. Overall, 28% (126 of 449) of all medical intensive care unit patients who underwent echocardiography had Doppler-defined PH, while 42% (126 of 299) of the patients eligible for the study had Doppler-defined PH. Baseline characteristics and admission diagnoses of patients with and without Doppler-defined PH are similar [Table 1]. The distribution of TR jet velocities in the study cohort (mean 2.88±0.57 m/sec) is displayed in Figure 2. Only 17 of the 299 study eligible patients (6%) had a pre-existing diagnosis of PH. The prevalence of other comorbid conditions in the study population was as follows: systemic hypertension (55%), coronary artery disease (28%), chronic obstructive pulmonary disease (22%), congestive heart failure (19%), immunosuppression (19%), chronic kidney disease (17%), and interstitial lung disease (5%).
Figure 1.
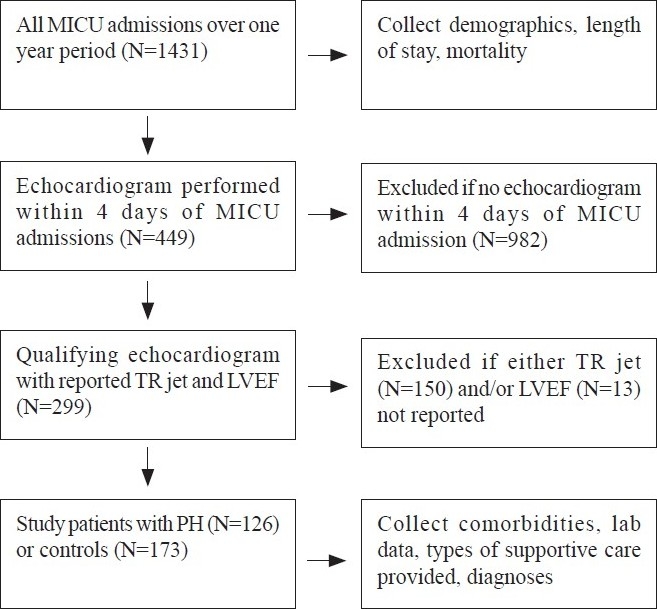
Study flow diagram. Pulmonary hypertension defined as TR jet ≥3 m/sec (LVEF – Left ventricular ejection fraction; MICU – Medical intensive care unit; PH – Pulmonary hypertension; TR – tricuspid regurgitant)
Table 1.
Characteristics of patients with Doppler-defined pulmonary hypertension and controls
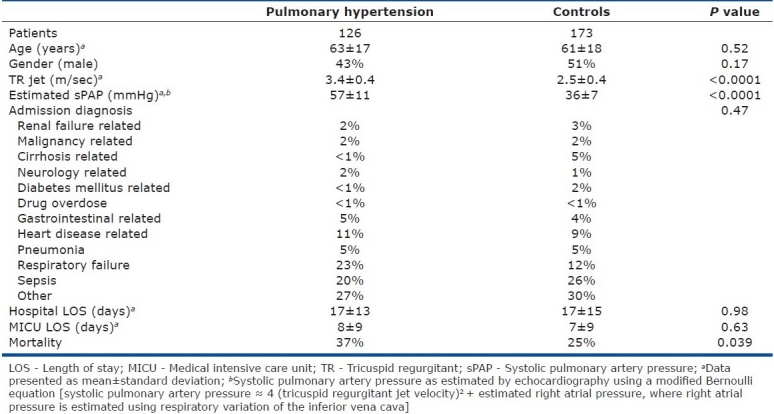
Figure 2.
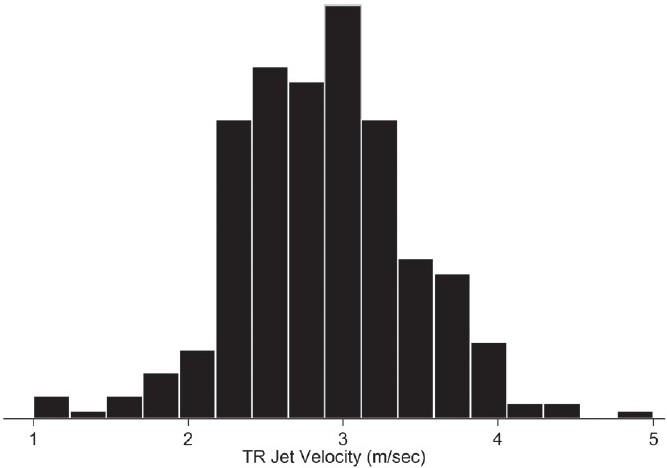
Distribution of tricuspid regurgitant (TR) jet velocities in medical intensive care unit population. Mean TR jet velocity = 2.88±0.57 m/sec; median TR jet velocity=2.80 m/sec
Right heart catheterization
Twenty-one of 299 patients (7%) underwent right heart catheterization during the study admission (11 in Doppler-defined PH group and 10 in control group). The mean duration between echocardiogram and catheterization was 10±10 days. The positive predictive value of a TR jet velocity ≥3 m/sec to diagnose PH, defined as a mean pulmonary artery pressure ≥25 mmHg, was 91% while the negative predictive value was 20% in this critically ill cohort. A moderate correlation existed between estimated and measured values of systolic pulmonary artery pressures (r=0.65, P=0.002).
Risk factors for Doppler-defined PH
The distribution of measured ejection fraction was markedly skewed; categorization of the ejection fraction variable around the 25th percentile (ejection fraction<50%) revealed that 29 patients (23%) in the PH group and 22 patients (13%) in the control group had a depressed left ventricular ejection fraction (P=0.019). Laboratory data associated with Doppler-defined PH included lowest recorded partial pressure of arterial oxygen (P=0.01), lowest recorded serum bicarbonate value (P=0.04), and a trend toward association with highest recorded brain natriuretic peptide (P=0.07). Multiple patients did not have blood gas (21% of study cohort) or brain natriuretic peptide (74% of study cohort) testing performed during their intensive care unit admission. Pulmonary vasodilator, used in a total of 12 patients (11 sildenafil and 1 bosentan), was significantly associated with the Doppler-defined PH (P<0.001). Of the reviewed diagnostic codes, only pulmonary embolus (Doppler-defined PH in 71% of those with a pulmonary embolus compared to 40% of those without a pulmonary embolus, P=0.005) was significantly associated with Doppler-defined PH. Those with ARDS displayed a trend toward reduced PH (Doppler-defined PH in 28% of those with compared to 43% of those without ARDS, P=0.14). Mechanical ventilation, vasopressor use, and renal replacement therapy were not associated with Doppler-defined PH [Table 2].
Table 2.
Clinical variables and their association with pulmonary hypertension
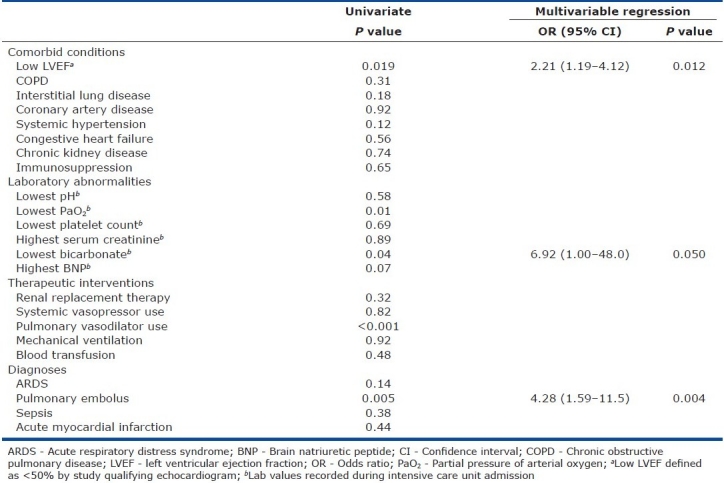
Multivariable regression analysis of Doppler-defined PH risk factors
The following clinical characteristics were included in a regression analysis of Doppler-defined PH risk factors: age, gender, low ejection fraction, history of interstitial lung disease, lowest serum bicarbonate value, and diagnosis of pulmonary embolus. Partial pressure of arterial oxygen and brain natriuretic peptide values were excluded due to multiple missing values. Pulmonary vasodilator was considered a consequence rather than an indicator of PH and was not included in the regression model. When controlling for these clinical features, low ejection fraction, lowest serum bicarbonate, and diagnosis of pulmonary embolus remained independently associated with Doppler-defined PH [Table 2].
Risk factors for mortality
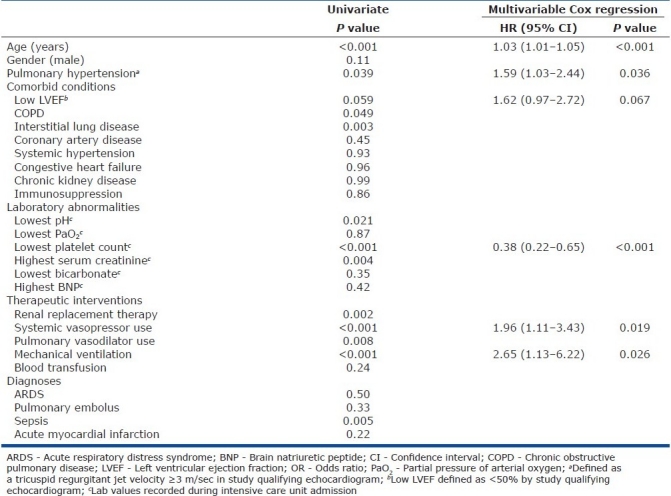
Of the examined variables, the following were significantly associated with in-hospital mortality in univariate analysis: age (P<0.001), chronic obstructive pulmonary disease (P=0.049) or interstitial lung disease (P=0.003), Doppler-defined PH (P=0.039), highest serum creatinine (P=0.004), lowest platelet count (P<0.001), diagnosis of sepsis (P=0.005), renal replacement therapy (P=0.002), mechanical ventilation (P<0.001), and use of either vasopressors (P<0.001) or pulmonary vasodilators (P=0.008) [Table 3].
Table 3.
Clinical variables and their association with mortality
Survival analysis
Kaplan–Meier survival analysis yielded significantly lower survival in those with Doppler-defined PH (log rank test P=0.05) [Figure 3]. The following clinical characteristics were included in a Cox proportional hazard analysis of variables associated with in-hospital mortality: age, gender, pre-existing history of chronic obstructive pulmonary disease or interstitial lung disease, Doppler-defined PH, low left ventricular ejection fraction, highest serum creatinine, lowest platelet count, diagnosis of sepsis, renal replacement therapy, mechanical ventilation and vasopressor use. When controlling for these clinical features, Doppler-defined PH remained significantly associated with mortality (HR=1.59, 95% CI=1.03–2.44, P=0.036)[Table 3].
Figure 3.
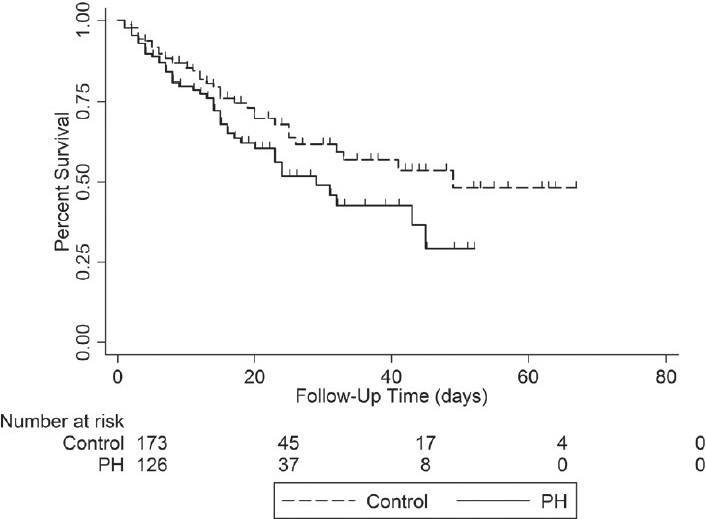
Kaplan–Meier survival curve of those with (PH) and without (control) Doppler-defined pulmonary hypertension. Log-rank test=0.05. Time of survival calculated as date of intensive care unit admission until death or hospital discharge (censoring)
DISCUSSION
This is the first report to indicate that Doppler-defined PH is common in a general medical intensive care unit population, with 126 of 449 patients (28%) undergoing echocardiography having evidence of PH, as defined by a TR jet velocity ≥3 m/sec. It is important to note that Doppler-defined PH in this investigation includes both pulmonary venous and pulmonary arterial hypertension, given the diagnostic reliance on echocardiography. The presence of Doppler-defined PH is associated with an increased risk of death, an effect that remains even after controlling for other clinical variables associated with mortality. Risk factors for the development of Doppler-defined PH in the medical intensive care unit include low left ventricular ejection fraction and a diagnosis of pulmonary embolism.
The definition of PH as a TR jet velocity ≥3 m/sec, which corresponds approximately to a systolic pulmonary artery pressure of ≥40 mmHg, is consistent with prior literature in other patient populations[13,14] and is in accordance with recent evidenced-based guidelines.[11] The distribution of TR jet velocities in a general intensive care unit population has not been previously reported. A TR jet velocity of 2.64 m/sec is reported to be 2 standard deviations above the mean in a normal population.[12] The mean TR jet velocity of 2.88 m/sec in our patient population is markedly higher than that seen in non-critically ill patients and suggests that PH is prevalent in the critically ill.
PH is a known complication of certain diseases commonly seen in the medical intensive care unit, such as ARDS and pulmonary embolism.[2–6,15] However, less is known of the burden of PH in a general medical critical care population. Our finding that at least 28% of patients undergoing echocardiography have an elevated TR jet velocity suggests that the burden of PH in a general medical intensive care unit population may be significant. Our retrospective study design inherently results in selection bias, however, and likely inflates the apparent burden of PH in the critically ill. Similarly, as not all patients admitted to the intensive care unit underwent echocardiography, the actual prevalence could not be determined with this study.
Our findings that depressed left ventricular ejection fraction and a diagnosis of pulmonary embolism are risk factors for the development of PH are consistent with the reports in literature.[9,16] While in accordance with prior reports, only a minority of patients with PH in our study had either a pulmonary embolus (12%) or depressed ejection fraction (23%), suggesting that these disorders were not the predominant comorbidities in our cohort. Admittedly, given the reliance on non-invasively measured pulmonary vascular pressures, a reduced left ventricular ejection fraction does not necessarily relate to post-capillary PH and, conversely, those with normal left ventricular function can still have post-capillary PH.[17] The association between low arterial partial pressure of oxygen and PH has been previously reported[5,6] and may reflect hypoxia-mediated pulmonary vasoconstriction. Multiple patients in our cohort did not undergo arterial blood gas measurement, limiting further interpretation. Our finding that low serum bicarbonate is an independent risk factor for PH is also consistent with prior literature. Teplinsky and coworkers reported that metabolic acidemia leads to increased pulmonary arterial pressures and depressed left ventricular stroke volume in a canine model.[18] Although acidemia may result in increased pulmonary arterial pressure, our finding of a lack of an association between blood pH and Doppler-defined PH in those patients who underwent arterial blood gas analysis suggests that the result obtained could also be due to chance.
The diagnosis of ARDS was not associated with Doppler-defined PH in our study. Although PH is commonly reported in the ARDS literature, the heterogeneous definitions of acute lung injury used in these studies and the selective use of diagnostic right heart catheterization make extrapolations between studies difficult and determination of true prevalence impossible.[3–5,19] Our use of ICD-9 diagnostic codes to define ARDS is similar to that used in other studies but resulted in few patients being diagnosed with ARDS.[10] Thus, our study may have lacked sensitivity in detecting ARDS and be underpowered.
We found a significant association between Doppler-defined PH and increased mortality. Moreover, Doppler-defined PH remained an independent predictor of death when controlling for other clinical variables associated with mortality. While not previously reported in a general medical critical care population, PH is independently associated with mortality in multiple other disorders, including pulmonary embolism,[9,15] congestive heart failure,[20] and chronic obstructive pulmonary disease.[21] The ARDS literature suggests a correlation between the presence of PH and worse outcomes,[3,5,22] although this finding has not been seen in all studies.[4] Bull and colleagues recently reported in a secondary analysis of a modern cohort of patients with acute lung injury, who underwent right heart catheterization, that an elevated transpulmonary gradient or pulmonary vascular resistance (both hemodynamic measures of pulmonary vascular dysfunction) predicted increased morbidity and mortality, while independent hemodynamic measurements, such as mean pulmonary artery or pulmonary artery occlusion pressure, were not associated with worse outcomes.[19] While intriguing, only a randomized study will be able to address the unresolved issue of whether PH is casually related to mortality or is simply a marker of disease severity.
The results of right heart catheterization in a subset of patients demonstrated that the presence of PH by echocardiography is a reliable predictor of true elevated mean pulmonary arterial pressures in the critically ill, with a positive predictive value of over 90%. However, the absence of an elevated TR jet velocity does not rule out PH, based on the lower negative predictive value found in our study. While the total number of right heart catheterization studies is low and should be interpreted with caution, echocardiography estimated systolic pulmonary artery pressures showed good correlation with invasive measurements.
The results of our investigation demonstrate robust external validity. Features that were found to be associated with Doppler-defined PH, including hypoxemia, reduced ejection fraction, and pulmonary embolus, are known risk factors for PH.[5,9,16,20] Clinical markers of increased mortality in the critically ill, including advanced age, requirement for mechanical ventilation, systemic vasopressor support, and renal replacement therapy, were found to predict mortality in our study cohort.[23,24]
Despite the strengths of our analysis and the validity of our data, the study has several limitations worth consideration. The single center retrospective design, and in particular, the inability to control which patients received an echocardiogram, introduces significant selection bias. Clearly, the patients in this study who underwent echocardiography were older and had higher mortality than those patients who did not; thus, our results cannot be extrapolated to all medical intensive care unit patients. Although echocardiography is a diagnostic tool available to most intensivists, the reliance on routine clinical echocardiography in this study prohibits further characterization of the contribution of left and right heart disease to the diagnosis of PH, information that may be obtained from invasive hemodynamic measurements and/or specialized echocardiographic techniques. Finally, the reliance on the electronic medical record for clinical information, with the attendant issues of missing data, accuracy, and use of ICD-9 codes, is a documented source of potential study error.[25,26]
In conclusion, in this initial hypothesis-generating investigation, we have shown that Doppler-defined PH in a general medical intensive care unit population is common and associated with an increased risk of mortality. Future prospective studies should attempt to more clearly define the prevalence of and risk factors for PH in a medical intensive care unit population. In addition, the balance of pulmonary venous versus pulmonary arterial hypertension, with clinical associations of each, needs to be defined. Given the lack of current knowledge regarding the balance and outcomes associated with pulmonary arterial versus pulmonary venous hypertension, empiric therapy of Doppler-detected PH in the critically ill with targeted pulmonary vascular medications cannot be recommended at this time. If pulmonary arterial hypertension independently predicts worsened outcomes in the critically ill, therapeutic trials of hemodynamic goal-directed therapy, using pulmonary vascular targeted medication and aimed at reducing pulmonary artery pressures and improving right ventricular function, may be indicated.
Acknowledgments
The authors would like to thank Thomas Richards, PhD, for his thoughtful review of the study's statistical plan and results.
Footnotes
Source of Support: None declared.
Conflict of Interest: Dr. Mathier reports receiving consulting fees (all less than US$10,000) from Actelion, Gilead, and United Therapeutics. Dr. Gladwin further reports receiving grant support from the Institute of Transfusion Medicine and the Hemophilia Center of Western Pennsylvania, and federal funding by the NHLBI, NIDDK and NIAMS of the National Institutes of Health (HL098032, HL096973, DK085852, AR058910). None of Dr. Gladwin's grant resources were used in this project and there is no funding source for this study.
REFERENCES
- 1.Zamanian RT, Haddad F, Doyle RL, Weinacker AB. Management strategies for patients with pulmonary hypertension in the intensive care unit. Crit Care Med. 2007;35:2037–50. doi: 10.1097/01.ccm.0000280433.74246.9e. [DOI] [PubMed] [Google Scholar]
- 2.Zapol WM, Snider MT. Pulmonary hypertension in severe acute respiratory failure. N Engl J Med. 1977;296:476–80. doi: 10.1056/NEJM197703032960903. [DOI] [PubMed] [Google Scholar]
- 3.Sloane PJ, Gee MH, Gottlieb JE, Albertine KH, Peters SP, Burns JR, et al. A multicenter registry of patients with acute respiratory distress syndrome. Physiology and outcome. Am Rev Respir Dis. 1992;146:419–26. doi: 10.1164/ajrccm/146.2.419. [DOI] [PubMed] [Google Scholar]
- 4.Suchyta MR, Clemmer TP, Elliott CG, Orme JF, Jr, Weaver LK. The adult respiratory distress syndrome. A report of survival and modifying factors. Chest. 1992;101:1074–9. doi: 10.1378/chest.101.4.1074. [DOI] [PubMed] [Google Scholar]
- 5.Squara P, Dhainaut JF, Artigas A, Carlet J. Hemodynamic profile in severe ARDS: Results of the European Collaborative ARDS Study. Intensive Care Med. 1998;24:1018–28. doi: 10.1007/s001340050710. [DOI] [PubMed] [Google Scholar]
- 6.Vieillard-Baron A, Schmitt JM, Augarde R, Fellahi JL, Prin S, Page B, et al. Acute cor pulmonale in acute respiratory distress syndrome submitted to protective ventilation: Incidence, clinical implications, and prognosis. Crit Care Med. 2001;29:1551–5. doi: 10.1097/00003246-200108000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Wynne R, Botti M. Postoperative pulmonary dysfunction in adults after cardiac surgery with cardiopulmonary bypass: Clinical significance and implications for practice. Am J Crit Care. 2004;13:384–93. [PubMed] [Google Scholar]
- 8.Subramaniam K, Yared JP. Management of pulmonary hypertension in the operating room. Semin Cardiothorac Vasc Anesth. 2007;11:119–36. doi: 10.1177/1089253207301733. [DOI] [PubMed] [Google Scholar]
- 9.Kucher N, Rossi E, De Rosa M, Goldhaber SZ. Prognostic role of echocardiography among patients with acute pulmonary embolism and a systolic arterial pressure of 90 mm Hg or higher. Arch Intern Med. 2005;165:1777–81. doi: 10.1001/archinte.165.15.1777. [DOI] [PubMed] [Google Scholar]
- 10.Reynolds HN, McCunn M, Borg U, Habashi N, Cottingham C, Bar-Lavi Y. Acute respiratory distress syndrome: Estimated incidence and mortality rate in a 5 million-person population base. Crit Care. 1998;2:29–34. doi: 10.1186/cc121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Badesch DB, Champion HC, Sanchez MA, Hoeper MM, Loyd JE, Manes A, et al. Diagnosis and assessment of pulmonary arterial hypertension. J Am Coll Cardiol. 2009;54:S55–66. doi: 10.1016/j.jacc.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 12.McQuillan BM, Picard MH, Leavitt M, Weyman AE. Clinical correlates and reference intervals for pulmonary artery systolic pressure among echocardiographically normal subjects. Circulation. 2001;104:2797–802. doi: 10.1161/hc4801.100076. [DOI] [PubMed] [Google Scholar]
- 13.Arcasoy SM, Christie JD, Ferrari VA, Sutton MS, Zisman DA, Blumenthal NP, et al. Echocardiographic assessment of pulmonary hypertension in patients with advanced lung disease. Am J Respir Crit Care Med. 2003;167:735–40. doi: 10.1164/rccm.200210-1130OC. [DOI] [PubMed] [Google Scholar]
- 14.Allanore Y, Borderie D, Meune C, Cabanes L, Weber S, Ekindjian OG, et al. N-terminal pro-brain natriuretic peptide as a diagnostic marker of early pulmonary artery hypertension in patients with systemic sclerosis and effects of calcium-channel blockers. Arthritis Rheum. 2003;48:3503–8. doi: 10.1002/art.11345. [DOI] [PubMed] [Google Scholar]
- 15.Vieillard-Baron A, Page B, Augarde R, Prin S, Qanadli S, Beauchet A, et al. Acute cor pulmonale in massive pulmonary embolism: Incidence, echocardiographic pattern, clinical implications and recovery rate. Intensive Care Med. 2001;27:1481–6. doi: 10.1007/s001340101032. [DOI] [PubMed] [Google Scholar]
- 16.Konstantinides S, Geibel A, Heusel G, Heinrich F, Kasper W. Heparin plus alteplase compared with heparin alone in patients with submassive pulmonary embolism. N Engl J Med. 2002;347:1143–50. doi: 10.1056/NEJMoa021274. [DOI] [PubMed] [Google Scholar]
- 17.Hoeper MM, Barberà JA, Channick RN, Hassoun PM, Lang IM, Manes A, et al. Diagnosis, assessment, and treatment of non-pulmonary arterial hypertension pulmonary hypertension. J Am Coll Cardiol. 2009;54:S85–96. doi: 10.1016/j.jacc.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 18.Teplinsky K, O’Toole M, Olman M, Walley KR, Wood LD. Effect of lactic acidosis on canine hemodynamics and left ventricular function. Am J Physiol. 1990;258:H1193–9. doi: 10.1152/ajpheart.1990.258.4.H1193. [DOI] [PubMed] [Google Scholar]
- 19.Bull TM, Clark B, McFann K, Moss M. National Institutes of Health/National Heart, Lung, and Blood Institute ARDS Network. Pulmonary vascular dysfunction is associated with poor outcomes in patients with acute lung injury. Am J Respir Crit Care Med. 2010;182:1123–8. doi: 10.1164/rccm.201002-0250OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grigioni F, Potena L, Galiè N, Fallani F, Bigliardi M, Coccolo F, et al. Prognostic implications of serial assessments of pulmonary hypertension in severe chronic heart failure. J Heart Lung Transplant. 2006;25:1241–6. doi: 10.1016/j.healun.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 21.Oswald-Mammosser M, Weitzenblum E, Quoix E, Moser G, Chaouat A, Charpentier C, et al. Prognostic factors in COPD patients receiving long-term oxygen therapy. Importance of pulmonary artery pressure. Chest. 1995;107:1193–8. doi: 10.1378/chest.107.5.1193. [DOI] [PubMed] [Google Scholar]
- 22.Villar J, Blazquez MA, Lubillo S, Quintana J, Manzano JL. Pulmonary hypertension in acute respiratory failure. Crit Care Med. 1989;17:523–6. doi: 10.1097/00003246-198906000-00007. [DOI] [PubMed] [Google Scholar]
- 23.Ware LB. Prognostic determinants of acute respiratory distress syndrome in adults: Impact on clinical trial design. Crit Care Med. 2005;33:S217–22. doi: 10.1097/01.ccm.0000155788.39101.7e. [DOI] [PubMed] [Google Scholar]
- 24.Vincent JL, Ferreira F, Moreno R. Scoring systems for assessing organ dysfunction and survival. Crit Care Clin. 2000;16:353–66. doi: 10.1016/s0749-0704(05)70114-7. [DOI] [PubMed] [Google Scholar]
- 25.Ward NS. The accuracy of clinical information systems. J Crit Care. 2004;19:221–5. doi: 10.1016/j.jcrc.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 26.Howard AE, Courtney-Shapiro C, Kelso LA, Goltz M, Morris PE. Comparison of 3 methods of detecting acute respiratory distress syndrome: Clinical screening, chart review, and diagnostic coding. Am J Crit Care. 2004;13:59–64. [PubMed] [Google Scholar]


