Dysfunctional bone morphogenetic protein (BMP) signaling has been found in patients with pulmonary arterial hypertension (PAH); however, the exact role of BMP signaling in the treatment of PAH remains unknown. The BMP receptor type II (BMPR-II) is a member of the TGF-β family of signaling molecules. Functional receptors are heterodimers composed of a BMPR-II subunit and a serine–threonine kinase type I subunit, of which there are three members: BMPR-Ia, BMPR-Ib and Alk2.[1,2] Both BMPR-II and BMPR-Ia/Ib are highly expressed in the pulmonary vascular smooth muscle and endothelium. The discovery of heterozygous mutations of the BMPR-II gene (BMPR2) in patients with hereditary (or familial) PAH and patients with idiopathic PAH[3,4] represented a significant advance in the understanding of the genetic contributions to PAH.
The two main pathways downstream of BMP signaling are the Smad-dependent pathway, which uses Smad-signaling proteins, and the Smad-independent pathway, which involves p38, MAPK, ERK and JNK proteins.[1,5,6] In the Smad pathway, activation of the receptor complex by ligand binding results in the recruitment and phosphorylation of regulatory Smad proteins (Smads-1, 5 or 8). These Smad proteins recruit Smad-4, and the resulting complex is translocated to the nucleus, where it regulates transcription of target genes containing the Smad-binding sequence. Although BMP signaling may provide a common pathway in PAH pathogenesis, it is unclear whether current treatments targeting different pathways lead to an increase in BMP signal transduction in the lung tissues. A treatment that targets a common underlying cause of PAH, such as BMP dysfunction, may prove efficacious.
Both sildenafil, a phosphodiesterase-5 inhibitor, and simvastatin, a cholesterol-lowering drug, have therapeutic effects on PAH. In our previous studies, we investigated three different therapeutic regimens for treating pulmonary hypertension (PH) in rats injected with monocrotaline (MCT) using sildenafil, simvastatin and a combined sildenafil and simvastatin treatment. MCT injections cause a significant increase in the mean pulmonary arterial pressure (PAP) and pulmonary vascular medial thickening. The time course for the MCT-induced increase in mean PAP is associated with the MCT-induced increase in pulmonary vascular wall thickness, occurring after 2 weeks of the injection of MCT.[7] Although all treatment regimens have an effect on right ventricular systolic pressure (RVSP) in rats with MCT-mediated PH, the combined drug treatment of both sildenafil and simvastatin is more effective in reducing RVSP than either drug alone.[7]
The purpose of this study was to investigate the changes in BMP signaling, including both Smad and ERK downstream pathways, in rats with MCT-induced PH treated with sildenafil and simvastatin.
We first examined whether key BMP molecules (BMPR-II, BMPR-Ia and BMP-2) were changed in lung tissues over a 4-week time course in rats subcutaneously injected with MCT (50 mg/kg or 25 ml/kg of 2% MCT). Interestingly, animals at both weeks 1 and 2 (after injection of MCT) had significantly increased levels of BMPR-II (3.655±1.008 in week 1 group and 2.164±0.508 in week 2 group compared to 1.874±0.121 in the saline injected control), BMPR-Ia (1.451±0.334 in week 1 group and 1.082±0.106 in week 2 group compared to 0.625±0.188 in the saline injected control animals), and BMP-2 (1.500±0.243 in the week 1 group and 1.428±0.056 in week 2 group vs. 0.696±0.068 in the saline injected control group); however, by the third week, BMPR-II had declined to levels below those of the control group and, by the fourth week, BMP-2 had also declined to below the control levels [Figure 1]. Our observations indicate that MCT injection caused a transient increase in the mRNA and protein expression of BMP-2, BMPR-II and BMPR-Ia, which correlates with a transient increase in Smad1 phosphorylation.[7] The changes in BMP signaling molecules correlate well with the observed MCT-induced PH at 3 and 4 weeks.[7] Thus, both hemodynamic indicators of PAH and underlying molecular abnormalities (e.g., BMP-signaling molecules and Smad) correlate with each other in rats during the development of MCT-induced PH.
Figure 1.
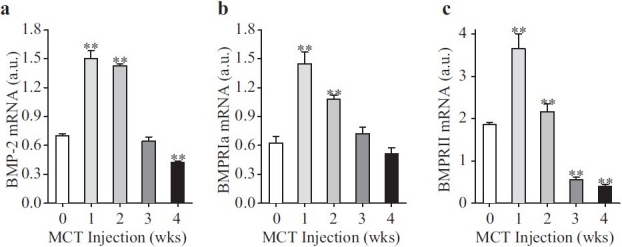
Bone morphogenetic protein (BMP) signaling proteins in rat lung initially increase and then decrease after monocrotaline (MCT) injection. mRNA was collected from whole lung from rats injected with MCT at the indicated time points after injection. The week 0 group was injected with saline and RNA was isolated the next day. mRNA was used in reverse transcriptase-polymerase chain reaction to quantify BMP-2 (a), BMPR-Ia (b) and BMPR-II (c) mRNA levels. All BMP signaling molecule mRNA levels were normalized against Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA levels. Data are presented in arbitrary units (a.u.) as mean±SD; n=8/group; **P<0.01 vs. 0 week control group
Then, we examined the effects of sildenafil, simvastatin and combination treatment on BMPR-Ia, BMPR-II and BMP-2 mRNA levels in our MCT model of PH. Compared with rats in the sham group (normotensive saline-injected rats), lung tissue BMPR-Ia, BMPR-II and BMP-2 mRNA levels detected by real-time reverse transcriptase polymerase chain reaction (RT-PCR) in the MCT-injected control group decreased significantly (BMPR-Ia: 0.50±0.09 compared with 0.39±0.11; BMPR-II: 1.75±0.28 compared with 0.40±0.02; BMP-2: 0.68±0.02 compared with 0.37±0.05 arbitrary unit) [Figure 2A(a–c)]. Either sildenafil or simvastatin treatment alone prevented decreases in BMPR-Ia, BMPR-II and BMP-2 mRNA levels, but the combination therapy was more effective at preventing the decrease in BMPR-II and BMPR-Ia [Figure 2B(b and c)]. The level of BMP-2 in the combination group, while being higher than that in the MCT-injected control animals, was not significantly different from either treatment alone [Figure 2B(a)].
Figure 2.
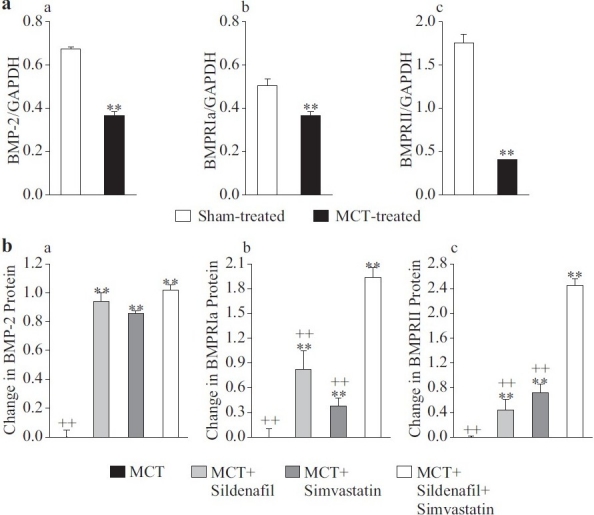
Combined sildenafil and simvastatin treatment prevents the monocrotaline (MCT)-induced decrease in bone morphogenetic protein (BMP)-signal pathway molecules to a greater extent than either drug alone. mRNA was collected from whole lung tissue from saline-injected sham treated rats, MCT-injected control rats and rats treated with sildenafil, simvastatin or both. mRNA levels of BMP-2 (Aa), BMPR-Ia (Ab) and BMPR-II (Ac) were determined using reverse transcriptase-polymerase chain reaction and normalized to GAPDH. Increases in BMP-2 (a), BMPR-Ia (b) and BMPR-II (c) are shown in (B) as the change compared with the levels in the MCT-injected control group. Data are presented in arbitrary units (A) as mean±SD; n=8/group; **P<0.01 vs. sham-injected (A) or vs. MCT-injected control (B); ++P<0.01 vs. combination group (B)
BMP signaling, including BMP-2 and its receptors, plays an important role in maintaining the normal structure of the pulmonary vasculature through its regulation of apoptosis and suppression of cell proliferation.[8–10] In the pulmonary vasculature, BMP-2 is predominantly expressed in endothelial cells, with a low level of expression in vascular smooth muscle cells, whereas BMPR-II localizes to endothelial cells, smooth muscle cells and adventitial fibroblasts.[8] In both apical and basal membranes of the arteriolar endothelium, BMPR-II colocalizes with caveolin-1,[11] indicating that BMPR-II may have a hemodynamic regulatory role because caveolae also contains hemodynamically relevant signaling molecules, including eNOS,[12,13] the serotonin transporter,[14–16] transient receptor potential (TRP) channels[17,18] and endothelin receptors.[19,20]
In our study, the expression of BMP-2, BMPR-Ia and BMPR-II mRNAs during the 4-week course of MCT-induced PH increased significantly in the first week after MCT injection. At week 2, these levels were still above baseline, although they had started to decline from week 1. This initial increase was followed by a significant decrease 3–4 weeks after MCT injection. We did not study BMP signaling or pulmonary artery smooth muscle cell (PASMC) proliferation in MCT-treated rats beyond 4 weeks after injection; however, within the 4-week time course, we observed an important difference between MCT-induced PAH and clinical PAH caused by defective BMP signaling. In many patients, dysfunctional BMP signaling due to BMPR-II mutations and/or downregulated BMPR-Ia causes PAH.[21–24] However, in the MCT model, there was an initial increase in BMP signaling molecules as previously noted. The subsequent decline in these correlated with the development of elevated RVSP, right ventricular hypertrophy (RVH) and remodeling.[7] An initial increase in BMP signaling molecules in models of PAH is not without precedent, however, as hypoxia induces an initial rise (~3 weeks) in BMP4 in mouse lung followed by a decrease in BMP4 mRNA after week 3.[25] It may be that initial MCT-induced endothelial injury causes the subsequent rise in BMP signaling. Lung tissue p-Smad1, a downstream signaling molecule involved in proapoptotic BMPR-II signaling, paralleled the changes of BMP2, BMPR-Ia and BMPR-II mRNAs, consistent with the observation of previous studies.[26,27] This pattern suggests that acute injury to the pulmonary vascular endothelium may stimulate BMPR-II, BMPR-Ia and BMP-2 expression in lung vascular endothelial cells, whereas chronic injury results in a decline of these molecules.
BMPR-II signaling in pulmonary artery endothelial cells (PAEC) promotes cell survival and protects against apoptosis, whereas it is proapoptotic (through Smad signaling) in PASMC.[10,28] It is therefore tempting to speculate that PAEC respond to MCT injury initially by increasing BMP signaling to promote endothelial protection; the subsequent MCT-induced decline in BMP-signaling molecules may then tip the balance toward an increase in PASMC proliferation, which overtakes the already injured endothelium, thus leading to muscularization and PH by week 3 after MCT injection. Indeed, the pulmonary vascular remodeling associated with clinical PAH is believed to result largely from increased proliferation and decreased apoptosis of PASMC that could be caused by defects in the proapoptotic BMPR-II/Smad signaling pathway.[6,21–23,28,29]
Our results suggest that targeting BMPR-II deficiencies in PAH may provide a useful therapeutic approach to treat the disease. Current PAH medications do not target the BMP pathway, but a more complete understanding of BMPR-II signaling in the MCT model of PH, such as provided here, will prove useful in future investigations aimed at developing interventions that target this pathway.
The levels of lung tissue BMPR-II, BMPR-Ia and BMP-2 mRNA and p-Smad1 protein were much higher in both the sildenafil and the simvastatin groups compared with the MCT-injected control group, indicating that either drug alone inhibits the decrease in the expression of the BMP signaling molecules. The inhibition of the loss of BMPR-II signaling may be an important mechanism underlying the prevention of PASMC overproliferation and pulmonary vascular remodeling in the sildenafil or simvastatin treatment groups. The low levels of matrix metalloproteinases (MMPs) may be due to the concomitant increase in BMP-signal transduction, as the reduction in BMP signaling has been shown to reduce MMP activity.[30] Furthermore, reductions in BMP signaling and/or MMP activity confer resistance to apoptosis,[30] indicating that sildenafil and simvastatin may reduce PASMC proliferation at least in part by maintaining BMP signaling molecules.
Because combination treatment was able to prevent the decrease in lung tissue BMPR-II and BMPR-Ia to a greater extent than either treatment alone, it is likely that the additive effect of combination treatment in preventing the development of PH involves BMP signaling.
Acknowledgments
We would like to thank Dr. Ling Zhu for her helpful comments in preparing this manuscript. This work was supported in part by grants from the National Natural Science Foundation of China (NSFC-30810103904) and 973 Program (2009CB522107).
Footnotes
Source of Support: National Natural Science Foundation of China (NSFC-30810103904) and 973 Program (2009CB522107)
Conflict of Interest: None declared.
REFERENCES
- 1.Nohe A, Keating E, Knaus P, Petersen NO. Signal transduction of bone morphogenetic protein receptors. Cell Signal. 2004;16:291–9. doi: 10.1016/j.cellsig.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 2.Yu PB, Beppu H, Kawai N, Li E, Bloch KD. Bone morphogenetic protein (BMP) type II receptor deletion reveals BMP ligand-specific gain of signaling in pulmonary artery smooth muscle cells. J Biol Chem. 2005;280:24443–50. doi: 10.1074/jbc.M502825200. [DOI] [PubMed] [Google Scholar]
- 3.Machado RD, Pauciulo MW, Thomson JR, Lane KB, Morgan NV, Wheeler L, et al. BMPR2 haploinsufficiency as the inherited molecular mechanism for primary pulmonary hypertension. Am J Hum Genet. 2001;68:92–102. doi: 10.1086/316947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Newman JH, Wheeler L, Lane KB, Loyd E, Gaddipati R, Phillips JA, 3rd, et al. Mutation in the gene for bone morphogenetic protein receptor II as a cause of primary pulmonary hypertension in a large kindred. N Engl J Med. 2001;345:319–24. doi: 10.1056/NEJM200108023450502. [DOI] [PubMed] [Google Scholar]
- 5.Nohe A, Hassel S, Ehrlich M, Neubauer F, Sebald W, Henis YI, et al. The mode of bone morphogenetic protein (BMP) receptor oligomerization determines different BMP-2 signaling pathways. J Biol Chem. 2002;277:5330–8. doi: 10.1074/jbc.M102750200. [DOI] [PubMed] [Google Scholar]
- 6.Mandegar M, Fung YC, Huang W, Remillard CV, Rubin LJ, Yuan JX. Cellular and molecular mechanisms of pulmonary vascular remodeling: Role in the development of pulmonary hypertension. Microvasc Res. 2004;68:75–103. doi: 10.1016/j.mvr.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 7.Kuang T, Wang J, Pang B, Huang X, Burg ED, Yuan JX, et al. Combination of sildenafil and simvastatin ameliorates monocrotaline-induced pulmonary hypertension in rats. Pulm Pharmacol Ther. 2010;23:456–64. doi: 10.1016/j.pupt.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takahashi H, Goto N, Kojima Y, Tsuda Y, Morio Y, Muramatsu M, et al. Downregulation of type II bone morphogenetic protein receptor in hypoxic pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2006;290:L450–8. doi: 10.1152/ajplung.00206.2005. [DOI] [PubMed] [Google Scholar]
- 9.Fukui N, Zhu Y, Maloney WJ, Clohisy J, Sandell LJ. Stimulation of BMP-2 expression by pro-inflammatory cytokines IL-1 and TNF-a in normal and osteoarthritic chondrocytes. J Bone Joint Surg Am. 2003;85-A:59–66. doi: 10.2106/00004623-200300003-00011. [DOI] [PubMed] [Google Scholar]
- 10.Zhang S, Fantozzi I, Tigno DD, Yi ES, Platoshyn O, Thistlethwaite PA, et al. Bone morphogenetic proteins induce apoptosis in human pulmonary vascular smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2003;285:L740–54. doi: 10.1152/ajplung.00284.2002. [DOI] [PubMed] [Google Scholar]
- 11.Ramos M, Lame MW, Segall HJ, Wilson DW. The BMP type II receptor is located in lipid rafts, including caveolae, of pulmonary endothelium in vivo and in vitro. Vascul Pharmacol. 2006;44:50–9. doi: 10.1016/j.vph.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 12.Garcia-Cardena G, Oh P, Liu J, Schnitzer JE, Sessa WC. Targeting of nitric oxide synthase to endothelial cell caveolae via palmitoylation: Implications for nitric oxide signaling. Proc Natl Acad Sci U S A. 1996;93:6448–53. doi: 10.1073/pnas.93.13.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shaul PW, Smart EJ, Robinson LJ, German Z, Yuhanna IS, Ying Y, et al. Acylation targets emdothelial nitric-oxide synthase to plasmalemmal caveolae. J Biol Chem. 1996;271:6518–22. doi: 10.1074/jbc.271.11.6518. [DOI] [PubMed] [Google Scholar]
- 14.Fiorica-Howells E, Hen R, Gingrich J, Li Z, Gershon MD. 5-HT2A receptors: Location and functional analysis in intestines of wild-type and 5-HT2A knockout mice. Am J Physiol Gastrointest Liver Physiol. 2002;282:G877–93. doi: 10.1152/ajpgi.00435.2001. [DOI] [PubMed] [Google Scholar]
- 15.Dreja K, Voldstedlund M, Vinten J, Tranum-Jensen J, Hellstrand P, Sward K. Cholesterol depletion disrupts caveolae and differentially impairs agonist-induced arterial contraction. Arterioscler Thromb Vasc Biol. 2002;22:1267–72. doi: 10.1161/01.atv.0000023438.32585.a1. [DOI] [PubMed] [Google Scholar]
- 16.Bhatnagar A, Sheffler DJ, Kroeze WK, Compton-Toth B, Roth BL. Caveolin-1 interacts with 5-HT2A serotonin receptors and profoundly modulates the signaling of selected Gaq-coupled protein receptors. J Biol Chem. 2004;279:34614–23. doi: 10.1074/jbc.M404673200. [DOI] [PubMed] [Google Scholar]
- 17.Lockwich TP, Liu X, Singh BB, Jadlowiec J, Weiland S, Ambudkar IS. Assembly of Trp1 in a signaling complex associated with caveolin-scaffolding lipid raft domains. J Biol Chem. 2000;275:11934–42. doi: 10.1074/jbc.275.16.11934. [DOI] [PubMed] [Google Scholar]
- 18.Bergdahl A, Gomez MF, Dreja K, Xu SZ, Adner M, Beech DJ, et al. Cholesterol depletion impairs vascular reactivity to endothelin-1 by reducing store-operated Ca2+ entry dependent on TRPC1. Circ Res. 2003;93:839–47. doi: 10.1161/01.RES.0000100367.45446.A3. [DOI] [PubMed] [Google Scholar]
- 19.Chun M, Liyanage UK, Lisanti MP, Lodish HF. Signal transduction of a G protein-coupled receptor in caveolae: Colocalization of endothelin and its receptor with caveolin. Proc Natl Acad Sci U S A. 1994;91:11728–32. doi: 10.1073/pnas.91.24.11728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okamoto Y, Ninomiya H, Miwa S, Masaki T. Cholesterol oxidation switches the internalization pathway of endothelin receptor type A from caveolae to clathrin-coated pits in Chinese hamster ovary cells. J Biol Chem. 2000;275:6439–46. doi: 10.1074/jbc.275.9.6439. [DOI] [PubMed] [Google Scholar]
- 21.Long L, Crosby A, Yang X, Southwood M, Upton PD, Kim DK, et al. Altered bone morphogenetic protein and transforming growth factor-b signaling in rat models of pulmonary hypertension: Potential for activin receptor-like kinase-5 inhibition in prevention and progression of disease. Circulation. 2009;119:566–76. doi: 10.1161/CIRCULATIONAHA.108.821504. [DOI] [PubMed] [Google Scholar]
- 22.Schwappacher R, Weiske J, Heining E, Ezerski V, Marom B, Henis YI, et al. Novel crosstalk to BMP signalling: cGMP-dependent kinase I modulates BMPR receptor and Smad activity. EMBO J. 2009;28:1537–50. doi: 10.1038/emboj.2009.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morrell NW. Pulmonary hypertension due to BMPR2 mutation: A new paradigm for tissue remodeling? Proc Am Thorac Soc. 2006;3:680–6. doi: 10.1513/pats.200605-118SF. [DOI] [PubMed] [Google Scholar]
- 24.Davies RJ, Morrell NW. Molecular mechanisms of pulmonary arterial hypertension: Role of mutations in the bone morphogenetic protein type II receptor. Chest. 2008;134:1271–7. doi: 10.1378/chest.08-1341. [DOI] [PubMed] [Google Scholar]
- 25.Frank DB, Abtahi A, Yamaguchi DJ, Manning S, Shyr Y, Pozzi A, et al. Bone morphogenetic protein 4 promotes pulmonary vascular remodeling in hypoxic pulmonary hypertension. Circ Res. 2005;97:496–504. doi: 10.1161/01.RES.0000181152.65534.07. [DOI] [PubMed] [Google Scholar]
- 26.Ramos MF, Lame MW, Segall HJ, Wilson DW. Smad signaling in the rat model of monocrotaline pulmonary hypertension. Toxicol Pathol. 2008;36:311–20. doi: 10.1177/0192623307311402. [DOI] [PubMed] [Google Scholar]
- 27.Morty RE, Nejman B, Kwapiszewska G, Hecker M, Zakrzewicz A, Kouri FM, et al. Dysregulated bone morphogenetic protein signaling in monocrotaline-induced pulmonary arterial hypertension. Arterioscler Thromb Vasc Biol. 2007;27:1072–8. doi: 10.1161/ATVBAHA.107.141200. [DOI] [PubMed] [Google Scholar]
- 28.Eickelberg O, Morty RE. Transforming growth factor b/bone morphogenic protein signaling in pulmonary arterial hypertension: Remodeling revisited. Trends Cardiovasc Med. 2007;17:263–9. doi: 10.1016/j.tcm.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 29.Rubin LJ. Pulmonary arterial hypertension. Proc Am Thorac Soc. 2006;3:111–5. doi: 10.1513/pats.200510-112JH. [DOI] [PubMed] [Google Scholar]
- 30.El-Bizri N, Guignabert C, Wang L, Cheng A, Stankunas K, Chang CP, et al. SM22a-targeted deletion of bone morphogenetic protein receptor 1A in mice impairs cardiac and vascular development, and influences organogenesis. Development. 2008;135:2981–91. doi: 10.1242/dev.017863. [DOI] [PMC free article] [PubMed] [Google Scholar]


