Abstract
The acute respiratory distress syndrome (ARDS) is a complex disorder of heterogeneous etiologies characterized by a consistent, recognizable pattern of lung injury. Extensive epidemiologic studies and clinical intervention trials have been conducted to address the high mortality of this disorder and have provided significant insight into the complexity of studying new therapies for this condition. The existing clinical investigations in ARDS will be highlighted in this review. The limitations to current definitions, patient selection, and outcome assessment will be considered. While significant attention has been focused on the parenchymal injury that characterizes this disorder and the clinical support of gas exchange function, relatively limited focus has been directed to hemodynamic and pulmonary vascular dysfunction equally prominent in the disease. The limited available clinical information in this area will also be reviewed. The current standards for cardiopulmonary management of the condition will be outlined. Current gaps in our understanding of the clinical condition will be highlighted with the expectation that continued progress will contribute to a decline in disease mortality.
Keywords: hypoxia, hypoxic pulmonary vasoconstriction, clinical trial, gas exchange, ARDS, adult respiratory distress syndrome, acute lung injury, positive end expiratory pressure, acute cor pulmonale
INTRODUCTION
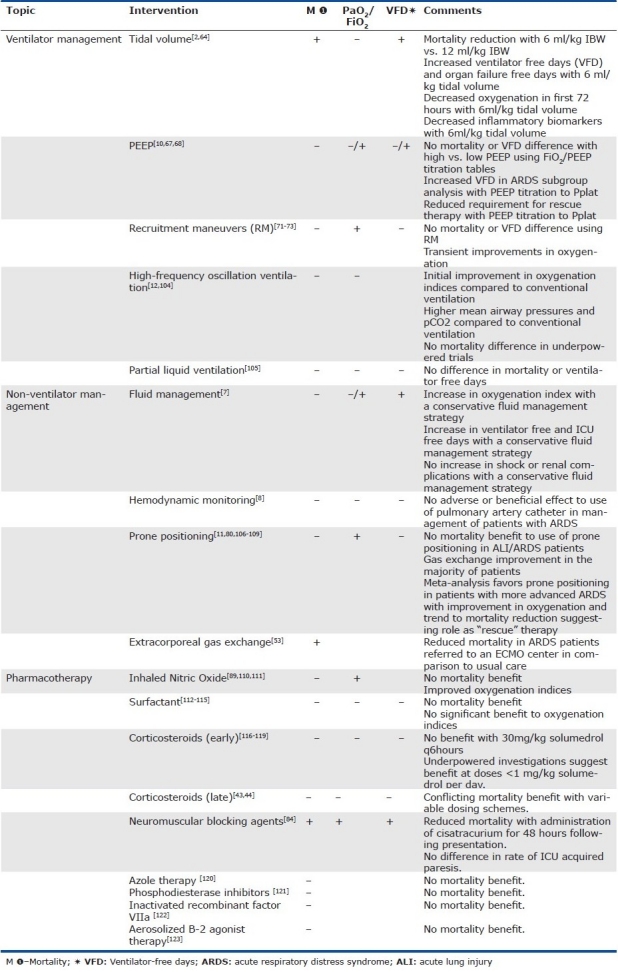
The acute respiratory distress syndrome (ARDS) is a rapidly progressive form of acute respiratory failure characterized by severe hypoxemia and non-hydrostatic pulmonary edema. The syndrome represents a recognizable common pattern of acute alveolar-capillary injury in critically ill patients, yet a pathway triggered by a wide range of primary disease processes. The mechanisms by which diverse etiologies such as chest trauma, sepsis, and pancreatitis lead to a common clinical and pathologic syndrome remains unclear. Epidemiologic surveys confirm the impact of this clinical syndrome is significant at ~200,000 cases per year in the US alone, leading to significant patient morbidity and health care burden.[1] Grouping a diverse set of disparate illnesses into a common syndrome has allowed investigation of ARDS as a final common pathway. The syndrome has facilitated a broad range of clinical investigations into the epidemiology, basic biology, and clinical support measures for this syndrome. Yet, despite numerous randomized clinical trials aimed at regulating the lung inflammatory response, the only proven therapy to consistently reduce mortality is a protective ventilation strategy.[2] The risk of linking multiple diverse etiologies as a single common pathway is an enhanced focus on the syndrome and its clinical management, with a diminished view of the importance of the underlying cause. Specific treatments, when applied to a non-specific condition, might be expected to show variable effectiveness. This may explain the relative paucity of successful therapeutic interventions in ARDS to date (Table 1). In this review, we will explore the clinical features of ARDS including the evolution of the ARDS definition, the limitations to investigation as a common disease pathway, the current evidence to guide cardiopulmonary management in this disorder, and consider directions for future clinical investigation.
Table 1.
Select clinical trials in ARDS
DEFINING A SYNDROME
Ashbaugh and colleagues established ARDS as a clinical entity in a case series reported in 1967.[3] They highlighted a respiratory distress syndrome in 12 patients manifested by the acute onset of tachypnea, hypoxemia, and loss of compliance after a variety of stimuli. The syndrome proved to be unresponsive to usual and ordinary methods of respiratory therapy. The clinical and pathological features resembled those seen in infants with respiratory distress and to conditions in congestive atelectasis and post perfusion lung injury. A theoretical relationship of this syndrome to alveolar surface-active agent was postulated. The ARDS syndrome was based upon five key clinical features: (1) the presence of a defined risk factor; (2) severe hypoxemia despite administration of supplemental oxygen; (3) bilateral pulmonary infiltrates; (4) reduced lung compliance; and (5) the absence of congestive heart failure.
In 1988, Murray and colleagues, attempted to expand the definition of ARDS to incorporate the risk factor, the relative acuteness of the disease process, and measures of severity.[4] The severity was graded using a Lung Injury Score (LIS), incorporating physiologic data representing oxygenation, positive end-expiratory pressure, compliance, and radiographic distribution. The LIS is often referenced in clinical trials of ARDS, but remains invalidated as a marker of mortality risk.[5]
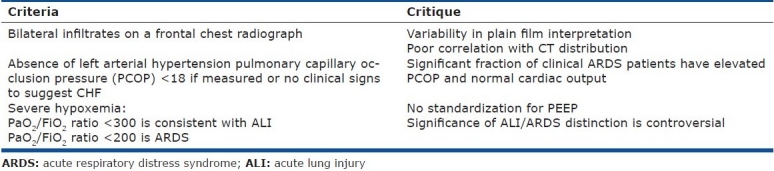
In 1994, a joint American-European Consensus Conference (AECC) met to refine the definition of ARDS to standardize clinical research trials for the disease. The definition is summarized in Table 2.[6] The definition has subsequently been widely employed to define patient enrollment in a broad range of ARDS therapeutic trials.[2,7–12] Despite the apparent simplicity of this definition, a number of clinical limitations are recognized.
Table 2.
American European consensus definition of ARDS
DIAGNOSTIC LIMITATIONS
The pathology of ARDS is characterized by the evolution of interstitial and alveolar edema to advanced fibrosis. The characteristic lesion, termed diffuse alveolar damage, undergoes progression from an exudative, to proliferative, to a fibrotic phase. Pathologically, the lung evolves through these phases of injury and remodeling independent of the inciting cause, supporting the ARDS syndrome classification. Yet, studies of clinical–pathologic correlation have shown only modest agreement between the pathologic finding of diffuse alveolar damage and the AECC diagnostic criteria.[13–16] More than half of patients referred for open lung biopsy in ARDS of unknown etiology, prove to have unanticipated diagnoses.[14,17,18] These published series of lung biopsy in acute lung injury (ALI)/ARDS criteria patients provide two important insights into the management of these patients. First, consideration can be given to open lung biopsy in ARDS patients, often performed at the bedside in reported series, as an important diagnostic tool when an exhaustive clinical workup including chest CT, bronchoalveolar lavage, and laboratory investigation fails to yield a specific inciting agent. Published series suggest that biopsy under these conditions can be safely performed and provides a significant diagnosis to alter therapy in 70-80% of patients.[13–16] Biopsies have occurred a median of 1-2 weeks into ARDS therapy based upon the lack of an inciting agent and limited clinical improvement. However, a lack of randomization in these trials makes a definitive comparison to empiric therapy difficult to determine.
Secondly, the variable clinical-pathologic correlation between the AECC clinical criteria for ALI/ARDS and lung pathology suggests that we might expect clinical trials using AECC inclusion criteria to have a broader range of pulmonary disease than expected. Do the AECC diagnostic criteria have additional limitations?
The AECC oxygenation criteria do not account for variations in the PaO2/FiO2 in response to varying levels of positive end-expiratory pressure (PEEP). A patient with a PaO2/FiO2 ratio <200 on 15 cm H2O PEEP is considered equivalent to a patient with a similar ratio on a PEEP of 5 cm H2O. Investigators have advocated that a standardized PEEP/FiO2 assessment is necessary to accurately classify ARDS severity to match prognostic outcome for study groups in clinical trials.[19,20] In one population of 170 patients that met AECC criteria for ARDS, a standardized assessment on PEEP>10 cm H20 and FiO2 >0.5 demonstrated 99 patients (58.2%) continued to meet the AECC definition of ARDS, 55 patients (32%) were reclassified as acute lung injury (ALI), and 16 patients (9.4%) no longer met criteria for either (Fig. 1).[19] Most importantly, the reclassification was associated with a mortality rate of 45.5% in the reclassified ARDS group, 20% in the ALI group, and 6.3% in patients reclassified as acute respiratory failure without ALI. Failure to standardize the assessment of the PaO2/FiO2 in the diagnosis of ARDS could lead to a significantly imbalanced randomization in ARDS clinical trials with a bias that over or underestimates the expected outcome. A lack of standardization may also explain why the PaO2/FiO2 ratio has failed to consistently predict outcome in epidemiologic studies of patients with ARDS.[21] The ARDS Network, which has enrolled exclusively based upon AECC criteria, has retrospectively reviewed their study population and suggested FiO2, but not PEEP, could be used in addition to PaO2/FiO2 to select patient populations with high or low predicted mortality.[22] Yet, addition of this criteria did not appear to change conclusions regarding the effectiveness of their reported interventions.
Figure 1.
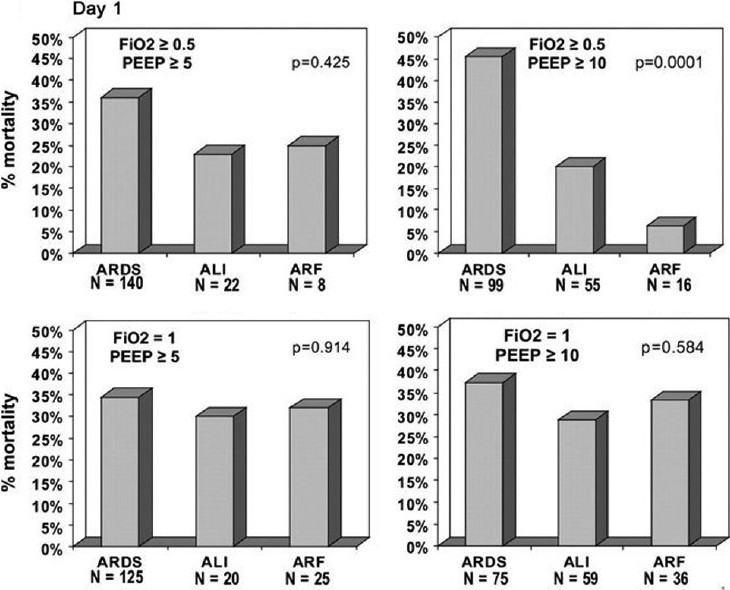
Reclassification of patients meeting AECC ARDS criteria into acute respiratory distress syndrome (ARDS), acute lung injury (ALI), or acute respiratory failure (ARF) categories based upon response to four standard ventilator settings on Day 1. Mortality rate for individual groups is shown based upon the reclassification. P values refer to the differences in mortality rates. Reference 19 with permission.
The classic radiographic feature of ARDS also introduces some controversy. The AECC radiographic definition includes “bilateral infiltrates consistent with pulmonary edema on a frontal chest radiograph.” No attempt is made to grade the severity or distribution of the infiltrates. Comparative studies using blinded radiographic interpretation show only modest agreement between radiologists on studies that fulfill the AECC criteria.[23–25] Further, agreement between plain film chest radiographs and chest computed tomography (CT) with respect to infiltrate distribution in ARDS patients is poor.[26] Classification of ARDS by CT into diffuse and lobar infiltrative patterns appears to predict outcome, so accurate radiographic classification may be important in the analysis of comparative populations.[26]
The AECC criteria excludes hydrostatic edema with the requirement that left atrial hypertension is not present based upon either clinical assessment or by measurement of a left atrial pressure (LAP)<18 mm Hg. However, this variable may not be easy to assess non-invasively or even an important distinction clinically. In patients randomized to a fluid and catheter therapy trial using only the clinical exclusion of elevated LAP, a full 29% of the subjects subsequently were shown to have a pulmonary capillary occlusion pressure (PCOP)>18 mm Hg, 8% had a cardiac index <2.5 L/minute, and 3% had both when measured post randomization.[8] As the vast majority of the patients had normal cardiac function with an elevated PCOP, in a clinical condition consistent with ARDS, this appears to represent a normal variation of the syndrome classification. The prognostic significance of this variation remains undetermined.
In conclusion, the clinical-pathologic correlation between the AECC definition and the gold standard DAD pathology is only modest. Three of the critical components of the AECC definition of ARDS, specifically oxygenation (PaO2/FiO2 ratio), the chest radiograph, and estimates of LAP may all be subject to significant interobserver variability. These factors must be considered in the design of ARDS clinical trials to avoid unintended randomization bias.
PREDICTIVE LIMITATIONS
Can we use a single variable to define the severity of ARDS? The AECC definition establishes a grading system for the severity of acute lung injury based solely upon the measurement of gas exchange indices. Acute lung injury is defined by a PaO2/FiO2<300 with ARDS defined by a PaO2/FiO2<200. The validity of this distinction remains controversial. While some series suggest the presence of ALI without ARDS (200<PaO2/FiO2<300) does not influence prognosis, other investigators note a clear distinction.[1,21,27,28] This inconsistency in the predictive value of PaO2/FiO2 may reflect the limitations of a single static measurement. The progression in gas exchange indices over time may provide a more accurate assessment.[29] Likewise, the clinical transition from ALI without ARDS to ARDS has also been identified as an important trend in gas exchange which adversely impacts prognosis.[1]
The inciting cause is also an important variable in ARDS progression and prognosis. When specific etiologies of ARDS are compared, trauma-associated ALI has been consistently associated with a better prognosis in comparison to sepsis related ALI.[30–32] Patients with traumatic injuries demonstrated a lower odds ratio of death than patients with non-trauma related injury despite controlling for baseline demographic and clinical variables. Trauma patients have distinct biomarker profiles including reduced plasma markers traditionally associated with poor clinical outcomes in ALI including ICAM-1, SP-D, sTNFr-1, and vWF.[30] This pattern of biomarker distinction may signal a reduced magnitude of both epithelial and endothelial injury in the trauma patient. These data suggest an improved prognosis in trauma associated ALI can be attributed to the mechanism of lung injury rather than the characteristics of the population. Could the mechanism of lung injury distinguish the outcome in other ARDS populations?
In a comparative ARDS animal model, the physiologic and pathologic characteristics of ARDS induced by a pulmonary and extra pulmonary trigger have been compared.[33] Despite relatively similar levels of functional lung change compared to placebo treated animals, the epithelial insult (pulmonary) demonstrated greater inflammatory and ultrastructural change in the lung compared to an extrapulmonary injury. In human studies, lung compliance and the radiologic recruitment response to PEEP titration is reduced in ARDS secondary to a pulmonary cause (ARDSp) compared to an extrapulmonary (ARDSexp) insult.[34] The computed tomography (CT) appearance of these two ARDS conditions may also differ. ARDS of a pulmonary origin appears to be characterized by more frequent asymmetric lung consolidation and ground glass infiltrates in contrast to the more homogenous pattern of ARDSexp.[35] Comparison of outcome in pulmonary versus extrapulmonary triggers for ARDS patients has demonstrated conflicting results, but suggests a trend towards higher mortality in the ARDSp patient population when other prognostic variables are controlled.[5,29,36,37]
If the inciting agent is an important prognostic variable, this must be considered in both clinical trial design and study analysis. As one example, the ARDSNet low tidal volume trial contained only 59 trauma patients in 432 total subjects randomized to the 6ml/kg treatment arm of this investigation.[2] Despite this small subgroup size, the therapeutic benefit of low tidal volume ventilation in ARDS appeared to be independent of the inciting agent.[32] Subgroup analysis is important to consider in the planning phase of ARDS trials, where possible, to determine the benefit of an ARDS intervention across different inciting agents.
In addition to influencing the prognosis, the inciting agent is also important in defining the risk of progression to ALI/ARDS. The most common risk factor for ARDS development is infection. Pulmonary infection has been associated with a higher risk of ARDS progression in comparison to non-pulmonary infections in “at risk” populations.[38] A more comprehensive, multi-center risk assessment, excluding patients with ALI/ARDS on presentation, has suggested the highest rate of ALI occurs after smoke inhalation (26%), shock (18%), aspiration (17%), aortic surgery (17%, and lung contusion (14%). The lowest rate of progression is seen with pancreatitis (3%).[39] The presence of gastroesophageal reflux appears to be a key clinical feature in patients that develop recurrent acute lung injury.[40]
Understanding the risk factors for progression to ALI/ARDS is especially important, considering only 6.8% of patients with a recognized risk factor on hospital admission progress to ALI and only 4% develop ARDS.[39] This gap between “at risk” patients and ALI/ARDS development makes ALI/ARDS prevention studies not feasible based solely on investigation of patients “at risk.” The Lung Injury Predictor Score (LIPS) attempts to incorporate both specific known risk factors (i.e., pneumonia, sepsis, trauma) and recognized risk modifiers (i.e., alcohol, smoking, hypoalbuminemia) to better define patients at risk for ALI/ARDS.[41] The LIPS model discriminates patients with a small chance of developing ALI/ARDS (good specificity), while maintaining appropriate sensitivity as a screening tool. The model has now been validated in a larger multi-center cohort and appears to retain a similar level of calibration as the original derivation cohort.[39]
Compared with at-risk patients that did not develop ALI, those who develop lung injury have an increased mortality (23 vs. 4%) and increased resource use as reflected in longer ICU (8 vs. 2 d) and hospital (15 vs. 6 d) stays.[39] When adjusted for severity of illness using APACHE II score, and predisposing conditions (LIPS), the development of ALI markedly increases the risk of in-hospital death (odds ratio, 4.1; 95% CI, 2.9–5.7).
Other clinical features, in additional to the inciting agent, have been examined for their ability to predict outcome in the ARDS population. ARDS has a recognized time course from onset, through the pathophysiologic exudative, proliferative and fibrotic phases. Clinical observations suggest the timing of ARDS in relation to disease onset may be an important variable to consider. Classification of disease by onset in the clinical course as early (<48 hours) versus late (> 48 hours) appears to describe two different disease patterns and patient outcomes in a trauma population.[42] Patients with early post-traumatic ARDS appeared to have hemorrhagic shock with capillary leak as the most common etiologic agent, while a later onset was more frequently associated with infection/pneumonia and progressive multiple organ failure. The timing in evolution of ARDS from an exudative to proliferative process may also influence the response to therapeutic interventions.[43,44]
Race and ethnicity may be additional important clinical variables which influence outcome from ALI/ARDS. In a retrospective analysis, African-American and Hispanic patients had a significantly higher risk of death than white patients.[45] The increased mortality risk for African Americans was attributable to illness severity on presentation but could not be explained for the Hispanic population.
The AECC criteria provide a framework to define the ARDS patient population. Considered in isolation, the criteria do not address specific variables recognized to influence mortality risk in the population including the inciting agent, timing of the injury, and race and ethnicity factors. Clinical studies in ARDS must consider these factors in the design and interpretation of clinical trials for this disorder.
ARDS OUTCOME AND PULMONARY VASCULAR DISEASE
While the AECC definition of ARDS focuses on the clinical manifestations of alveolar edema with radiographic and gas exchange criteria, ARDS is also a disease of the pulmonary circulation. Is the response of the pulmonary circulation an important variable in the clinical course of the patient with ARDS?
Histologic studies in ARDS have demonstrated a pattern of diffuse pulmonary endothelial injury associated with both macro and microscopic thrombi formation. These early changes progress to fibrocellular intimal proliferation that can obliterate small vessels. Radiographic imaging confirms the vascular changes can be manifested as actual filling defects in the distal pulmonary vasculature.[46]
The vascular changes of ARDS could lead to a type of ventilation/perfusion (V/Q) mismatch contributing to an increase in physiologic dead space. In contrast to the variable results with oxygenation indices, an increase in pulmonary dead space fraction (Vd/Vt) has proven to be a powerful predictor of mortality in patients with ALI/ARDS.[47] For every 0.05 increase in the deadspace fraction, the odds of death in an ARDS study population increased by 45 percent (odds ratio, 1.45; 95 percent confidence interval, 1.15 to 1.83; P=0.002). The widespread acceptance of Vd/Vt as a prognostic tool for ARDS has been limited by the requirement for measuring mixed expired carbon dioxide. A modification of the Vd/Vt equation using readily available clinical data has been described and this modified Vd/Vt remains predictive of ARDS outcome in a dose responsive manner.[48] The value of Vd/Vt as a predictive indictor requires further validation in larger populations. However, these findings do support the importance of vascular derangements as an important component of the ARDS phenotype and likely a significant predictor of outcome.
Both pulmonary vascular hemodynamic variables and right ventricular dysfunction have been studied in the ARDS population as clinical markers of pulmonary vascular injury.[31,49] Pulmonary hypertension is recognized in a significant fraction of ARDS patients and the potential causes are quite diverse. These have been hypothesized to include altered vasomotor tone due to hypoxemia and/or hypercapnia, altered intrathoracic pressures in association with ventilator support, and in situ thrombosis. Without consideration of cause, early clinical studies suggested elevated pulmonary artery systolic pressure in ARDS patients was associated with an adverse prognosis.[31] These data have been further supported by a more recent analysis of hemodynamic data from the ARDSNet Fluids and Catheter Therapy Trial (FACTT).[50] The investigators assessed the transpulmonary gradient (TPG) (mean PA pressure-pulmonary capillary occlusion pressure [PCOP]) and the pulmonary vascular resistance index (PVRi) in a group of patients randomized to receive a pulmonary artery catheter to guide their ARDS management. Of note, all patients received a consistent protective ventilator strategy with target tidal volume ~ 6ml/kg ideal body weight and plateau pressures maintained < 30 cm H20. The highest recorded daily value of TPG and PVRi was used for the analysis. In the population of 475 patients randomized to receive a pulmonary artery catheter for ARDS management, none of the baseline measures of cardiopulmonary dysfunction, including central venous pressure, PA systolic or diastolic pressure, pulmonary capillary occlusion pressure (PAOP), or cardiac index distinguished survivors from non-survivors. In the pulmonary artery catheter population, 73% demonstrated an elevated transpulmonary gradient (TPG>12). Patients with a TPG > 12 mm Hg had a significantly greater mortality rate than patients with a TPG<12 mm Hg (30% vs. 19%; P=0.02). Patients with a persistently elevated TPG through day #7 of therapy had a significantly greater mortality than patients with an elevated TPG at day 0-1 which subsequently normalized. In multivariate analysis, pulmonary vascular dysfunction, as represented by an elevated TPG and PVRi remained an independent predictor of an adverse outcome in the ARDS population. These data further support an important predictive role for pulmonary vascular disease in ARDS outcome and a potential target for therapeutic intervention.
If pulmonary hypertension is an important clinical parameter in ARDS patients, then logically, right heart dysfunction will be frequent in the population. ARDS has been associated with acute right heart dysfunction assessed either by hemodynamic indices or by echocardiography.[5,49,51] An elevated right atrial:pulmonary capillary occlusion pressure ratio was a strong predictor of mortality in one series of patients meeting AECC criteria.[5] The value of this parameter as a predictive variable for mortality was not confirmed in the FACTT patient population, however.[50] Acute cor pulmonale (ACP), defined echocardiographically as RV dilatation with paradoxical septal motion, occurs in 22-25% of the ARDS population.[49] Although echocardiographic findings of ACP are associated with significant morbidity (increased length of stay (LOS)), the finding is not clearly predictive of an adverse outcome.[49,51] In the largest published echocardiography series of ARDS, patients receiving a consistent lung protective ventilation strategy (mean PEEP of 10 cm H20 and mean plateau pressure (Pplat) of 23 cm H20), 22% of patients had evidence for acute cor pulmonale. Of this population, 19% demonstrated evidence of a moderate-to-large patent foramen ovale (PFO).[51] The incidence of right to left shunting increased to 34% in patients with echocardiograpic evidence of acute cor pulmonale.
Although limited in scope, the available data suggest the presence of pulmonary vascular disease, especially with evidence for right heart dysfunction, holds important prognostic information for the ARDS patient outcome. The interaction of ventilator support variables, pulmonary artery pressures, and risk for right to left shunting, is a complex interaction to challenge the management of hypoxemia in this disorder. This interaction would be expected to influence the success of many of the treatment strategies for hypoxemia in ARDS including PEEP, prone ventilation, and vasodilator therapy.
DISEASE PREDICTION MODELS
Because the PaO2/FiO2 ratio has been inconsistently associated with defining prognosis in ARDS, clinical investigators have sought other markers to define severe ARDS for enrollment in clinical trials.[52,53]
Both traditional severity of illness models applied to ARDS (i.e., APACHE) and disease specific ARDS models have been considered to predict ARDS mortality from clinical data available early in the disease course.[54–56] The goals of a prediction model, if accurate, would be twofold. An accurate prediction model would provide an important resource to enhance surrogate discussion making regarding prognosis for the clinical management of ARDS. A well-calibrated model could also provide a tool to stratify cohorts of ARDS patients in clinical trials according to their mortality risk.
Using the ARDSNet low tidal volume population, a simple model incorporating the parameters of age, serum bilirubin (mg/dL), net 24-hour urine volume (in-out [mL]), and hematocrit was devised to predict mortality in non-trauma ARDS patients with good calibration.[55] The model has been validated in a population of non-trauma patients from a second ARDSNet clinical trial (ALVEOLI). The main appeal of this model is the simplicity of the calculation rather than superiority to the more generalized ICU predictions models, such as APACHE III which also shows good prediction for the ARDS population.[29] The transportability of the ARDS specific model was subsequently examined in a non ARDSNet cohort of non-trauma patients.[56] The model showed an equal ability to discriminate between survivors and non-survivors with excellent calibration in high and low risk patients. However, for intermediate risk patients, the observed mortality was substantially higher than predicted by the model. The variability in calibration of disease specific models in different populations may reflect the unique characteristics of the development cohort. The more general populations, in contrast to the ARDSNet study populations, may have a different racial distribution, different clinical characteristics, and different severity of illness, all factors which might impact the calibration of a mortality prediction model.
In addition to clinical variables, numerous plasma biomarkers have been investigated in ARDS populations due to their hypothesized relationship to the disease pathogenesis. These biomarkers have included a broad array of mediators reflective of lung injury and repair mechanisms and provide insight to disease pathogenesis (Table 3). A major limitation of the current literature supporting these biomarkers is that the majority have been derived from a single ARDSNet population data set with little confirmation in more diverse populations. While statistically significant, the utility of these mediators individually or in combination to refine patient selection for clinical trials remains to be determined.
Table 3.
Biomarkers associated with ALI/ARDS prognosis
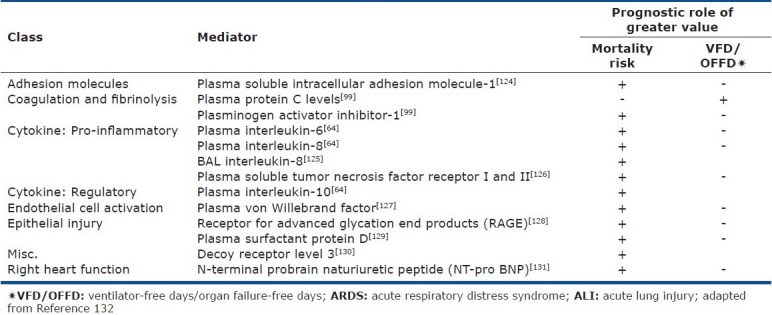
A combination of clinical and biologic markers for risk prediction may provide a more accurate assessment of disease outcome in the population. In an ARDSNet population randomized to unique PEEP strategies, the addition of the biologic markers IL-8, SP-D, PAI-1 and TNFR1 to clinical predictive variables provided a stronger predictive calibration of patient mortality than clinical variables alone (AUC 0.850 vs. 0.815).[57] In ARDSNet populations from the tidal volume and PEEP trials, incorporation of five biomarkers (soluble intercellular adhesion molecule-1, von Willebrand factor antigen, IL-8, SP-D, and sTNFr-1) significantly improved risk prediction when compared to the use of the Acute Physiology and Chronic Health Evaluation Score III alone.[58]
Imaging characteristics of the ARDS population have also been identified as important contributors to mortality prediction. Recognition of the limits of plain film radiographs has prompted the use of CT imaging in ARDS to provide a more refined description of the lung injury pattern and determination of lung recruitability.[26,59] The CT Scan ARDS Study Group has defined an ARDS severity score (ARDS-SS) based upon a combined assessment of physiologic and imaging characteristics.[26] ARDS mortality was higher in patients with diffuse attenuations (76%) in comparison to those with lobar and patchy attenuations (41 and 42%). The ARDS-SS appeared to discriminate patients with a high mortality rate >60% and may serve to identify patients for therapeutic trials of higher risk interventions.
There is also a rapidly growing body of literature exploring genetic factors in patients who develop ARDS. Genome-wide association screening studies of patients who either have ARDS or are at risk of developing ARDS have been summarized in a recent review.[60] These studies in ARDS are challenged by a heterogeneous phenotype, selection of the appropriate control population, inconsistent replication studies, insufficient population sampling, and complex interactions between the genetic risk and clinical variables. Additional variability can be seen in relation to race and population stratification. A more refined phenotype characterization will likely be needed to improve the success of genetic replication studies to confirm specific variants that contribute to prognosis.
The development of models which incorporate additional descriptive parameters beyond the AECC criteria for ALI/ARDS are needed. By combining physiologic data with biomarkers, imaging characteristics, and genetic information investigators hope to create a more homogeneous disease model for targeted intervention. These models will require validation in large ALI/ARDS cohorts. A more homogeneous patient selection will allow investigation of both high and low risk interventions in a patient group specifically targeted based upon disease pathophysiology and course.
THERAPEUTIC STRATEGIES FOR RESPIRATORY MANAGEMENT
Tidal volume
Correction of hypoxemia and hypercapnia are integral to ARDS management and the majority of patients with more advanced ALI and ARDS require mechanical ventilatory support. Over the past 30 years, accumulating basic science and clinical evidence has confirmed that mechanical ventilation can extend the inflammatory response of ARDS in response to cyclic tidal alveolar hyperinflation and recruiting/decrecruiting injury.[61] The cyclic overdistention produced by excessive transpulmonary pressure has been identified as one of the major determinants of ventilator induced lung injury (VILI).
A landmark paper published by Webb and Tierney in 1974 examined the response of normal lungs to incremental peak inflation pressures (PIP) of 14, 30, or 45 cmH2O without positive end-expiratory pressure (PEEP), as well as with PIP of 30 or 45 cmH2O with 10 cmH2O of PEEP.[62] The deadspace of the ventilatory circuit was adjusted to provide a consistent PaCO2 with all ventilation strategies. Ventilation at low inflation pressures (PIP 14 cmH2O) did not cause significant injury in comparison to ventilation with higher inflation pressures (30 or 45 cmH2O) which produced hypoxemia and perivascular edema. Ventilation at high inflation pressures (45 cmH2O) without PEEP produced severe lung injury and death within 35 min. The use of PEEP with the same inflation pressures conferred protection from the alveolar edema.
Dreyfuss et al. extended these observations by examining whether VILI resulted from a pressure mediated or lung volume (stretch) mediated injury.[63] Rats were subjected to incremental PIP but tidal volume could be restricted in one group using a thoracoabdominal binder to limit chest wall excursion. This study confirmed that high tidal volume ventilation, irrespective of airway pressure, produced severe lung injury characterized by pulmonary edema, increased alveolar-capillary permeability, and structural abnormalities. In addition, PEEP once again was found to be “protective,” as the presence of PEEP prevented pulmonary epithelial damage and alveolar edema and significantly reduced interstitial edema and endothelial cell changes. As a result of these investigations, clinical researchers began to focus on the importance of “volutrauma” as an important clinical parameter to avoid in ARDS ventilator management.
Although numerous ventilatory strategies have been investigated, the ARDSNetwork low tidal volume (ARMA) trial comparing 6 ml/kg ideal body weight (IBW) tidal volume versus 12 ml/kg IBW tidal volume established a clinical relevance to the animal models of ventilator induced lung injury (VILI).[2] Each patient group also had their respective Pplat restrictions (<30 for 6ml/kg and <50 cm H20 for 12 ml/kg). The 6ml/kg IBW tidal volume group showed a marked absolute survival benefit (31 vs. 40%, P=0.007). The low tidal volume strategy was also associated with a reduction in measured plasma biomarkers (tumor necrosis factor receptor (TNF 1r), interleukin-6, and interleukin-8), inflammatory mediators typically reflective of more severe lung injury.[64] This latter finding established a clinical biologic relevance between the lung protective ventilator strategy and the systemic inflammatory response of ARDS. The elevated blood inflammatory markers provide the link between the ventilator management strategy and progression of organ failure in ARDS. The enhanced inflammation associated with VILI, leading to the release of inflammatory mediators from the lung into the bloodstream, has been called biotrauma.[61]
The clinical data supporting the importance of tidal volume and Plat control in ARDS is supported by assessment of lung metabolic activity. By combining CT and PET imaging, investigators have determined that ARDS lung metabolic activity is increased in aerated regions in proportion to the tidal volume and Pplat.[65] Plat >26-27 cm H20 correlate with greater lung inflammation in these well ventilated regions consistent with an injury signal. These imaging data provide further support for a lung origin to changing systemic inflammatory mediators in response to tidal volume change.
Positive end-expiratory pressure
While the ARMA trial addressed the issue of tidal hyperinflation of the alveoli, it did not address the role of PEEP in regulating lung injury. Both groups in the ARMA trial were managed with identical protocolized changes in PEEP/FiO2 combinations, so the impact of PEEP on minimizing VILI could not be assessed.
The evidence suggesting that large tidal volumes cause lung injury (volutrauma) is accompanied by evidence from animal models that recruitment/derecruitment cycling of atelectatic, edematous lung can also be harmful (atelectrauma).[66] As previously noted, evidence from animal models suggested that higher PEEP could prevent ventilator induced lung injury, independent of PEEP associated benefits to oxygenation.[63] The heterogeneous nature of ARDS, however, complicates the interaction of PEEP with the injured lung. In diseased regions, PEEP acts to stabilize lung volume and reduce the amount of lung volume undergoing tidal cycling opening and closing. In normal regions, PEEP leads to overdistention and exacerbates tidal hyperinflation. In contrast to functional metabolic imaging with tidal overdistention, the role of alveolar recruitment/derecruitment in enhancing lung metabolic activity and injury is less clear.[65]
ALI/ARDS investigators have extensively investigated the potential benefits of PEEP in patient management. A follow-up trial to ARMA, termed the Assessment of Low Tidal Volume and Elevated End-Expiratory Volume to Obviate Lung Injury (ALVEOLI) trial, randomized ALI patients to a high and low PEEP strategy. The randomization employed a consistent low tidal volume/Pplat strategy matched to two different PEEP /oxygenation tables for titration. The higher PEEP strategy was the intervention compared to the control, or lower PEEP/high FiO2 strategy of the ARMA trial.[10] The higher PEEP strategy did not show an improvement in outcome over the original ARMA PEEP management.
The Lung Open Ventilation Study (LOV), employed a level of PEEP, either higher or lower, based upon an oxygenation scale conceptually similar to the ALVEOLI trial.[67] The intervention group received a 40-sec breath hold at 40 cm H20 with PEEP set at 20 cm H20. The patients were then treated with FiO2/PEEP titration based upon a table. Despite the lack of a clear mortality benefit, this strategy did result in a significant improvement in secondary endpoints of reduced refractory hypoxemia, reduced death due to refractory hypoxemia, and reduced requirement for rescue therapy due to intractable hypoxemia, barotrauma, or acidosis. Rescue therapies included inhaled nitric oxide, prone ventilation, high-frequency oscillation, high-frequency jet ventilation, and extracorporeal membrane oxygenation.
The Expiratory Pressure Study Group (EXPRESS) randomized an ALI population to achieve a high PEEP strategy based upon lung mechanics.[68] The randomization achieved a minimal distention strategy (low PEEP) and an increased recruitment strategy (high PEEP). In the minimal distention strategy, PEEP and inspiratory Pplat were kept as low as possible without falling below oxygenation targets. External PEEP was set to maintain total PEEP (the sum of external and intrinsic PEEP) between 5 and 9 cm H2O. In the recruitment strategy, PEEP was adjusted based on airway pressure and was kept as high as possible without increasing the maximal inspiratory Pplat above 28 to 30 cm H2O. The recruitment strategy was titrated based on Pplat, regardless of its effect on oxygenation. Overall, this high PEEP recruitment strategy resulted in no effect on mortality in the randomized population. The recruitment strategy did result in better oxygenation, more ventilator free days, more organ failure free days, and a reduced requirement for rescue therapy.
Collectively, these three trials have studied 2,229 patients with a comparative hospital mortality of 33.9% in the high PEEP strategy and 36.3% in the lower PEEP strategy. A meta-analysis of the available clinical trials comparing PEEP levels in the setting of low tidal volume ventilation has concluded that a higher PEEP strategy is associated with improved survival in the subset of patients with ARDS.[69] In contrast, patients with ALI without ARDS may not benefit or may actually experience harm from higher PEEP levels. The higher PEEP strategy is associated with no evidence of serious adverse effects although a slight increase in pneumothorax was noted (absolute risk difference, 1.6%) A second meta-analysis of similar data has reached relatively similar conclusions although contradicts the mortality benefit.[70]
A supplement to high PEEP ventilator management has been the use of recruitment maneuvers. A recruitment maneuver periodically, but briefly, raises the transpulmonary pressure to higher levels than being used for tidal inflation. Theoretically, intermittent recruitment maneuvers could open collapsed alveoli, minimize the cycling stretch associated with recurrent airway opening, and improve respiratory system compliance. Three randomized trials have examined the use of recruitment maneuvers in ARDS patients.[71–73] As might be expected, transient recruitment maneuvers are associated with transient improvements in gas exchange but no apparent sustained benefit. The risks associated with recruitment maneuvers include both pulmonary risks, in relation to VILI, and hemodynamic risks secondary to compromised cardiac output.
How should the three “negative” trials of high PEEP therapy be interpreted in the face of abundant animal studies favoring this strategy in the regulation of lung edema? Are the negative clinical trials limited by the heterogeneity of the PEEP response of the target ALI study population? Are oxygenation parameters inadequate to guide optimal lung recruitment and minimize VILI? These questions have prompted investigators to explore alternative strategies for PEEP titration.
Radiographic studies using CT imaging, under conditions of increasing PEEP, have suggested the potential for alveolar recruitment is quite variable among patients with ARDS.[59,74] A poor correlation between radiographic recruitment of lung parenchyma and changes in gas exchange indices (PaO2/FiO2 or PaCO2) has been suggested.[59] A lobar or heterogeneous radiographic pattern is associated with overdistention of aerated lung regions during the application of PEEP, in contrast to a more diffuse pattern of lung injury.[74] These data suggest CT imaging may be a critically important tool to define recruitability of the ARDS lung and titrate PEEP to minimize risk.
The use of quantitative CT in ARDS, despite its critical importance in defining ARDS pathophysiology, has not generally been accepted as a clinical tool. This may be related to perceived disadvantages for patient care including risk of patient transfer, radiation exposure, cost, and processing limitations. Alternative techniques, such as electrical impedance tomography (EIT) and lung ultrasound are, therefore, being explored as alternative tools to guide PEEP titration in the critically ill patient.[75]
In contrast to imaging strategies, the analysis of pressure:volume relationships has been proposed to titrate PEEP using a variety of methods. Both the lower inflection point of maximum curvature on the pressure-volume curve and the stress index have been employed with variable results.[76,77] The stress index, has been advocated as a measurement to more optimally set PEEP and avoid potential hyperinflation in patients with a more focal ARDS distribution (Fig. 2).[78] Unlike traditional static-pressure volume curves, the stress index is measured under conditions of constant flow, volume controlled ventilation. The stress index defines the slope of the airway opening pressure during a period of constant flow. A stress index>or<1 suggests a changing lung elastance during the inflation period. Values<1 suggest a continuous decrease in elastance during lung inflation and are consistent with hyperinflation. Values>1 suggest an increase in lung elastance consistent with tidal opening and closing of alveoli. In contrast to the ARMA PEEP/FiO2 titration tables in patients with more focal ARDS, the stress index led to consistent reduction in the prescribed PEEP level in order to avoid hyperinflation. Titration of PEEP to the stress index also led to reductions in plasma inflammatory mediators including interleukin-6, interleukin- 8, and soluble tumor necrosis factor receptor. These same biomarkers were reduced in association with low tidal volume ventilation in the ARMA trial.[64] The validity of the stress index technique as a more optimal measure for PEEP titration remains to be confirmed by other investigators. If validated, in combination with low tidal volume ventilation, PEEP titration based upon mechanical indices could significantly further regulate biotrauma in VILI.
Figure 2.
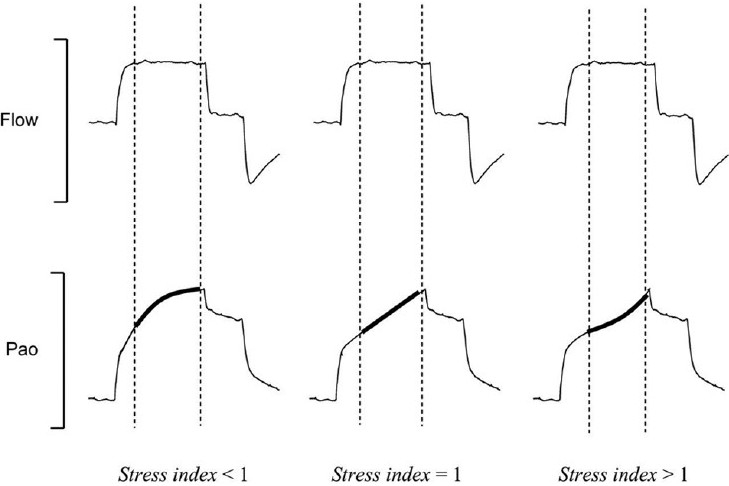
The stress index is the coefficient b of a power equation (airway pressure = a · inspiratory time b+c), fitted on the airway opening pressure (Pao) segment (bold lines) corresponding to the period of constant-flow inflation (dotted lines), during constant-flow, volume-cycled mechanical ventilation. For stress index values of less than 1, the Pao curve presents a downward concavity, suggesting a continuous decrease in elastance during constant-flow inflation. For stress index values higher than 1, the curve presents an upward concavity suggesting a continuous increase in elastance. Finally, for a stress index value equal to 1, the curve is straight, suggesting the absence of tidal variations in elastance. Reference 78, with permission.
Airway pressure measurements reflect the elastance properties of both the lung and chest wall and higher airway pressure targets may be needed in patients with altered extrathoracic mechanics. Because ICU patients are characterized by widely variable abdominal and pleural pressures, ideally, ventilator settings could be optimized to achieve a targeted transpulmonary pressure (airway pressure-pleural pressure) to minimize alveolar overdistention and cyclic alveolar collapse.[79] Pleural pressure is traditionally estimated in humans by measurement of esophageal pressure using an esophageal balloon catheter. The use of transpulmonary pressure measurements to titrate PEEP demonstrated improved oxygenation and lung compliance during the initial 72 hours of monitoring in comparison to the 6ml/kg tidal volume and ARMA PEEP/FiO2 oxygenation table. In the transpulmonary pressure group, PEEP levels were set to achieve a transpulmonary pressure of 0 to 10 cm of water at end expiration, and tidal volume was limited to keep transpulmonary pressure <25 cm of water at end inspiration. The mortality was reduced in the group randomized to transpulmonary pressure monitoring but the investigation was underpowered for this question. The measurement of transpulmonary pressures is not standard in most ICUs. Further confirmation of this technique in larger patient samples is needed.
At the current time, the strong animal data supporting the role of PEEP in limiting cycling opening/closing lung injury (atelectrauma) has not been confirmed in clinical trials. Titration of PEEP based upon oxygenation indices alone does not reveal a therapeutic benefit to higher PEEP levels. This may reflect a poor correlation between oxygenation indices and alveolar stability. Radiographic and physiologic techniques have been described to better titrate PEEP for minimal VILI. These techniques require validation in large populations for both general acceptance and a demonstrated mortality effect. The elusive PEEP strategy for ARDS management may be dependent on measurement of “recruitment” rather than oxygenation as the characteristic that determines PEEP's value (or detriment) in the management of the ARDS patients.
Prone positioning
The clinical investigations of prone positioning illustrate many of the challenges in patient selection and study design for ARDS clinical trials. Prone positioning has been recognized to improve oxygenation in animal models of ALI and in a significant fraction of patients with ALI/ARDS. The proposed mechanisms include an increase in end-expiratory lung volume, improved ventilation-perfusion matching, more uniform distribution of lung stress and strain with tidal cycling, and regional improvement in lung and chest wall mechanics. Regardless of mechanism, an improvement in oxygenation occurs in a majority of patients when this intervention is applied. The potential risks of this intervention are primarily pressure related injury and tube dislodgement with turning maneuvers.
Despite the improvements in oxygenation, early randomized clinical trials were unable to demonstrate a mortality benefit with this intervention.[11] The interpretation of these initial trials was limited by variable enrollment criteria (ALI/ARDS vs. ARDS alone), variable intervention duration (prone time), and lack of a consistent ventilation strategy (Pplat and tidal volume targets). These limitations were specifically addressed in the Prone-Supine II (PSII) investigation which randomized patients only meeting ARDS criteria (P/F ratio <200).[80] The patients were randomized according to the severity of the hypoxemia as moderate (P/F ratio of 100-200) and severe (P/F ratio <100). The randomization strategy was based upon prior RCT subgroup analysis that suggested more severely ill patients, and patients with improved CO2 exchange in response to prone positioning may benefit from this intervention.[11,81] Ventilation was standardized to a maximum tidal volume of 8 ml/kg and a Pplat of <30 cm H2O. PEEP and FiO2 settings were based upon oxygenation tables. Patients were ventilated in the prone position for a minimum of 20 hours per day. Despite controlling for many of the variables critiqued in prior RCT's of prone ventilation, the investigators remained unable to find a mortality benefit in the study population or in the subgroup analysis. Yet, the PaO2/FiO2 ratio was significantly higher in the prone group compared to the supine group, consistent with findings in earlier trials of prone ventilation. The beneficial effect of prone positioning on oxygenation was seen in both the moderate and severe hypoxemia study groups. Positive end expiratory pressure, tidal volume, and total minute ventilation were similar in the prone and supine groups. A significantly greater proportion of patients in the prone group, as compared with the supine group, experienced at least 1 complication (e.g., need for increased sedation, muscle paralysis, hemodynamic instability, device displacement). The investigation was admittedly underpowered to detect a mortality difference <15% in the population of very severe advanced hypoxemia.
To overcome the issue of sample size for the most severe ARDS populations, meta-analysis has been employed to pool study results. These analyses have suggested that prone positioning can be beneficial when restricted to patients with very advanced disease (i.e., P/F ratio <100).[82,83] Collectively, the existing data suggest prone positioning is best considered a “rescue” regimen employed for patients with intractable hypoxemia.
Pharmacologic paralysis
Neuromuscular blocking agents (NMBS) are frequently used in the management of ARDS patients to facilitate patient-ventilator synchrony and improve poor oxygenation when traditional sedation is not adequate. Under these conditions, NMBA are frequently effective. Less clear is their role in the management of ARDS patients with less severe disease. Given the frequent association of NMBA with critical illness myopathy, understanding the risk/benefit profile of these medications in the treatment of ARDS patients is especially important.
In a multi-center trial, patients with severe ARDS were defined as having a PaO2/FiO2 ratio of less than 150, a PEEP >5 cm of water, and a tidal volume of 6 to 8 ml per kilogram of predicted body weight.[84] Both groups continued to receive a lung protective ventilation strategy. These patients were then randomized to 48 hours treatment with cisatracurium compared to placebo. The primary outcome was the proportion of patients who died either before hospital discharge or within 90 days after study enrollment (i.e., the 90-day in-hospital mortality rate). The crude 90-day mortality was 31.6% (95% CI, 25.2 to 38.8) in the cisatracurium group and 40.7% (95% CI, 33.5 to 48.4) in the placebo group (P=0.08). No comparative increase in critical illness myopathy was seen in the cisatracurium population.
How do we explain the beneficial effect of short-term pharmacologic paralysis in this clinical trial? As gas exchange indices were similar in both populations, this mechanism does not seem to explain the reported benefit. Theoretically, short-term paralysis may facilitate patient-ventilator synchrony in the setting of lung protective ventilation. Short-term paralysis would eliminate patient triggering, active expiratory muscle activity, and overventilation. In combination, these effects may serve to limit regional overdistention (volutrauma) and cyclic alveolar collapse (atelectrauma). Paralysis may also act to lower metabolism and overall ventilatory demand.
The role of NMBS in the management of ARDS requires further exploration in additional clinical trials. Many questions remain in addition to the proposed mechanism of benefit. Whether the therapeutic benefit is drug (cisatracurium) or class specific remains undefined as does the optimal duration of therapy, and will require further studies.
High-frequency oscillatory ventilation
An alternative approach to the tidal cycling of conventional ventilation is the use of high-frequency oscillatory ventilation (HFOV). HFOV employs a relatively constant airway pressure, with CO2 exchange accomplished through non-convective mechanisms produced by rapid pressure oscillations (300-900 breaths per minute) in the airway. This lung protective strategy of HFOV is theoretically achieved by alveolar recruitment with a relatively constant mean airway pressure and avoiding the low and high tidal swings in alveolar pressure associated with conventional ventilation. Animal models of ALI have suggested HFOV reduces the level of inflammatory mediators produced by the injured lung in comparison to conventional mechanical ventilation.[85] The risks of HFOV relate to barotrauma and hemodynamic compromise in association with the sustained elevation in mean airway pressure.
A randomized trial confined to ARDS patients compared HFOV to conventional ventilation using a target tidal volume of <10 ml/kg in the conventional group.[12] This trial randomized 148 subjects with ARDS to the two ventilation strategies and confirmed an improvement in oxygenation indices with HFOV in the first 24 hours which was not sustained. No difference in mortality or ventilator free days could be confirmed in this relatively small sample size. A meta-analysis of published studies suggests HFOV applied early in ARDS patients (as opposed to rescue therapy) may be associated with a reduction in ARDS mortality and the need for alternative therapies, without any significant change in ventilator free days.[86] The average increase in PaO2/FiO2 ratio at 24-72 hours was 16-24% and the average increase in mean airway pressure was 22-33%. When the oxygenation index and PaCO2 were considered, however, HFOV demonstrated no advantage over conventional ventilation. No difference in the risk of barotrauma, hemodynamic compromise, or endotracheal tube obstruction was evident. Again, these data suggest HFOV is best considered a rescue regimen for patients with intractable hypoxemia. Ongoing clinical trials hope to address more specifically the role of this therapy in patients with ARDS.
Extracorporeal membrane oxygenation
If a lung protective ventilatory strategy is critical to the support of ARDS patients, then extracorporeal life support should provide the most optimal methodology to achieve lung “rest.” The potential benefit of extracorporeal membrane oxygenation (ECMO) is offset by an incremental bleeding risk related to the need for anticoagulation, and an additional infection risk related to the need for intravascular catheters. Early clinical trials of ECMO employed primarily an arterial-venous strategy with larger bore catheters for patients with intractable hypoxemia.[87] More modern investigations have used a safer venovenous access approach and have appropriately compared ECMO, or a modification of ECMO called extracorporeal CO2 removal, with a lung protective ventilation strategy.
The Conventional Ventilation or ECMO for Severe Adult Respiratory Failure (CESAR) trial randomized 180 patients with ARDS and a Murray Lung Injury Score > 3 or a pH <7.20 to either conventional therapy or transfer to an ECMO center for consideration of ECMO.[53] Patients were excluded from participation if they had been on high levels of inspired oxygen or high peak inspiratory pressure for longer than 7 days, had a contraindication to anticoagulation, or had a limited hope for recovery. The conventional therapy patients were assigned to a conventional ventilation strategy with target parameters (tidal volume 4-8 ml / kg and Pplat<30 cm H20) but received no standardized treatment protocol. The intervention group was transferred to an ECMO center for consideration of extracorporeal therapy. The ECMO center provided a comprehensive care program including lung protective ventilation, prone positioning, and nutrition support. Of the patients randomized to the ECMO arm, 63%, survived in comparison to 47% of those allocated to the conventional ventilation arm (relative risk 0·69; 95% CI 0·05–0·97, P=0·03). Of note, only 75% of the patients transferred for ECMO actually received the therapy, raising question as to whether the proposed intervention (ECMO) or better patient management in a highly specialized center was the most important intervention. Because of the extreme cost of the intervention, additional studies will be needed to define the role of extracorporeal support in the management of severe ARDS patients.
Inhaled vasodilators
The recognized pulmonary hypertension, right heart dysfunction, and severe hypoxemia which characterizes ARDS has prompted investigators to consider treatment strategies to address both parameters. The most promising agents for treatment of hypoxemia and pulmonary hypertension have been inhaled vasodilators. Systemic administration of vasodilators including prostagladin based vasodilators and sildenafil have been unable to show a therapeutic effect in ARDS and are often associated with worsening of oxygenation indices.[88,89] These medications should be used only with extreme caution in patients with advanced hypoxemia.
In contrast, inhaled vasodilators reduce pulmonary arterial pressure and redistribute blood flow to well ventilated lung regions with little to no systemic side effects. The two most frequently investigated agents are inhaled nitric oxide and inhaled prostacyclin.
Inhaled nitric oxide (iNO) improves oxygenation and reduces pulmonary artery pressure without lowering systemic blood pressure in select patients with ARDS.[89] Inhaled NO may also modify the host activation of neutrophils and platelets in the setting of inflammation. The results of numerous clinical trials examining the effects of iNO in ARDS patients are summarized in a review and meta-analysis.[90] The analysis combines 12 trials which have enrolled 1237 patients. The combined analysis suggests iNO has a small beneficial effect on oxygenation (PaO2/FiO2 ratio and Oxygenation Index) but no significant population effect on pulmonary artery pressures. There was no measurable effect on mortality or ventilator free days in the pooled analysis. The analysis raises concern regarding safety suggesting an increased rate of renal dysfunction in the study population randomized to receive iNO. The existing studies are limited by a fixed dosing schedule for the iNO administration of variable duration. Because the iNO dose response appears to vary with time in ARDS patients, the fixed-dose intervention design may have revealed adverse effects associated with long term administration.[91]
The inhaled prostacyclins, epoprostenol (prostaglandin I2 (PGI2) and alprostadil (PGE1) demonstrate similar vasodilator effects when compared to iNO including improved oxygenation and reduction in pulmonary hypertension.[92,93] However, these drugs lack the experience in randomized clinical trials characteristic of iNO.
Based upon the published trials to date, the use of inhaled vasodilators must be considered a rescue therapy for patients with intractable hypoxemia and/or pulmonary hypertension where other interventions such as high PEEP titration, prone positioning, and HFOV have been unsuccessful.
THERAPEUTIC STRATEGIES FOR HEMODYNAMIC MANAGEMENT
In addition to problems with gas exchange, ARDS patients frequently have evidence for cardiovascular failure. Although a large number of clinical trials and epidemiologic studies have been devoted to identifying therapeutic strategies for respiratory failure, much less investigation has been devoted to understanding the hemodynamic changes that characterize the ARDS population. Population studies suggest that over 1/2 of ARDS patients have evidence for cardiovascular dysfunction on presentation.[27] Comparison between survivors and non-survivors of ARDS suggest indices of right ventricular function and pulmonary hypertension distinguish these patients on presentation.[5,31,50] However, few intervention trials currently exist to direct therapy in the ALI/ARDS population.
The issue of optimal hemodynamic monitoring has been debated in ARDS, primarily focused on the need for a pulmonary artery catheter (PAC) in disease management. An initial randomized trial of PAC use in patients with sepsis and ARDS showed that PAC exposure did not confer a 28-day survival benefit, differences in organ dysfunction, need for vasoactive medications, or duration of ventilator/ICU/hospital days.[94] In this clinical trial, treatment decisions based upon the hemodynamic information were not determined by protocol but rather directed by the treating physician. .
The Fluid and Catheter Treatment Trial (FACTT), as part of the ARDSNetwork, compared specific management protocols guided by either a PAC or central venous catheter.[8] This study showed no differences in clinical outcomes with respect to 60-day survival, ventilator-free days, renal function, need for hemodialysis, or vasopressor therapy. This trial incorporated device specific estimates of preload and fluid management. No differences in fluid management were noted with the use of the respective monitoring devices. Patients with ARDS secondary to non-pulmonary causes are underrepresented in the study population. As a result of the two previously mentioned trials, current clinical guidelines have moved away from advocating the use of the PAC in sepsis and/or ARDS management.
ARDS fundamentally is characterized by increased capillary permeability. The permeability edema that characterizes ARDS is aggravated by any state which increases hydrostatic pressure. The inciting conditions of ARDS are typically associated with a systemic inflammatory response leading to a greater preload dependence of the ventricle for optimal function. Yet, elevations in pulmonary capillary occlusion pressure, to achieve greater preload response, are classically associated with increasing lung water in the setting of injury to the alveolar:capillary membrane. This conflict in therapeutic goals was addressed when the NHLBI ARDSNetwork published their findings from a prospective, randomized controlled trial of fluid conservative versus fluid liberal management strategies in ARDS patients.[7] The fluid conservative intervention was associated with a net even fluid balance in the ARDS population during the first week of therapy (Fig. 3). This contrasted with the liberal treatment group and past ARDS experience, where net fluid balance approximates 1 liter per day of hospitalization. Despite the lack of a true mortality benefit, the fluid conservative strategy improved oxygenation and reduced the duration of time on mechanical ventilation. The incidence of nonpulmonary organ failure, especially renal failure and shock did not increase.
Figure 3.
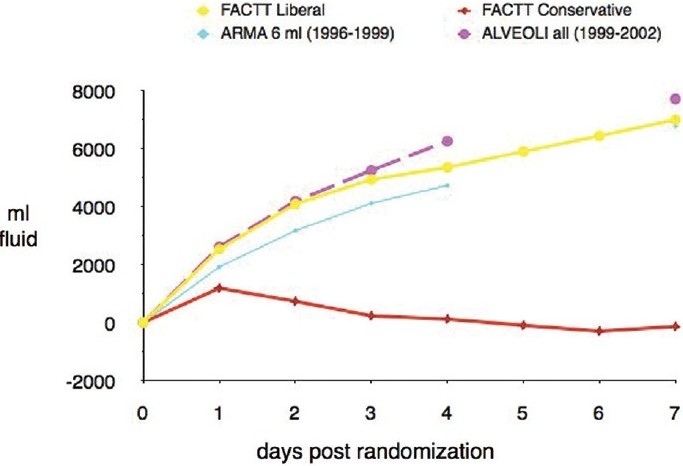
Cumulative fluid balance over first 7 days post randomization in FACTT patients in the liberal fluid management (FACTT-liberal) and conservative fluid management (FACTT-conservative) strategies of the Fluids and Catheter Therapy Trial. The two study groups are compared to fluid balance data available from two additional ARDSNet ventilator trials (ARMA and ALVEOLI). In comparison to all three other trials, the FACTTconservative arm ended up with an overall even fluid balance over the 7-day interval. Reference 8 with permission.
Early and aggressive fluid resuscitation of patients with sepsis, the most common etiology of ARDS, has been shown to improve patient outcome and limit progression to organ failure.[95] Are the aggressive fluid resuscitationrecommendations for sepsis treatment incompatible with the dry fluid strategy in ARDS? Actually, the findings are quite compatible if the timing of the intervention is considered. The ARDS Net conservative fluid strategy was initiated after the early period of resuscitation. The mean time from ICU admission to the first protocol instruction was 41.3±1.6 hours in the liberal-strategy group and 43.8±2.5 hours in the conservative-strategy group (P=0.42). The “dry” intervention strategy was implemented after the early aggressive resuscitation period had passed. These studies remind the clinician that ARDS is a dynamic disease process both clinically and pathologically so timing of the intervention is critically important in the design and analysis of clinical trials.
In addition to fluid management, the interaction of cardiopulmonary interventions is important to consider in ARDS management. The presence of acute cor pulmonale (ACP) is related to the Pplat associated with mechanical ventilation in ARDS patients.[96] ACP was uncommon when the Pplat was <27 cm H20, whereas ACP was seen in a high fraction of patients (~35%) when PPlat was between 27 and 35 cm H2O. In the setting of an elevated Pplat, ACP has an additive effect on mortality. The interaction of pulmonary hypertension, right to left shunting, and specific ARDS therapies has also been examined. Increasing PEEP to levels above 10 cm H2O has been associated with a progressive decline in cardiac output, mean arterial pressure, and LV dimensions secondary to RV systolic overload.[97] The effect of PEEP on right ventricular function may be related to the effect of PEEP on the lung. Recruitment of atelectatic alveoli could improve regional oxygenation, decrease pulmonary vascular resistance, and have no adverse effect on right ventricular function. Alternatively, augmentation of PEEP leading to overdistention of alveoli will increase pulmonary vascular resistance, creating a load on the right ventricle. The interaction of PEEP on the right ventricle may therefore be dependent on the balance of lung recruitment versus overdistention.[98]
Increasing the PEEP level induced PFO shunting in 9% of one study population without PFO shunting at baseline.[51] Reducing the PEEP level in patients with PFO shunting abolished the shunt in 13% of patients. The administration of inhaled nitric oxide abolished the shunt in 2 of 14 patients when this therapy was applied. The use of prone positioning did not abolish the PFO in any patients that received this intervention in contrast to prior investigations. In patients without a PFO, incremental PEEP titration was associated with improvements in the PaO2/FiO2 ratio. In contrast, for patients with a PFO, incremental PEEP titration was not associated with statistically significant change in the oxygenation indices (Fig. 4).[51]
Figure 4.
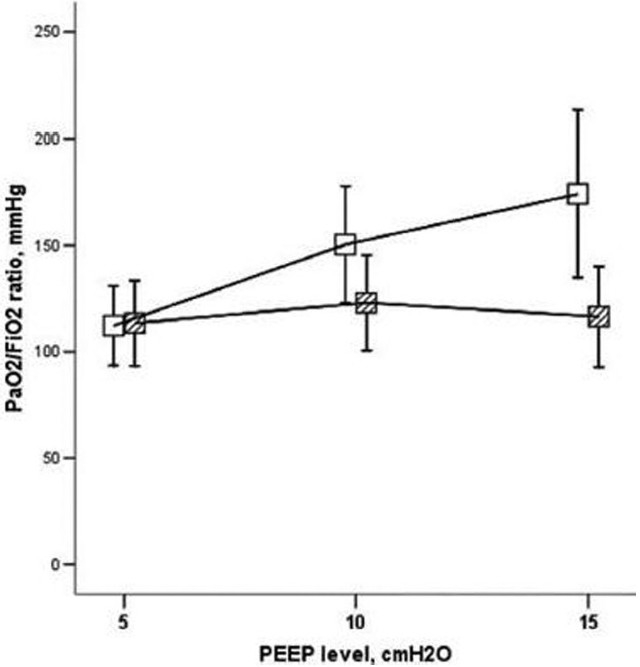
Ratio of PaO2/FIO2 during positive end-expiratory pressure (PEEP) titration in patients who had acute respiratory distress syndrome with echo findings of moderate-to-large shunting (shaded squares) or without shunting (white squares) across a patent foramen ovale. Reference 51 with permission.
These data illustrate the complex interaction of assessing PEEP response by solely considering improvements in the PaO2/FiO2 ratio. PEEP can improve oxygenation in ARDS by stabilizing alveolar volume and decreasing intrapulmonary shunting. In isolation, this would lead to improvements in oxygenation. However, PEEP could successfully achieve alveolar recruitment without improving oxygenation. Changes in intrathoracic pressure associated with PEEP could alter cardiovascular performance by regulating systemic venous return and right ventricular afterload. If PEEP contributes to a decline in cardiac output, an associated fall in mixed venous oxygen saturation could offset any beneficial effect of PEEP on intrapulmonary shunting. Alternatively, even in the setting of a constant cardiac index, if PEEP raises right atrial pressure in the setting of a PFO, this could lead to greater right to left shunting and a worsening of intracardiac shunting while PEEP improves intrapulmonary shunting. The net effect could be no change or even a deterioration in gas exchange in the setting of successful alveolar recruitment.
Very limited pharmacologic trials have been conducted specifically focused on the vascular manifestations of ARDS. The recognized fibrin deposition and small vessel thrombi within the lung circulation of patients with ALI, in addition to documented plasma protein C deficiency in these patients, prompted investigators to investigate a potential role for activated protein C in the treatment of this disorder.[99] Activated protein C (APC) is a novel therapy with anticoagulant and antiinflammatory properties approved for the treatment of patients with severe sepsis.[100] In a randomized clinical trial of ALI patients with an APACHE score <25, activated protein C demonstrated no benefit with regard to ventilator-free days (the study primary study endpoint), mortality, or lung injury score.[101] The trial was stopped after enrollment of 75 subjects by the DSMB for futility. Of interest in this investigation, activated protein C was associated with a reduction in the measured pulmonary deadspace fraction suggesting a physiologic signal despite the lack of change in other gas exchange parameters including the PaO2/FiO2 ratio and Lung Injury Score. The lack of therapeutic efficacy for activated protein C may reflect the complex nature of the vascular injury in ARDS analogous to similar single agent trials of anti-inflammatory therapeutics for this condition.
Currently available data highlights the interaction between ventilatory strategies in ARDS and right ventricular function. The majority of epidemiologic data has associated clinical markers of pulmonary hypertension and right ventricular dysfunction with an adverse outcome in patients with ARDS. A complex interaction between lung recruitment and cardiovascular function is recognized. Few intervention trials have studied the role of cardiovascular management strategies in the ARDS patient population. The intensivist must recognize the pulmonary-cardiovascular interaction and assess both the physiologic benefits of ventilation strategies on gas exchange indices and the deleterious effect on right ventricular function and tissue oxygenation. This dynamic interaction applies to both PEEP and recognized rescue regimens including prone ventilation, HFOV, and inhaled vasodilators. The routine use of echocardiography allows informed clinical decisions in critically ill patients. The advance of portable imaging techniques should bring this information more readily to the patient's bedside.
ARDS SURVIVORS
Despite the limited success of clinical intervention trials, the available clinical data suggests the prognosis from ALI/ARDS is improving. Analysis of mortality from the ARDSNetwork clinical trials, using a consistent disease definition, demonstrated a gradual decline from 35% mortality in 1996 to 26% in 2005.[102] This reduction persisted after adjustment for a low tidal volume ventilation strategy. As the mortality rate has shifted, attention has focused to the recovery process in ARDS. Despite the intensity of support needed to correct gas exchange deficits during the acute process, the respiratory system recovery appears to be relatively short-term and complete. However, the burden for long-term survivors of ARDS is focused on psychological and neuromuscular dysfunction. A carefully described ARDS cohort, tracked over 5 years, confirms near normal lung function recovery at both 1-year and 5-year intervals.[103] Despite this improvement, assessment of physical function in these survivors shows a plateau at year 2 with incomplete recovery to normal. Six minute walking distance remains reduced in comparison to normal individuals at 5-year follow-up. The majority of the surviving population was able to return to work at 1 year (78%) and 5 years (94%).[103] These data, and others, have provided a renewed focus on the non-ventilatory management of the ARDS patient to limit immobility and prevent neuromuscular function loss during the period of acute support.
SUMMARY
ARDS is a heterogeneous syndrome with common clinical and pathophysiologic components. Clinical investigations have sought to define the limitations of the current ALI/ARDS classification system to refine prediction models for disease outcome and better design and conduct clinical trials of promising new therapies. The pessimist views a large collection of negative ARDS clinical trials as a sign of limited progress. The realist accepts the complex biology of the clinical disorder and ongoing progress in defining our techniques of treatment, monitoring, and recovery in this complex patient population.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, et al. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;20(353):1685–93. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 2.Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342:1301–8. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 3.Ashbaugh DG, Bigelow DB, Petty TL, Levine BE. Acute respiratory distress in adults. Lancet. 1967;290:319–23. [Google Scholar]
- 4.Murray JF, Matthay MA, Luce JM, Flick MR. An expanded definition of the adult respiratory distress syndrome. Am Rev Respir Dis. 1988;138:720–3. doi: 10.1164/ajrccm/138.3.720. [DOI] [PubMed] [Google Scholar]
- 5.Monchi M, Bellenfant F, Cariou A, Joly L, Thebert D, Laurent I, et al. Early predictive factors of survival in the acute respiratory distress syndrome: A multivariate analysis. Am J Respir Crit Care Med. 1998;158:1076–81. doi: 10.1164/ajrccm.158.4.9802009. [DOI] [PubMed] [Google Scholar]
- 6.Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, et al. The American-European Consensus Conference on ARDS.Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149:818–24. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 7.Wiedemann HP, Wheeler AP, Bernard GR, Thompson BT, Hayden D, deBoisblanc B, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354:2564–75. doi: 10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]
- 8.Wheeler AP, Bernard GR, Thompson BT, Schoenfeld D, Wiedemann HP, deBoisblanc B, et al. Pulmonary-artery versus central venous catheter to guide treatment of acute lung injury. N Engl J Med. 2006;354:2213–24. doi: 10.1056/NEJMoa061895. [DOI] [PubMed] [Google Scholar]
- 9.Slutsky AS. Neuromuscular blocking agents in ARDS. N Engl J Med. 2010;363:1176–80. doi: 10.1056/NEJMe1007136. [DOI] [PubMed] [Google Scholar]
- 10.Brower RG, Lanken PN, MacIntyre N, Matthay MA, Morris A, Ancukiewicz M, et al. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med. 2004;351:327–36. doi: 10.1056/NEJMoa032193. [DOI] [PubMed] [Google Scholar]
- 11.Gattinoni L, Tognoni G, Pesenti A, Taccone P, Mascheroni D, Labarta V, et al. Effect of prone positioning on the survival of patients with acute respiratory failure. N Engl J Med. 2001;345:568–73. doi: 10.1056/NEJMoa010043. [DOI] [PubMed] [Google Scholar]
- 12.Derdak S, Mehta S, Stewart TE, Smith T, Rogers M, Buchman TG, et al. High-frequency oscillatory ventilation for acute respiratory distress syndrome in adults: A randomized, controlled trial. Am J Respir Crit Care Med. 2002;166:801–8. doi: 10.1164/rccm.2108052. [DOI] [PubMed] [Google Scholar]
- 13.de Hemptinne Q, Remmelink M, Brimioulle S, Salmon I, Vincent JL. ARDS: A clinicopathological confrontation. Chest. 2009;135:944–9. doi: 10.1378/chest.08-1741. [DOI] [PubMed] [Google Scholar]
- 14.Patel SR, Karmpaliotis D, Ayas NT, Mark EJ, Wain J, Thompson BT, et al. The role of open-lung biopsy in ARDS. Chest. 2004;125:197–202. doi: 10.1378/chest.125.1.197. [DOI] [PubMed] [Google Scholar]
- 15.Kao KC, Tsai YH, Wu YK, Chen NH, Hsieh MJ, Huang SF, et al. Open lung biopsy in early-stage acute respiratory distress syndrome. Crit Care. 2006;10:R106. doi: 10.1186/cc4981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Esteban A, Fernández-Segoviano P, Frutos-Vivar F, Aramburu JA, Nájera L, Ferguson ND, et al. Comparison of clinical criteria for the acute respiratory distress syndrome with autopsy findings. Ann Intern Med. 2004;141:440–5. doi: 10.7326/0003-4819-141-6-200409210-00009. [DOI] [PubMed] [Google Scholar]
- 17.Baumann HJ, Kluge S, Balke L, Yekebas E, Izbicki JR, Amthor M, et al. Yield and safety of bedside open lung biopsy in mechanically ventilated patients with acute lung injury or acute respiratory distress syndrome. Surgery. 2008;143:426–33. doi: 10.1016/j.surg.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 18.Papazian L, Doddoli C, Chetaille B, Gernez YL, Thirion X, Roch A, et al. A contributive result of open-lung biopsy improves survival in acute respiratory distress syndrome patients. Crit Care Med. 2007;35:755–62. doi: 10.1097/01.CCM.0000257325.88144.30. [DOI] [PubMed] [Google Scholar]
- 19.Villar J, Perez-Mendez L, Lopez J, Belda J, Blanco J, Saralegui I, et al. An Early PEEP/FIO2 Trial Identifies Different Degrees of Lung Injury in Patients with Acute Respiratory Distress Syndrome. Am J Respir Crit Care Med. 2007;176:795–804. doi: 10.1164/rccm.200610-1534OC. [DOI] [PubMed] [Google Scholar]
- 20.Ferguson ND, Kacmarek RM, Chiche J, Singh JM, Hallett DC, Mehta S, et al. Screening of ARDS patients using standardized ventilator settings: Influence on enrollment in a clinical trial. Intensive Care Med. 2004;30:1111–6. doi: 10.1007/s00134-004-2163-2. [DOI] [PubMed] [Google Scholar]
- 21.Krafft P, Fridrich P, Pernerstorfer T, Fitzgerald RD, Koc D, Schneider B, et al. The acute respiratory distress syndrome: definitions, severity and clinical outcome.An analysis of 101 clinical investigations. Intensive Care Med. 1996;22:519–29. doi: 10.1007/BF01708091. [DOI] [PubMed] [Google Scholar]
- 22.Britos M, Smoot E, Liu KD, Thompson BT, Checkley W, Brower RG. The value of positive end-expiratory pressure and FiO2 criteria in the definition of the acute respiratory distress syndrome. Crit Care Med. 2011 Apr 28; doi: 10.1097/CCM.0b013e31821cb774. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rubenfeld GD, Caldwell E, Granton J, Hudson LD, Matthay MA. Interobserver variability in applying a radiographic definition for ARDS. Chest. 1999;116:1347–53. doi: 10.1378/chest.116.5.1347. [DOI] [PubMed] [Google Scholar]
- 24.Angoulvant F, Llor J, Alberti C, Kheniche A, Zaccaria I, Garel C, et al. Inter-observer variability in chest radiograph reading for diagnosing acute lung injury in children. Pediatr Pulmonol. 2008;43:987–91. doi: 10.1002/ppul.20890. [DOI] [PubMed] [Google Scholar]
- 25.Meade MO, Cook RJ, Guyatt GH, Groll R, Kachura JR, Bedard M, et al. Interobserver variation in interpreting chest radiographs for the diagnosis of acute respiratory distress syndrome. Am J Respir Crit Care Med. 2000;161:85–90. doi: 10.1164/ajrccm.161.1.9809003. [DOI] [PubMed] [Google Scholar]
- 26.Rouby JJ, Puybasset L, Cluzel P, Richecoeur J, Lu Q, Grenier P. Regional distribution of gas and tissue in acute respiratory distress syndrome. II. Physiological correlations and definition of an ARDS Severity Score. CT Scan ARDS Study Group. Intensive Care Med. 2000;26:1046–56. doi: 10.1007/s001340051317. [DOI] [PubMed] [Google Scholar]
- 27.Brun-Buisson C, Minelli C, Bertolini G, Brazzi L, Pimentel J, Lewandowski K, et al. Epidemiology and outcome of acute lung injury in European intensive care units: Results from the ALIVE study. Intensive Care Med. 2004;30:51–61. doi: 10.1007/s00134-003-2022-6. [DOI] [PubMed] [Google Scholar]
- 28.Bersten AD, Edibam C, Hunt T, Moran J. Incidence and mortality of acute lung injury and the acute respiratory distress syndrome in three Australian states. Am J Respir Crit Care Med. 2002;165:443–8. doi: 10.1164/ajrccm.165.4.2101124. [DOI] [PubMed] [Google Scholar]
- 29.Cooke CR, Kahn JM, Caldwell E, Okamoto VN, Heckbert SR, Hudson LD, et al. Predictors of hospital mortality in a population-based cohort of patients with acute lung injury. Crit Care Med. 2008;36:1412–20. doi: 10.1097/CCM.0b013e318170a375. [DOI] [PubMed] [Google Scholar]
- 30.Calfee CS, Eisner MD, Ware LB, Thompson BT, Parsons PE, Wheeler AP, et al. Trauma-associated lung injury differs clinically and biologically from acute lung injury due to other clinical disorders. Crit Care Med. 2007;35:2243–50. doi: 10.1097/01.ccm.0000280434.33451.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Squara P, Dhainaut JF, Artigas A, Carlet J. Hemodynamic profile in severe ARDS: results of the European Collaborative ARDS Study. Intensive Care Med. 1998;24:1018–28. doi: 10.1007/s001340050710. [DOI] [PubMed] [Google Scholar]
- 32.Eisner MD, Thompson T, Hudson LD, Luce JM, Hayden D, Schoenfeld D, et al. Efficacy of low tidal volume ventilation in patients with different clinical risk factors for acute lung injury and the acute respiratory distress syndrome. Am J Respir Crit Care Med. 2001;164:231–6. doi: 10.1164/ajrccm.164.2.2011093. [DOI] [PubMed] [Google Scholar]
- 33.Menezes SL, Bozza PT, Neto HC, Laranjeira AP, Negri EM, Capelozzi VL, et al. Pulmonary and extrapulmonary acute lung injury: Inflammatory and ultrastructural analyses. J Appl Physiol. 2005;98:1777–83. doi: 10.1152/japplphysiol.01182.2004. [DOI] [PubMed] [Google Scholar]
- 34.Gattinoni L, Pelosi P, Suter P, Pedoto A, Vercesi P, Lissoni A. Acute respiratory distress syndrome caused by pulmonary and extrapulmonary disease: Different syndromes? Am J Respir Crit Care Med. 1998;158:3–11. doi: 10.1164/ajrccm.158.1.9708031. [DOI] [PubMed] [Google Scholar]
- 35.Goodman LR, Fumagalli R, Tagliabue P, Tagliabue M, Ferrario M, Gattinoni L, et al. Adult respiratory distress syndrome due to pulmonary and extrapulmonary causes: CT, clinical, and functional correlations. Radiology. 1999;213:545–52. doi: 10.1148/radiology.213.2.r99nv42545. [DOI] [PubMed] [Google Scholar]
- 36.Suntharalingam G, Regan K, Keogh BF, Morgan CJ, Evans TW. Influence of direct and indirect etiology on acute outcome and 6-month functional recovery in acute respiratory distress syndrome. Crit Care Med. 2001;29:562–6. doi: 10.1097/00003246-200103000-00016. [DOI] [PubMed] [Google Scholar]
- 37.Sheu CC, Gong MN, Zhai R, Bajwa EK, Chen F, Thompson BT, et al. The influence of infection sites on development and mortality of ARDS. Intensive Care Med. 2010;36:963–70. doi: 10.1007/s00134-010-1851-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sheu CC, Gong MN, Zhai R, Chen F, Bajwa EK, Clardy PF, et al. Clinical characteristics and outcomes of sepsis-related vs.non-sepsis-related ARDS. Chest. 2010;138:559–67. doi: 10.1378/chest.09-2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gajic O, Dabbagh O, Park PK, Adesanya A, Chang SY, Hou P, et al. Early identification of patients at risk of acute lung injury: Evaluation of lung injury prediction score in a multicenter cohort study. Am J Respir Crit Care Med. 2011;183:462–70. doi: 10.1164/rccm.201004-0549OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bice T, Li G, Malinchoc M, Lee AS, Gajic O. Incidence and risk factors of recurrent acute lung injury. Crit Care Med. 2011;39:1069–73. doi: 10.1097/CCM.0b013e31820edf91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Trillo-Alvarez C, Cartin-Ceba R, Kor DJ, Kojicic M, Kashyap R, Thakur S, et al. Acute lung injury prediction score: Derivation and validation in a population-based sample. Eur Respir J. 2011;37:604–9. doi: 10.1183/09031936.00036810. [DOI] [PubMed] [Google Scholar]
- 42.Croce MA, Fabian TC, Davis KA, Gavin TJ. Early and late acute respiratory distress syndrome: Two distinct clinical entities. J Trauma. 1999;46:361–66. doi: 10.1097/00005373-199903000-00001. discussion 366-8. [DOI] [PubMed] [Google Scholar]
- 43.Meduri GU, Headley AS, Golden E, Carson SJ, Umberger RA, Kelso T, et al. Effect of prolonged methylprednisolone therapy in unresolving acute respiratory distress syndrome: A randomized controlled trial. JAMA. 1998;280:159–65. doi: 10.1001/jama.280.2.159. [DOI] [PubMed] [Google Scholar]
- 44.Steinberg KP, Hudson LD, Goodman RB, Hough CL, Lanken PN, Hyzy R, et al. Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. N Engl J Med. 2006;354:1671–84. doi: 10.1056/NEJMoa051693. [DOI] [PubMed] [Google Scholar]
- 45.Erickson SE, Shlipak MG, Martin GS, Wheeler AP, Ancukiewicz M, Matthay MA, et al. Racial and ethnic disparities in mortality from acute lung injury. Crit Care Med. 2009;37:1–6. doi: 10.1097/CCM.0b013e31819292ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Greene R, Lind S, Jantsch H, Wilson R, Lynch K, Jones R, et al. Pulmonary vascular obstruction in severe ARDS: angiographic alterations after i.v. fibrinolytic therapy. AJR Am J Roentgenol. 1987;148:501–8. doi: 10.2214/ajr.148.3.501. [DOI] [PubMed] [Google Scholar]
- 47.Nuckton TJ, Alonso JA, Kallet RH, Daniel BM, Pittet J, Eisner MD, et al. Pulmonary dead-space fraction as a risk factor for death in the acute respiratory distress syndrome. N Engl J Med. 2002;346:1281–6. doi: 10.1056/NEJMoa012835. [DOI] [PubMed] [Google Scholar]
- 48.Siddiki H, Kojicic M, Li G, Yilmaz M, Thompson TB, Hubmayr RD, et al. Bedside quantification of dead-space fraction using routine clinical data in patients with acute lung injury: Secondary analysis of two prospective trials. Crit Care. 2010;14:R141. doi: 10.1186/cc9206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vieillard-Baron A, Schmitt JM, Augarde R, Fellahi JL, Prin S, Page B, et al. Acute cor pulmonale in acute respiratory distress syndrome submitted to protective ventilation: Incidence, clinical implications, and prognosis. Crit Care Med. 2001;29:1551–5. doi: 10.1097/00003246-200108000-00009. [DOI] [PubMed] [Google Scholar]
- 50.Bull TM, Clark B, McFann K, Moss M. for the National Institutes of Health/National Heart, Lung, Blood Institute ARDS Network. Pulmonary Vascular Dysfunction Is Associated with Poor Outcomes in Patients with Acute Lung Injury. Am J Respir Crit Care Med. 2010;182:1123–8. doi: 10.1164/rccm.201002-0250OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mekontso Dessap A, Boissier F, Leon R, Carreira S, Roche Campo F, Lemaire F, et al. Prevalence and prognosis of shunting across patent foramen ovale during acute respiratory distress syndrome. Crit Care Med. 2010;38:1786–92. doi: 10.1097/CCM.0b013e3181eaa9c8. [DOI] [PubMed] [Google Scholar]
- 52.Meade MO, Cook DJ, Guyatt GH, Slutsky AS, Arabi YM, Cooper DJ, et al. Ventilation strategy using low tidal volumes, recruitment maneuvers, and high positive end-expiratory pressure for acute lung injury and acute respiratory distress syndrome: A randomized controlled trial. JAMA. 2008;299:637–45. doi: 10.1001/jama.299.6.637. [DOI] [PubMed] [Google Scholar]
- 53.Peek G, Mugford M, Tiruvoipati R, Wilson A, Allen E, Thalanany M, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet. 2009;374:1351–63. doi: 10.1016/S0140-6736(09)61069-2. [DOI] [PubMed] [Google Scholar]
- 54.Gong MN, Thompson BT, Williams P, Pothier L, Boyce PD, Christiani DC. Clinical predictors of and mortality in acute respiratory distress syndrome: Potential role of red cell transfusion. Crit Care Med. 2005;33:1191–8. doi: 10.1097/01.ccm.0000165566.82925.14. [DOI] [PubMed] [Google Scholar]
- 55.Cooke CR, Shah CV, Gallop R, Bellamy S, Ancukiewicz M, Eisner MD, et al. A Simple Clinical Predictive Index for Objective Estimates of Mortality in Acute Lung Injury. Crit Care Med. 2009;37:1913–20. doi: 10.1097/CCM.0b013e3181a009b4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Damluji A, Colantuoni E, Mendez-Tellez PA, Sevransky JE, Fan E, Shanholtz C, et al. Short-term mortality prediction for acute lung injury patients: External validation of the ARDSNet prediction model. Crit Care Med. 2011;39:1023–8. doi: 10.1097/CCM.0b013e31820ead31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ware LB, Koyama T, Billheimer DD, Wu W, Bernard GR, Thompson BT, et al. Prognostic and pathogenetic value of combining clinical and biochemical indices in patients with acute lung injury. Chest. 2010;137:288–96. doi: 10.1378/chest.09-1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Calfee CS, Ware LB, Glidden DV, Eisner MD, Parsons PE, Thompson BT, et al. Use of risk reclassification with multiple biomarkers improves mortality prediction in acute lung injury. Crit Care Med. 2011;39:711–7. doi: 10.1097/CCM.0b013e318207ec3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gattinoni L, Caironi P, Cressoni M, Chiumello D, Ranieri VM, Quintel M, et al. Lung recruitment in patients with the acute respiratory distress syndrome. N Engl J Med. 2006;354:1775–86. doi: 10.1056/NEJMoa052052. [DOI] [PubMed] [Google Scholar]
- 60.Gao L, Barnes KC. Recent advances in genetic predisposition to clinical acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2009;296:L713–5. doi: 10.1152/ajplung.90269.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tremblay LN, Slutsky AS. Ventilator-induced lung injury: from the bench to the bedside. Intensive Care Med. 2006;32:24–33. doi: 10.1007/s00134-005-2817-8. [DOI] [PubMed] [Google Scholar]
- 62.Webb HH, Tierney DF. Experimental pulmonary edema due to intermittent positive pressure ventilation with high inflation pressures.Protection by positive end-expiratory pressure. Am Rev Respir Dis. 1974;110:556–65. doi: 10.1164/arrd.1974.110.5.556. [DOI] [PubMed] [Google Scholar]
- 63.Dreyfuss D, Soler P, Basset G, Saumon G. High inflation pressure pulmonary edema.Respective effects of high airway pressure, high tidal volume, and positive end-expiratory pressure. Am Rev Respir Dis. 1988;137:1159–64. doi: 10.1164/ajrccm/137.5.1159. [DOI] [PubMed] [Google Scholar]
- 64.Parsons PE, Eisner MD, Thompson BT, Matthay MA, Ancukiewicz M, Bernard GR, et al. Lower tidal volume ventilation and plasma cytokine markers of inflammation in patients with acute lung injury. Crit Care Med. 2005;33:1–6. doi: 10.1097/01.ccm.0000149854.61192.dc. discussion 230-2. [DOI] [PubMed] [Google Scholar]
- 65.Bellani G, Guerra L, Musch G, Zanella A, Patroniti N, Mauri T, et al. Lung regional metabolic activity and gas volume changes induced by tidal ventilation in patients with acute lung injury. Am J Respir Crit Care Med. 2011;183:1193–9. doi: 10.1164/rccm.201008-1318OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Muscedere JG, Mullen JB, Gan K, Slutsky AS. Tidal ventilation at low airway pressures can augment lung injury. Am J Respir Crit Care Med. 1994;149:1327–34. doi: 10.1164/ajrccm.149.5.8173774. [DOI] [PubMed] [Google Scholar]
- 67.Meade MO, Cook DJ, Guyatt GH, Slutsky AS, Arabi YM, Cooper DJ, et al. Ventilation strategy using low tidal volumes, recruitment maneuvers, and high positive end-expiratory pressure for acute lung injury and acute respiratory distress syndrome: A randomized controlled trial. JAMA. 2008;299:637–45. doi: 10.1001/jama.299.6.637. [DOI] [PubMed] [Google Scholar]
- 68.Mercat A, Richard JC, Vielle B, Jaber S, Osman D, Diehl JL, et al. Positive End-Expiratory Pressure Setting in Adults With Acute Lung Injury and Acute Respiratory Distress Syndrome: A Randomized Controlled Trial. JAMA. 2008;299:646–55. doi: 10.1001/jama.299.6.646. [DOI] [PubMed] [Google Scholar]
- 69.Briel M, Meade M, Mercat A, Brower RG, Talmor D, Walter SD, et al. Higher vs.lower positive end-expiratory pressure in patients with acute lung injury and acute respiratory distress syndrome: Systematic review and meta-analysis. JAMA. 2010;303:865–73. doi: 10.1001/jama.2010.218. [DOI] [PubMed] [Google Scholar]
- 70.Dasenbrook EC, Needham DM, Brower RG, Fan E. Higher positive end-expiratory pressure in patients with acute lung injury: A systematic review and meta-analysis. Respir Care. 2011 doi: 10.4187/respcare.01011. (Jan 27 e-pub) [DOI] [PubMed] [Google Scholar]
- 71.Meade MO, Cook DJ, Griffith LE, Hand LE, Lapinsky SE, Stewart TE, et al. A study of the physiologic responses to a lung recruitment maneuver in acute lung injury and acute respiratory distress syndrome. Respir Care. 2008;53:1441–9. [PubMed] [Google Scholar]
- 72.Brower RG, Morris A, MacIntyre N, Matthay MA, Hayden D, Thompson T, et al. Effects of recruitment maneuvers in patients with acute lung injury and acute respiratory distress syndrome ventilated with high positive end-expiratory pressure. Crit Care Med. 2003;31:2592–7. doi: 10.1097/01.CCM.0000090001.91640.45. [DOI] [PubMed] [Google Scholar]
- 73.Oczenski W, Hörmann C, Keller C, Lorenzl N, Kepka A, Schwarz S, et al. Recruitment maneuvers after a positive end-expiratory pressure trial do not induce sustained effects in early adult respiratory distress syndrome. Anesthesiology. 2004;101:620–5. doi: 10.1097/00000542-200409000-00010. [DOI] [PubMed] [Google Scholar]
- 74.Puybasset L, Gusman P, Muller JC, Cluzel P, Coriat P, Rouby JJ. Regional distribution of gas and tissue in acute respiratory distress syndrome. III. Consequences for the effects of positive end-expiratory pressure. Care Med. 2000;26:1215–27. doi: 10.1007/s001340051340. [DOI] [PubMed] [Google Scholar]
- 75.Domenighetti G, Maggiorini M. Electrical impedance tomography to guide ventilation in ALI-ARDS patients: A research tool for zealous physiologists or an imminent support for the real world intensivist? Minerva Anestesiol. 2010;76:986–8. [PubMed] [Google Scholar]
- 76.Maggiore SM, Jonson B, Richard JC, Jaber S, Lemaire F, Brochard L. Alveolar derecruitment at decremental positive end-expiratory pressure levels in acute lung injury: Comparison with the lower inflection point, oxygenation, and compliance. Am J Respir Crit Care Med. 2001;164:795–801. doi: 10.1164/ajrccm.164.5.2006071. [DOI] [PubMed] [Google Scholar]
- 77.Harris RS, Hess DR, Vengas JG. An objective analysis of the pressure-volume curve in the acute respiratory distress syndrome. Am J Respir Crit Care Med. 2000;161:432–9. doi: 10.1164/ajrccm.161.2.9901061. [DOI] [PubMed] [Google Scholar]
- 78.Grasso S, Stripoli T, de Michele M, Bruno F, Moschetta M, Angelelli G, et al. ARDSnet ventilatory protocol and alveolar hyperinflation: Role of positive end-expiratory pressure. Am J Respir Crit Care Med. 2007;176:761–7. doi: 10.1164/rccm.200702-193OC. [DOI] [PubMed] [Google Scholar]
- 79.Talmor D, Sarge T, Malhotra A, O’Donnell CR, Ritz R, Lisbon A, et al. Mechanical ventilation guided by esophageal pressure in acute lung injury. N Engl J Med. 2008;359:2095–104. doi: 10.1056/NEJMoa0708638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Taccone P, Pesenti A, Latini R, Polli F, Vagginelli F, Mietto C, et al. Prone positioning in patients with moderate and severe acute respiratory distress syndrome: A randomized controlled trial. JAMA. 2009;302:1977–84. doi: 10.1001/jama.2009.1614. [DOI] [PubMed] [Google Scholar]
- 81.Gattinoni L, Vagginelli F, Carlesso E, Taccone P, Conte V, Chiumello D, et al. Decrease in PaCO2 with prone position is predictive of improved outcome in acute respiratory distress syndrome. Crit Care Med. 2003;31:2727–33. doi: 10.1097/01.CCM.0000098032.34052.F9. [DOI] [PubMed] [Google Scholar]
- 82.Abroug F, Ouanes-Besbes L, Dachraoui F, Ouanes I, Brochard L. An updated study-level meta-analysis of randomised controlled trials on proning in ARDS and acute lung injury. Crit Care. 2011;15:R6. doi: 10.1186/cc9403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sud S, Friedrich JO, Taccone P, Polli F, Adhikari NK, Latini R, et al. Prone ventilation reduces mortality in patients with acute respiratory failure and severe hypoxemia: systematic review and meta-analysis. Intensive Care Med. 2010;36:585–99. doi: 10.1007/s00134-009-1748-1. [DOI] [PubMed] [Google Scholar]
- 84.Papazian L, Forel J, Gacouin A, Penot-Ragon C, Perrin G, Loundou A, et al. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med. 2010;363:1107–16. doi: 10.1056/NEJMoa1005372. [DOI] [PubMed] [Google Scholar]
- 85.Imai Y, Nakagawa S, Ito Y, Kawano T, Slutsky AS, Miyasaka K. Comparison of lung protection strategies using conventional and high-frequency oscillatory ventilation. J Appl Physiol. 2001;91:1836–44. doi: 10.1152/jappl.2001.91.4.1836. [DOI] [PubMed] [Google Scholar]
- 86.Sud S, Sud M, Friedrich JO, Meade MO, Ferguson ND, Wunsch H, et al. High-frequency oscillation in patients with acute lung injury and acute respiratory distress syndrome (ARDS): Systematic review and meta-analysis. BMJ. 2010;340:c2327. doi: 10.1136/bmj.c2327. [DOI] [PubMed] [Google Scholar]
- 87.Zapol WM, Snider MT, Hill JD, Fallat RJ, Bartlett RH, Edmunds LH, et al. Extracorporeal membrane oxygenation in severe acute respiratory failure.A randomized prospective study. JAMA. 1979;242:2193–96. doi: 10.1001/jama.242.20.2193. [DOI] [PubMed] [Google Scholar]
- 88.Cornet AD, Hofstra JJ, Swart EL, Girbes AR, Juffermans NP. Sildenafil attenuates pulmonary arterial pressure but does not improve oxygenation during ARDS. Intensive Care Med. 2010;36:758–64. doi: 10.1007/s00134-010-1754-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rossaint R, Falke KJ, López F, Slama K, Pison U, Zapol WM. Inhaled nitric oxide for the adult respiratory distress syndrome. N Engl J Med. 1993;328:399–405. doi: 10.1056/NEJM199302113280605. [DOI] [PubMed] [Google Scholar]
- 90.Adhikari NK, Burns KE, Friedrich JO, Granton JT, Cook DJ, Meade MO. Effect of nitric oxide on oxygenation and mortality in acute lung injury: Systematic review and meta-analysis. BMJ. 2007;334:779. doi: 10.1136/bmj.39139.716794.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gerlach H, Keh D, Semmerow A, Busch T, Lewandowski K, Pappert DM, et al. Dose-response characteristics during long-term inhalation of nitric oxide in patients with severe acute respiratory distress syndrome: A prospective, randomized, controlled study. Am J Respir Crit Care Med. 2003;167:1008–15. doi: 10.1164/rccm.2108121. [DOI] [PubMed] [Google Scholar]
- 92.Putensen C, Hörmann C, Kleinsasser A, Putensen-Himmer G. Cardiopulmonary effects of aerosolized prostaglandin e1 and nitric oxide inhalation in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 1998;157:1743–7. doi: 10.1164/ajrccm.157.6.9609017. [DOI] [PubMed] [Google Scholar]
- 93.Bein T, Metz C, Keyl C, Sendtner E, Pfeifer M. Cardiovascular and pulmonary effects of aerosolized prostacyclin administration in severe respiratory failure using a ventilator nebulization system. J Cardiovasc Pharmacol. 1996;27:583–6. doi: 10.1097/00005344-199604000-00019. [DOI] [PubMed] [Google Scholar]
- 94.Richard C, Warszawski J, Anguel N, Deye N, Combes A, Barnoud D, et al. Early use of the pulmonary artery catheter and outcomes in patients with shock and acute respiratory distress syndrome: A randomized controlled trial. JAMA. 2003;290:2713–20. doi: 10.1001/jama.290.20.2713. [DOI] [PubMed] [Google Scholar]
- 95.Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–77. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 96.Jardin F, Vieillard-Baron A. Is there a safe plateau pressure in ARDS.The right heart only knows? Intensive Care Med. 2007;33:444–7. doi: 10.1007/s00134-007-0552-z. [DOI] [PubMed] [Google Scholar]
- 97.Jardin F, Farcot JC, Boisante L, Curien N, Margairaz A, Bourdarias JP. Influence of positive end-expiratory pressure on left ventricular performance. N Engl J Med. 1981;304:387–92. doi: 10.1056/NEJM198102123040703. [DOI] [PubMed] [Google Scholar]
- 98.Mekontso Dessap A, Charron C, Devaquet J, Aboab J, Jardin F, Brochard L, et al. Impact of acute hypercapnia and augmented positive end-expiratory pressure on right ventricle function in severe acute respiratory distress syndrome. Intensive Care Med. 2009;35:1850–8. doi: 10.1007/s00134-009-1569-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ware LB, Matthay MA, Parsons PE, Thompson BT, Januzzi JL, Eisner MD. The National Heart L.Pathogenetic and prognostic significance of altered coagulation and fibrinolysis in acute lung injury/acute respiratory distress syndrome. Crit Care Med. 2007;35:1821–8. doi: 10.1097/01.CCM.0000221922.08878.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bernard GR, Vincent JL, Laterre PF, LaRosa SP, Dhainaut JF, Lopez-Rodriguez A, et al. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001;344:699–709. doi: 10.1056/NEJM200103083441001. [DOI] [PubMed] [Google Scholar]
- 101.Liu KD, Levitt J, Zhuo H, Kallet RH, Brady S, Steingrub J, et al. Randomized clinical trial of activated protein c for the treatment of acute lung injury. Am J Respir Crit Care Med. 2008;178:618–23. doi: 10.1164/rccm.200803-419OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Erickson SE, Martin GS, Davis JL, Matthay MA, Eisner MD. Recent trends in acute lung injury mortality: 1996-2005. Crit Care Med. 2009;37:1574–9. doi: 10.1097/CCM.0b013e31819fefdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Herridge MS, Tansey CM, Matte A, Tomlinson G, Diaz-Granados N, Cooper A, et al. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011;364:1293–304. doi: 10.1056/NEJMoa1011802. [DOI] [PubMed] [Google Scholar]
- 104.Bollen CW, van Vught AJ, Sherry T, Beale RJ, Shah S, Findlay G, et al. High-frequency oscillatory ventilation compared with conventional mechanical ventilation in adult respiratory distress syndrome: a randomized controlled trial. Crit Care. 2005;9:R430–9. doi: 10.1186/cc3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hirschl RB, Croce M, Gore D, Wiedemann H, Davis K, Zwischenberger J, et al. Prospective, randomized, controlled pilot study of partial liquid ventilation in adult acute respiratory distress syndrome. Am J Respir Crit Care Med. 2002;165:781–7. doi: 10.1164/ajrccm.165.6.2003052. [DOI] [PubMed] [Google Scholar]
- 106.Guérin C, Gaillard S, Lemasson S, Ayzac L, Girard R, Beuret P, et al. Effects of systematic prone positioning in hypoxemic acute respiratory failure: A randomized controlled trial. JAMA. 2004;292:2379–87. doi: 10.1001/jama.292.19.2379. [DOI] [PubMed] [Google Scholar]
- 107.Mancebo J, Fernández R, Blanch L, Rialp G, Gordo F, Ferrer M, et al. A multicenter trial of prolonged prone ventilation in severe acute respiratory distress syndrome. Am J Respir Crit Care Med. 2006;173:1233–39. doi: 10.1164/rccm.200503-353OC. [DOI] [PubMed] [Google Scholar]
- 108.Fernández R, Trenchs X, Klamburg J, Castedo J, Serrano JM, Besso G, et al. Prone positioning in acute respiratory distress syndrome: A multicenter randomized clinical trial. Intensive Care Med. 2008;34:1487–91. doi: 10.1007/s00134-008-1119-3. [DOI] [PubMed] [Google Scholar]
- 109.Voggenreiter G, Aufmkolk M, Stiletto RJ, Baacke MG, Waydhas C, Ose C, et al. Prone positioning improves oxygenation in post-traumatic lung injury-a prospective randomized trial. J Trauma. 2005;59:333–43. doi: 10.1097/01.ta.0000179952.95921.49. [DOI] [PubMed] [Google Scholar]
- 110.Lundin S, Mang H, Smithies M, Stenqvist O, Frostell C. Inhalation of nitric oxide in acute lung injury: Results of a European multicentre study. Intensive Care Med. 1999;25:911–9. doi: 10.1007/s001340050982. [DOI] [PubMed] [Google Scholar]
- 111.Taylor RW, Zimmerman JL, Dellinger RP, Straube RC, Criner GJ, Davis K, et al. Low-dose inhaled nitric oxide in patients with acute lung injury: a randomized controlled trial. JAMA. 2004;291:1603–9. doi: 10.1001/jama.291.13.1603. [DOI] [PubMed] [Google Scholar]
- 112.Anzueto A, Baughman RP, Guntupalli KK, Weg JG, Wiedemann HP, Raventós AA, et al. Aerosolized surfactant in adults with sepsis-induced acute respiratory distress syndrome.Exosurf Acute Respiratory Distress Syndrome Sepsis Study Group. N Engl J Med. 1996;334:1417–21. doi: 10.1056/NEJM199605303342201. [DOI] [PubMed] [Google Scholar]
- 113.Spragg RG, Lewis JF, Wurst W, Hafner D, Baughman RP, Wewers MD, et al. Treatment of acute respiratory distress syndrome with recombinant surfactant protein C surfactant. Am J Respir Crit Care Med. 2003;167:1562–6. doi: 10.1164/rccm.200207-782OC. [DOI] [PubMed] [Google Scholar]
- 114.Spragg RG, Lewis JF, Walmrath H, Johannigman J, Bellingan G, Laterre P, et al. Effect of recombinant surfactant protein C-based surfactant on the acute respiratory distress syndrome. N Engl J Med. 2004;351:884–92. doi: 10.1056/NEJMoa033181. [DOI] [PubMed] [Google Scholar]
- 115.Spragg RG, Taut FJ, Lewis JF, Schenk P, Ruppert C, Dean N, et al. Recombinant surfactant protein C-based surfactant for patients with severe direct lung injury. Am J Respir Crit Care Med. 2011;183:1055–61. doi: 10.1164/rccm.201009-1424OC. [DOI] [PubMed] [Google Scholar]
- 116.Bernard GR, Luce JM, Sprung CL, Rinaldo JE, Tate RM, Sibbald WJ, et al. High-dose corticosteroids in patients with the adult respiratory distress syndrome. N Engl J Med. 1987;317:1565–70. doi: 10.1056/NEJM198712173172504. [DOI] [PubMed] [Google Scholar]
- 117.Luce JM, Montgomery AB, Marks JD, Turner J, Metz CA, Murray JF. Ineffectiveness of high-dose methylprednisolone in preventing parenchymal lung injury and improving mortality in patients with septic shock. Am Rev Respir Dis. 1988;138:62–8. doi: 10.1164/ajrccm/138.1.62. [DOI] [PubMed] [Google Scholar]
- 118.Meduri GU, Golden E, Freire AX, Taylor E, Zaman M, Carson SJ, et al. Methylprednisolone infusion in early severe ARDS: Results of a randomized controlled trial. Chest. 2007;131:954–63. doi: 10.1378/chest.06-2100. [DOI] [PubMed] [Google Scholar]
- 119.Confalonieri M, Urbino R, Potena A, Piattella M, Parigi P, Puccio G, et al. Hydrocortisone infusion for severe community-acquired pneumonia: A preliminary randomized study. Am J Respir Crit Care Med. 2005;171:242–8. doi: 10.1164/rccm.200406-808OC. [DOI] [PubMed] [Google Scholar]
- 120.Ketoconazole for early treatment of acute lung injury and acute respiratory distress syndrome: A randomized controlled trial. The ARDS Network. JAMA. 2000;283:1995–2002. doi: 10.1001/jama.283.15.1995. [DOI] [PubMed] [Google Scholar]
- 121.Randomized, placebo-controlled trial of lisofylline for early treatment of acute lung injury and acute respiratory distress syndrome. Crit Care Med. 2002;30:1–6. doi: 10.1097/00003246-200201000-00001. [DOI] [PubMed] [Google Scholar]
- 122.Vincent JL, Artigas A, Petersen LC, Meyer C. A multicenter, randomized, double-blind, placebo-controlled, dose-escalation trial assessing safety and efficacy of active site inactivated recombinant factor VIIa in subjects with acute lung injury or acute respiratory distress syndrome. Crit Care Med. 2009;37:1874–80. doi: 10.1097/CCM.0b013e31819fff2c. [DOI] [PubMed] [Google Scholar]
- 123.Matthay MA, Brower RG, Carson S, Douglas IS, Eisner M, Hite D, et al. Randomized, placebo-controlled clinical trial of an aerosolized beta-2 agonist for treatment of acute lung injury. Am J Respir Crit Care Med. 2011 May 11; doi: 10.1164/rccm.201012-2090OC. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Calfee CS, Eisner MD, Parsons PE, Thompson BT, Conner ER, Jr, Matthay MA, et al. Soluble intercellular adhesion molecule-1 and clinical outcomes in patients with acute lung injury. Intensive Care Med. 2008;35:248–57. doi: 10.1007/s00134-008-1235-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lin WC, Lin CF, Chen CL, Chen CW, Lin YS. Prediction of outcome in patients with acute respiratory distress syndrome by bronchoalveolar lavage inflammatory mediators. Exp Biol Med (Maywood) 2010;235:57–65. doi: 10.1258/ebm.2009.009256. [DOI] [PubMed] [Google Scholar]
- 126.Parsons PE, Matthay MA, Ware LB, Eisner MD. Elevated plasma levels of soluble TNF receptors are associated with morbidity and mortality in patients with acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2005;288:L426–31. doi: 10.1152/ajplung.00302.2004. [DOI] [PubMed] [Google Scholar]
- 127.Ware LB, Eisner MD, Thompson BT, Parsons PE, Matthay MA. Significance of von Willebrand factor in septic and nonseptic patients with acute lung injury. Am J Respir Crit Care Med. 2004;170:766–72. doi: 10.1164/rccm.200310-1434OC. [DOI] [PubMed] [Google Scholar]
- 128.Calfee CS, Ware LB, Eisner MD, Parsons PE, Thompson BT, Wickersham N, et al. Plasma receptor for advanced glycation end products and clinical outcomes in acute lung injury. Thorax. 2008;63:1083–9. doi: 10.1136/thx.2008.095588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Eisner MD, Parsons P, Matthay MA, Ware L, Greene K. Plasma surfactant protein levels and clinical outcomes in patients with acute lung injury. Thorax. 2003;58:983–8. doi: 10.1136/thorax.58.11.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Chen CY, Yang KY, Chen MY, Chen HY, Lin MT, Lee YC, et al. Decoy Receptor 3 levels in peripheral blood predict outcomes of acute respiratory distress syndrome. Am J Respir Crit Care Med. 2009;180:751–60. doi: 10.1164/rccm.200902-0222OC. [DOI] [PubMed] [Google Scholar]
- 131.Bajwa EK, Januzzi JL, Gong MN, Thompson BT, Christiani DC. Prognostic value of plasma N-terminal probrain natriuretic peptide levels in the acute respiratory distress syndrome. Crit Care Med. 2008;36:2322–7. doi: 10.1097/CCM.0b013e318181040d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Spragg RG, Bernard GR, Checkley W, Curtis JR, Gajic O, Guyatt G, et al. Beyond Mortality: Future Clinical Research in Acute Lung Injury. Am J Respir Crit Care Med. 2010 May 15;181(10):1121. doi: 10.1164/rccm.201001-0024WS. [DOI] [PMC free article] [PubMed] [Google Scholar]


