Abstract
N-terminal pro B-type natriuretic peptide (NT-proBNP) is a product of cleavage of the cardiac prohormone pro B-type natriuretic peptide into its active form. It has proven to be a useful biomarker in left heart failure. However, studies examining the utility of serial measurements of NT-proBNP in pulmonary arterial hypertension (PAH) patients have shown mixed results. We compared three methods of predicting adverse clinical outcomes in PAH patients: the change in 6 minute walk distance (6MWD), the change in absolute levels of NT-proBNP and the change in log-transformed levels of NT-proBNP. All PAH patients presenting from March-June 2007 were screened. Patients who were clinically unstable, had abnormal renal function or hemoglobin levels or lacked a prior NT-proBNP were excluded. 63 patients were followed up for adverse clinical outcomes (defined as death, transplantation, hospitalisation for right heart failure, or need for increased therapy). Three methods were used to predict adverse events, i.e.: (a) comparing a 6MWD performed in March-June 2007 and a previous 6MWD. A decrease in 6MWD of ≥30m was used to predict clinical deterioration; (b) comparing a NT-proBNP value measured in March-June 2007 and a previous NT-proBNP. An increase in NT-proBNP of ≥250pg/ml was used to predict clinical deterioration (250pg/ml represented approximately 30% change from the baseline median value of NT-proBNP for this cohort); and (c) comparing the loge equivalents of two consecutive NT-proBNP values. We used the formula: loge(current NT-proBNP) - loge(previous NT-proBNP)=x. A value of x≥+0.26 was used to predict adverse events. This is equivalent to a 30% change from baseline, and hence is comparable to the chosen cut-off for absolute levels of NT-proBNP. A loge difference of ≥+0.26 identifies patients at risk of adverse events with a specificity of 98%, a sensitivity of 60%, a positive predictive value of 89%, and a negative predictive value of 90%. A drop in 6MWD of ≥30m has a specificity of 29%, a sensitivity of 73%, a positive predictive value of 24% and a negative predictive value of 24%. It seems possible to risk-stratify apparently stable PAH patients by following the changes in their serial log-transformed NT-proBNP values. In this small pilot study, this method was better than relying on changes in the actual levels of NT-proBNP or changes in 6MWD. This needs to be validated prospectively in a larger cohort.
Keywords: N-terminal pro B-type natriuretic peptide, 6-minute walk distance, biomarker
INTRODUCTION
Pulmonary arterial hypertension (PAH) is the term used to describe a group of rare conditions characterized by increased pulmonary artery pressures which lead to progressive right ventricular failure and death. Assessment of hemodynamic indices and exercise tolerance as measured by the six minute walk distance (6MWD) at baseline allows prediction of prognosis[1,2] to a certain extent. However, additional simple, accurate and non-invasive predictors of prognosis are still required. The need for this is accentuated by the fact that some treatments for PAH are inherently dangerous (e.g. heart-lung transplantation) and an accurate predictor of prognosis would be invaluable in establishing the optimal time to implement such measures.
N-terminal pro B-type natriuretic peptide (NT-proBNP) is a product of cleavage of the cardiac prohormone pro B-type natriuretic peptide into its active form. It is a valuable prognostic biomarker in patients with left heart failure.[3,4] Indeed, treatment guided by serial monitoring of NT-proBNP has been suggested to be superior to treatment guided by a clinical disease severity score based on Framingham criteria.[5,6] As a result of such studies, NT-proBNP has been investigated as a biomarker in PAH. Baseline levels of NT-proBNP have been shown to correlate with hemodynamic indices, 6MWD and right ventricular dimensions.[7,8,9,10] Elevated baseline levels of NT-proBNP are also an independent predictor of mortality in patients with idiopathic PAH and PAH associated with scleroderma.[7,11,12] Despite this, studies examining the utility of serial measurements of NT-proBNP in PAH patients have shown mixed results. A small study following 20 patients with PAH established that a reduction in levels of BNP of ≥50% from baseline following the introduction of epoprostenol was strongly indicative of event-free survival for the following year.[13] However a larger study in 94 PAH patients concluded that changes in serial NT-proBNP levels were not sensitive enough to detect clinical changes in World Health Organisation (WHO) heart failure class or 6MWD for the individual patient.[14]
Recently it has been shown that plasma concentrations of NT-proBNP follow a log-normal distribution in patients with chronic left heart failure.[15] This has led us to surmise that using the change in log-transformed NT-proBNP might be a better prognostic method. We sought to compare this novel method with using the change in untransformed values of NT-proBNP, and the traditional method of using changes in 6MWD. 6MWD has been a traditional surrogate end-point for trials in PAH and a change of at least 30m has been accepted as clinically significant.[16,17,18,19]
MATERIALS AND METHODS
Subjects
A cross-sectional study was performed. All patients with PAH admitted to Papworth Hospital from March to June 2007 were screened. The inclusion criteria were: (a) a diagnosis of pulmonary arterial hypertension (either idiopathic or associated with connective tissue disease, CTD or congenital heart disease, CHD) or chronic thromboembolic pulmonary hypertension (CTEPH); (b) a clinically stable condition at the time of review in March to June 2007. Stability was defined as a lack of need for intervention, i.e. no change in types or doses of medication for PAH (including diuretics and excluding warfarin); and (c) an ability to perform a 6MWD not limited by any other condition other than their PAH.
The exclusion criteria were: (a) a diagnosis of pulmonary venous hypertension; (b) lack of a prior NT-proBNP level within the 6 months preceding their review; (c) a history of chronic renal problems and/or a creatinine of >150micromoll-1; and (d) an abnormal haemoglobin level, defined as ≤13.0 or ≥18.0 (men), or ≤11.5 or ≥16.0 (women). The latter were exclusion criteria since renal failure and anaemia have been shown to be related to increased NT-proBNP levels, independent of the severity of heart failure.[20,21]
Measurement of NT-proBNP
Peripheral venous blood was drawn at admission and stored in serum gel tubes. This was analysed using a sandwich immunoassay (Elecsys 1010) produced by Roche. The measuring range was 5-35,000pg/ml. The assay did not have any significant cross-reactions with atrial natriuretic peptide, B-type natriuretic peptide or N-terminal pro-atrial natriuretic peptide.
Study design
63 patients fulfilled the criteria for inclusion. Their NT-proBNP levels from their admission in March-June 2007 and the NT-proBNP level measured within the previous 6 months were extracted by a single researcher. Two other researchers who were blinded to the NT-proBNP data examined the clinical course of these patients for fifteen months. Patients were classified as having an adverse event if any of the following occurred: (a) death, (b) transplantation, (c) need for additional targeted therapy for PAH, or (d) hospitalisation for right heart failure.
Three methods were used to predict the occurrence of clinical adverse events. The first involved the comparison of two consecutive 6MWD, i.e. the difference between a 6MWD performed in March-June 2007 and a 6MWD performed up to 6 months previously. A cut-off of 30m was used, i.e., a decrease in 6MWD of 30m or greater was used to predict the occurrence of clinical adverse events and an increase in 6MWD of 30m or greater was used to predict a stable clinical course. 30m was chosen as a cut-off point as many trials have demonstrated that a change of ≥30m or ≥10% from baseline is required in the 6MWD to be clinically significant.[16,17,18,19]
The second method involved the comparison of two consecutive NT-proBNP values, i.e. the difference between a NT-proBNP value measured in March-June 2007 and a NT-proBNP measured up to 6 months previously. An increase in NT-proBNP of ≥250pg/ml was used to predict the occurrence of adverse events and a drop of ≥250pg/ml was used to predict stability. These cut-offs were chosen as a change of 250pg/ml represents approximately 30% change from the baseline median value of NT-proBNP for this cohort.
The third method involved comparison of the two consecutive NT-proBNP values as above but transformed by taking their loge equivalents, i.e. values for NT-proBNP were transformed to loge equivalents and the difference determined using the formula: loge(current NT-proBNP) - loge(previous NT-proBNP)=x. A cut-off value of 0.26 was used, i.e. a value of x≥+0.26 was used to predict the occurrence of adverse events and a value of x≤-0.26 was used to predict stability. The cut-off levels of +0.26 and -0.26 were chosen as they were equivalent to a 30% change from baseline, and hence comparable to the chosen cut-offs for absolute levels of NT-proBNP.
Statistical Analysis
Descriptive statistical analyses were carried out on the study population of 63 patients. Continuous variables are expressed as mean (SD) if normally distributed and as median (interquartile range) if not. The three methods were compared using receiver operating characteristic and Kaplan-Meier curves, and these were produced using Prism (version 5.0).
RESULTS
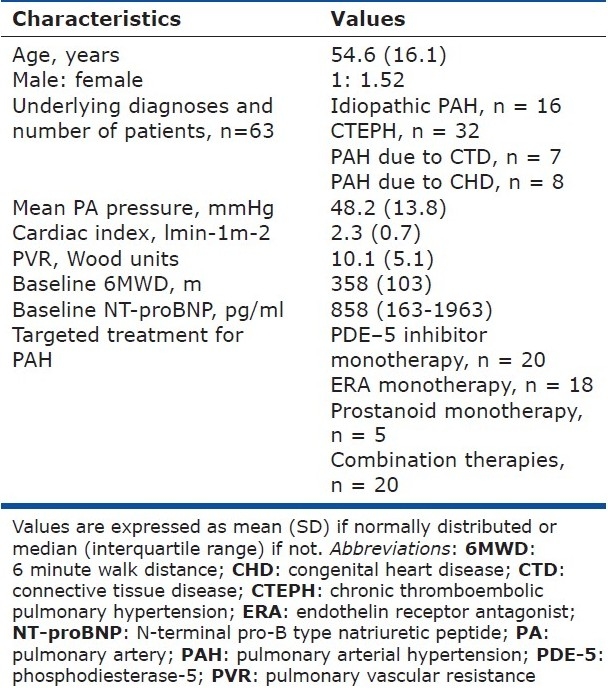
The 63 patients included in this study represented a mixture of idiopathic PAH, CTEPH and PAH associated with CTD and CHD. Their baseline characteristics are summarized in Table 1. Hemodynamic values were taken from the latest right heart catheter available. As a whole they had moderate-to-severe PAH with an average mean PA pressure of 48.2±13.8 mmHg and a cardiac index of 2.3±0.7 lmin-1m-2.
Table 1.
Baseline characteristics of the patients
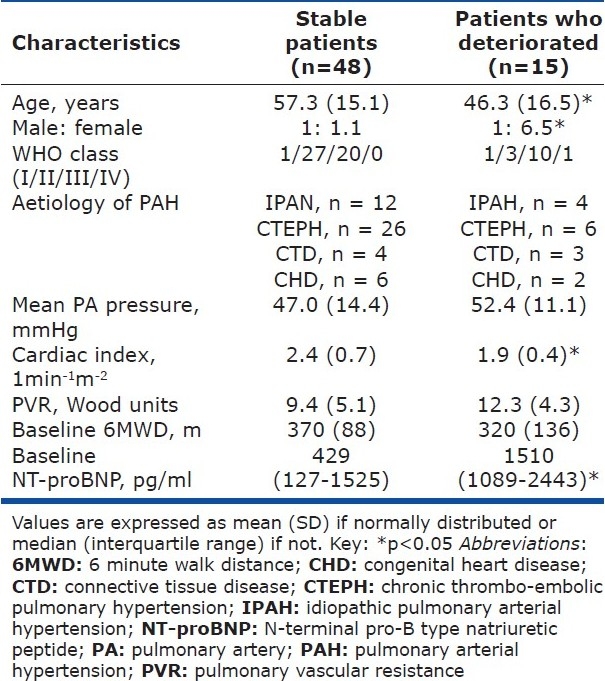
15 patients suffered an adverse event. Their characteristics as compared to the stable patients are shown in Table 2. As a whole the patients who deteriorated were younger but had a worse cardiac index (1.9±0.4 versus 2.4±0.7 lmin-1m-2, P<0.05). However, their other characteristics including mean PA pressures, WHO class and aetiologies of their PAH were not significantly different. There were also no significant differences in any of the 6MWD or the change in 6MWD.
Table 2.
Comparison of the characteristics of stable and deteriorating patients
The best method at predicting the occurrence of adverse events was a change in loge NT-proBNP values of ≥+0.26. This had a specificity of 98%, a sensitivity of 60%, a positive predictive value of 90% and a negative predictive value of 89%. A drop in 6MWD of ≥30m had a corresponding specificity of 29%, a sensitivity of 73%, a positive predictive value of 24% and a negative predictive value of 78%.
Conversely a change in loge NT-proBNP values of ≤ -0.26 was better than an increase in 6MWD of ≥30m at predicting a stable clinical course. The sensitivity for the loge NT-proBNP method was 93% (cf. 73% for 6MWD), specificity was 42% (cf. 29% for 6MWD), positive predictive value was 33% (also 33% for 6MWD) and the negative predictive value of 95% (cf. 78% for 6MWD).
Receiver operating characteristic (ROC) curves were plotted for each of the three methods (Fig. 1A–1C). A ROC curve is a graphical plot of the sensitivity versus (1-specificity). A ROC curve representing a perfect prediction method would be a point in the upper left hand corner of the plot, representing 100% sensitivity and 100% specificity.[22] Conversely a ROC curve based on random chance would yield the ‘line of no-discrimination’, which is a diagonal line connecting the left bottom and top right corners. Therefore for a perfect prediction method the area under curve (AUC) would be 1.0 while a prediction based on random chance would have an AUC of 0.5. -P-values can also be calculated based on ROC curves to show whether a prediction method is significantly better than pure chance. The method using the change in loge NT-proBNP has an AUC of 0.82 (P=0.0002). Conversely the ROC based on the change in 6MWD has an AUC of 0.59 (P=0.31).
Figure 1.
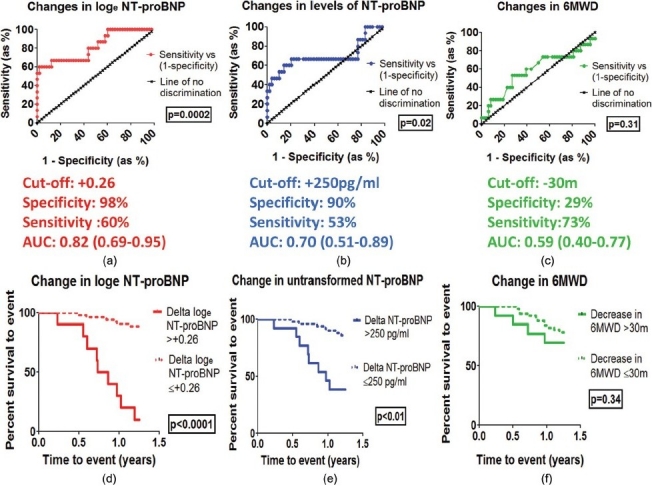
Top section: ROC curves for predictions based on the change in log-transformed NT-proBNP (a), the change in untransformed levels of NT-proBNP (b), and changes in 6MWD (c). Middle section: sensitivities, specificities and AUC values for each method. Bottom section: Kaplan-Meier curves showing cumulative survival to event for each method i.e. change in log-transformed NT-proBNP (d), change in untransformed NT-proBNP (e), and change in 6MWD (f).
Finally, Kaplan-Meier curves were constructed for each of these methods. The best method in discriminating between patients who deteriorated and those who did not was based on the change in log-transformed NT-proBNP (Fig. 1D, P<0.001). Kaplan-Meiers based on the change in untransformed levels of NT-proBNP (Fig. 1E) did show significant discriminatory effects but this was not as significant as those based on the change in log-transformed NT-proBNP. The Kaplan-Meiers based on a decrease in 6MWD of ≥30m (Fig. 1F) failed to show significant discrimination.
DISCUSSION
This study was originally triggered by our observation that there are some PAH patients with a stable 6MWD but increasing NT-proBNP levels who then go on to suffer a significant adverse event within the following year. There is also no current guidance as to what constitutes a significant change in NT-proBNP levels for PAH patients.
We accept that thinking in terms of a log change is not instinctive for clinicians. Therefore this concept is much more interpretable when one realises that a +0.26 difference in loge NT-proBNP is equivalent to a 30% change from baseline. This can be proven mathematically (Table 3).
Table 3.
Proof that a change in log-transformed levels of 0.26 is equal to a 30% change from baseline levels of NT-proBNP

The main drawback of this study is that it is based on a small cohort which was reviewed retrospectively. However this study is meant to be a proof-of-concept model that demonstrates the applicability of this pragmatic approach to a wide population of PAH patients at different stages of their disease. Another drawback is that this method is not useful if the change in loge NT-proBNP lie between the chosen cut-off points. 28 out of 63 patients studied fell into this category. However this limitation also applies to other methods. When the 6MWD model was examined, 34 of the 63 patients had changes in their 6MWD between +30m and -30m. For this group, careful clinical monitoring with a low threshold for right heart catheterization remains the mainstay. Also, this study has necessarily excluded patients who were unable to perform a 6MWD as we were attempting to compare the use of 6MWD versus the use of NT-proBNP and loge NT-proBNP.
This study has also highlighted one of the aspects regarding 6MWD that is open to debate; namely that the change in 6MWD did not appear to track with clinical outcome. This has been suggested before by Sitbon,[23] who noted in their cohort of 178 IPAH patients that the increase from baseline of the 6MWD performed after three months of epoprostenol therapy did not correlate with survival. A meta-analysis of 16 trials involving 1962 PAH patients by Macchia[24] did not find a significant association between the placebo-corrected increase in 6MWD and estimated time to death. However a larger meta-analysis by Galiè[25] including 3199 PAH patients showed a significant reduction in overall mortality of 43%, associated with a significant average improvement in 6MWD of 35.6m. Therefore the relationship between changes in 6MWD and time to clinical worsening remains unclear.
In our cohort, there are a few plausible explanations for this finding. Firstly, the 6MWD is easily affected by factors other than PAH, such as musculoskeletal problems and psychological factors. In this study we have attempted to screen for such problems and to exclude patients in whom a 6MWD was felt to be unreliable. However a bias may still exist. Secondly, the patients in this study represented a mixture of aetiologies and treatments. This was done deliberately to capture a snapshot of the general population of patients with PAH that are seen in our centre and to prove that the novel method we proposed was generally applicable. Most of the validation of 6MWD as a prognostic tool has been done in selected and relatively homogeneous populations of different types of PAH.[18,19,26,27,28] The third explanation could be that this is a true finding, i.e. that the change in 6MWD is simply not very good at predicting clinical worsening. This is supported by preliminary findings from another study[29] which demonstrated that the AUC for using changes in 6MWD to predict clinical worsening was 0.56 (comparable with our value of 0.59).
Finally, one may speculate on the implications of this pilot study. It potentially provides a simple and pragmatic method to keep apparently stable patients under surveillance. It may prove useful in situations where a patient lives a significant distance away from their specialist centre. A positive test would then serve as a “red flag” that the patient needs to be reviewed urgently.
CONCLUSIONS
It seems possible to risk-stratify apparently stable PAH patients by following the changes in their serial log-transformed NT-proBNP values. The formula used was:
loge (current NT-proBNP) - loge (previous NT-proBNP)=x.
A value of x≥+0.26; which is equivalent to a 30% increase from baseline, had a specificity of 98%, a sensitivity of 60%, a positive predictive value of 90% and a negative predictive value of 89% at predicting the occurrence of adverse events. In this small pilot study, this method was better than relying on changes in the actual levels of NT-proBNP or changes in 6MWD. This needs to be validated prospectively in a larger cohort.
Footnotes
Source of Support: This work was funded by an MRC (UK) Research Training Fellowship and the Sackler studentship (Elaine Soon), the British Heart Foundation and the NIHR Biomedical Research Centre (UK). Conflicts of Interest: Elaine Soon has received travel awards from GlaxoSmithKline and Encysive, an unrelated research award from Pfizer and the Sackler studentship. Natalie Doughty has received educational awards from Actelion, Pfizer, GlaxoSmithKline and Bayer and served on advisory panels for Actelion, Pfizer and GlaxoSmithKline. Carmen Treacy has received travel grants from Actelion. Robert MacKenzie-Ross has received travel grants from Pfizer and GlaxoSmithKline. Mark Toshner has received travel awards from Pfizer and GlaxoSmithKline. Paul Upton has received a research grant from Novartis and a travel grant from Actelion. Karen Sheares has received honoraria and travel grants from Actelion, United Therapeutics, Encysive and GlaxoSmithKline. Nicholas Morrell has received a research grant from Novartis. Joanna Pepke-Zaba has received honoraria from Actelion, Pfizer, GlaxoSmithKline and Bayer and research grants from Pfizer, Actelion, Schering and United Therapeutics
Conflict of Interest: None declared.
REFERENCES
- 1.Barst RJ, McGoon M, Torbicki A, Sitbon O, Krowka MJ, Olschewski H, et al. Diagnosis and differential assessment of pulmonary arterial hypertension. J Am Coll Cardiol. 2004;43:40S–70. doi: 10.1016/j.jacc.2004.02.032. [DOI] [PubMed] [Google Scholar]
- 2.McLaughlin VV, Presberg KW, Doyle RL, Abman SH, McCrory DC, Fortin T, et al. Prognosis of pulmonary arterial hypertension: ACCP evidence-based clinical practice guidelines. Chest. 2004;126:78S–92. doi: 10.1378/chest.126.1_suppl.78S. [DOI] [PubMed] [Google Scholar]
- 3.Gardner RS, Ozalp F, Murday AJ, Robbs SD, McDonagh TA. N-terminal pro-brain natriuretic peptide.A new gold standard in predicting mortality in patients with advanced heart failure. Eur Heart J. 2003;24:1735–43. doi: 10.1016/j.ehj.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 4.Bayes-Genis A, Pascual-Figal DA, Fabregat J, Domingo M, Planas F, Casas T, et al. Serial NT-proBNP monitoring and outcomes in outpatients with decompensation of heart failure. Int J Cardiol. 2007;120:338–43. doi: 10.1016/j.ijcard.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 5.Troughton RW, Frampton CM, Yandle TG, Espiner EA, Nicolls MG, Richards AM. Treatment of heart failure guided by amino-terminal brain natriuretic peptide (N-BNP) concentrations. Lancet. 2000;355:1126–30. doi: 10.1016/s0140-6736(00)02060-2. [DOI] [PubMed] [Google Scholar]
- 6.Pascual-Figal DA, Domingo M, Casas T, Gich I, Ordonez-Llanos J, Martinez P, et al. Usefulness of clinical and NT-proBNP monitoring for prognostic guidance in destabilized heart failure outpatients. Eur Heart J. 2008;29:1011–8. doi: 10.1093/eurheartj/ehn023. [DOI] [PubMed] [Google Scholar]
- 7.Fijalkowska A, Kurzyna M, Torbicki A, Szewczyk G, Florczyk M, Pruszczyk P, et al. Serum N-terminal brain natriuretic peptide as a prognostic parameter in patients with pulmonary hypertension. Chest. 2006;129:1313–21. doi: 10.1378/chest.129.5.1313. [DOI] [PubMed] [Google Scholar]
- 8.Blyth KG, Groenning BA, Mark PB, Martin TN, Foster JE, Steedman T, et al. NT-proBNP can be used to detect right ventricular systolic dysfunction in pulmonary hypertension. Eur Respir J. 2007;29:737–44. doi: 10.1183/09031936.00095606. [DOI] [PubMed] [Google Scholar]
- 9.Andreassen AK, Wergeland R, Simonsen S, Geiran O, Guevara C, Ueland T. N-terminal pro-B-type natriuretic peptide as an indicator of disease severity in a heterogeneous group of patients with chronic precapillary pulmonary hypertension. Am J Cardiol. 2006;98:525–9. doi: 10.1016/j.amjcard.2006.02.061. [DOI] [PubMed] [Google Scholar]
- 10.Leuchte HH, Holzapfel M, Baumgartner RA, Ding I, Neurohr C, Vogeser M, et al. Clinical significance of brain natriuretic peptide in primary pulmonary hypertension. J Am Coll Cardiol. 2004;43:764–70. doi: 10.1016/j.jacc.2003.09.051. [DOI] [PubMed] [Google Scholar]
- 11.Nagaya N, Nishikimi T, Uematsu M, Satoh T, Kyotani S, Sakamaki F, et al. Plasma brain natriuretic peptide as a prognostic indicator in primary pulmonary hypertension. Circulation. 2000;102:865–70. doi: 10.1161/01.cir.102.8.865. [DOI] [PubMed] [Google Scholar]
- 12.Williams MH, Handler CE, Akram R, Smith CJ, Das C, Smee J, et al. Role of N-terminal brain natriuretic peptide (N-TproBNP) in scleroderma-associated pulmonary hypertension. Eur Heart J. 2006;27:1485–94. doi: 10.1093/eurheartj/ehi891. [DOI] [PubMed] [Google Scholar]
- 13.Park MH, Scott RL, Uber PA, Ventura HO, Mehra MR. Usefulness of B-type natriuretic peptide as a predictor of treatment outcomes in pulmonary arterial hypertension. Congest Heart Fail. 2008;10:221–5. doi: 10.1111/j.1527-5299.2004.03881.x. [DOI] [PubMed] [Google Scholar]
- 14.Mackay LS, Hughes R, Peaston R, MacGowan G, Parry G, Fisher AJ, et al. Serial NT-probrain natriuretic peptide levels in patients with pulmonary arterial hypertension. J Heart Lung Transplant. 2007;26:S248. [Google Scholar]
- 15.Schou M, Gustafsson F, Kjaer A, Hildebrandt P. Long-term clinical variation of NT-proBNP in stable chronic heart failure patients. Eur Heart J. 2007;28:177–82. doi: 10.1093/eurheartj/ehl449. [DOI] [PubMed] [Google Scholar]
- 16.Provencher S, Sitbon O, Humbert M, Cabrol S, Jais X, Simmoneau G. Long-term outcome with first-line bosentan therapy in idiopathic pulmonary arterial hypertension. Eur Heart J. 2006;27:589–95. doi: 10.1093/eurheartj/ehi728. [DOI] [PubMed] [Google Scholar]
- 17.Galiè N, Ghofrani HA, Torbicki A, Barst RJ, Rubin LJ, Badesch D, et al. Sildenafil Use in Pulmonary Arterial Hypertension (SUPER) Study Group. Sildenafil citrate therapy for pulmonary arterial hypertension. N Engl J Med. 2006;353:2148–57. doi: 10.1056/NEJMoa050010. [DOI] [PubMed] [Google Scholar]
- 18.Olschewski H, Simonneau G, Galiè N, Higgenbottam T, Naeije R, Rubin LJ, et al. Aerosolized Iloprost Randomized Study Group. Inhaled iloprost for severe pulmonary hypertension. N Engl J Med. 2002;347:322–9. doi: 10.1056/NEJMoa020204. [DOI] [PubMed] [Google Scholar]
- 19.Barst RJ, Rubin LJ, Long WA, McGoon MD, Rich S, Badesch DB, et al. A comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertension: The Primary Pulmonary Hypertension Study Group. N Engl J Med. 1996;334:296–301. doi: 10.1056/NEJM199602013340504. [DOI] [PubMed] [Google Scholar]
- 20.Luchner A, Hengstenberg C, Lowel H, Riegger GA, Schunkert H, Holmer S. Effect of compensated renal dysfunction on approved heart failure markers: Direct comparison of brain natriuretic peptide (BNP) and N-terminal pro-BNP. Hypertension. 2005;46:118–23. doi: 10.1161/01.HYP.0000170140.36633.8f. [DOI] [PubMed] [Google Scholar]
- 21.Hogenhuis J, Voors AA, Jaarsma T, Hoes AW, Hillege HL, Kragten JA, et al. Anaemia and renal failure are independently associated with BNP and NT-proBNP levels in patients with heart failure. Eur J Heart Fail. 2007;9:787–94. doi: 10.1016/j.ejheart.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 22.Zweig MH, Campbell G. Receiver-operating characteristic (ROC) plots: A fundamental evaluation tool in clinical medicine. Clin Chem. 1993;39:561–77. [PubMed] [Google Scholar]
- 23.Sitbon O, Humbert M, Nunes H, Parent F, Garcia G, Herve P, et al. Long-term intravenous epoprostenol infusion in primary pulmonary hypertension. J Am Coll Cardiol. 2002;40:780–8. doi: 10.1016/s0735-1097(02)02012-0. [DOI] [PubMed] [Google Scholar]
- 24.Macchia A, Marchioli R, Marfisi RM, Scarano M, Levantesi G, Tavazzi L, et al. A meta-analysis of trials of pulmonary hypertension: A clinical condition looking for drugs and research methodology. Am Heart J. 2007;153:1037–47. doi: 10.1016/j.ahj.2007.02.037. [DOI] [PubMed] [Google Scholar]
- 25.Galiè N, Manes A, Negro L, Palazzini M, Bacchi-Reggiani ML, Branzi A. A meta-analysis of randomised controlled trials in pulmonary arterial hypertension. Eur Heart J. 2009;30:394–403. doi: 10.1093/eurheartj/ehp022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miyamoto S, Nagaya N, Satoh T, Kyotani S, Sakamaki F, Fujita M, et al. Clinical correlates and prognostic significance of six-minute walk test in patients with primary pulmonary hypertension: Comparison with cardiopulmonary exercise testing. Am J Respir Crit Care Med. 2000;161:487–92. doi: 10.1164/ajrccm.161.2.9906015. [DOI] [PubMed] [Google Scholar]
- 27.Paciocco G, Martinez FJ, Bossone E, Pielsticker E, Gillespie B, Rubenfire M. Oxygen desaturation on the six-minute walk test and mortality in untreated primary pulmonary hypertension. Eur Respir J. 2001;17:647–52. doi: 10.1183/09031936.01.17406470. [DOI] [PubMed] [Google Scholar]
- 28.Badesch DB, Tapson VF, McGoon MD, Brundage BH, Rubin LJ, Wigley FM, et al. Continuous intravenous epoprostenol for pulmonary hypertension due to the scleroderma spectrum of disease: A randomized, controlled trial. Ann Intern Med. 2000;132:425–34. doi: 10.7326/0003-4819-132-6-200003210-00002. [DOI] [PubMed] [Google Scholar]
- 29.Carlin C, Blyth KG, McLure LE, Spooner R, Peacock AJ, Johnson MK. Serial NT-proBNP measurements predict early clinical worsening and mortality in pulmonary arterial and chronic thromboembolic pulmonary hypertension. Thorax. 2008;63:A44. [Google Scholar]


