Abstract
A prospective study was performed to evaluate the efficacy of neurophysiological monitoring (NPM) techniques in the detection of ischemic changes that may be seen during endovascular treatment of cerebral aneurysms. Sixty three patients underwent NPM during first-stage endovascular treatment of cerebral aneurysms. The endovascular procedures included coil embolization (26 patients), balloon-remodeling coiling (16 patients), stent-assisted coiling (10 patients), balloon-stent-assisted coiling (9 patients), and balloon test occlusion (2 patients). NPM included electroencephalography, somatosensory evoked potentials, and brain stem auditory evoked potentials, depending on the location of the aneurysm and its associated vascular territory. NPM changes were seen in 3 (4.8%) patients and the procedures were altered immediately. No neurological changes were found postendovascularly. Ten patients demonstrated abnormal angiographic findings without concurrent NPM changes, of which 5 patients developed visual disturbance or hemiparesis. It is concluded that NPM is a valuable monitoring tool for endovascular treatment of cerebral aneurysms.
Keywords: Cerebral Aneurysm, Endovascular treatment, Neurophysiological Monitoring
Introduction
Endovascular therapy of cerebral aneurysms may result in thromboembolic complications, or may impede blood flow partially or completely in the parent vessel. Recently, a neurophysiological technique to monitor significant changes in regional cerebral blood flow (rCBF) during endovascular treatment of aneurysms may prevent ischemic complications and improves patients outcome.
Neurophysiological monitoring (NPM), in addition to Transcranial Doppler Sonography, radionuclide CBF studies and xenon CT, can indirectly measure rCBF and thus detect regional ischemia. The correlation between changes in NPM and changes in rCBF has been well established [3,31]. The application of NPM [1,5,8,24–27,32] in interventional neuroradiology has been gradually developed, particularly in arteriovenous malformation embolization, balloon test occlusion, and coil embolization of aneurysms. The purpose of this study was to assess the effectiveness and efficacy of NPM in detecting significant ischemia during endovascular treatment of cerebral aneurysms.
Materials and Methods
The study was a prospective evaluation of patients treated during a 1-year period ending in July 2008. Sixty three patients ranging in age from 7 to 88 years (mean age, 56.5 years) who underwent NPM during endovascular treatment of cerebral aneurysms were included. Preoperative and postoperative imaging studies including DSA, CT, or MR were performed in each case. At the time of the study, only one physician was actively involved in NPM for all elective and emergency endovascular cases.
Of all, 11 patients had ruptured aneurysms (7 in Hunt-Hess II, 4 in Hunt-Hess III) which underwent emergency treatments, whereas 52 had unruptured aneurysms. All coil embolization procedures (including balloon-remodeling, stent-assisted, and combined balloon-stent-assisted) were performed with the patients under general anesthesia to minimize patient motion. Conscious sedation was used for patients who required balloon test occlusion before definitive treatment, to perform neurological examination in addition to NPM.
Propofol was used for induction of anesthesia. After opening of the dura mater, anesthesia was shifted to sevoflurane in order to reach a constant background EEG and to reduce pharmacologically induced EEG high frequencies and bursts [11]. During the temporary balloon occlusion, systemic arterial hypertension was induced (increase by 20% of the mean arterial pressure).
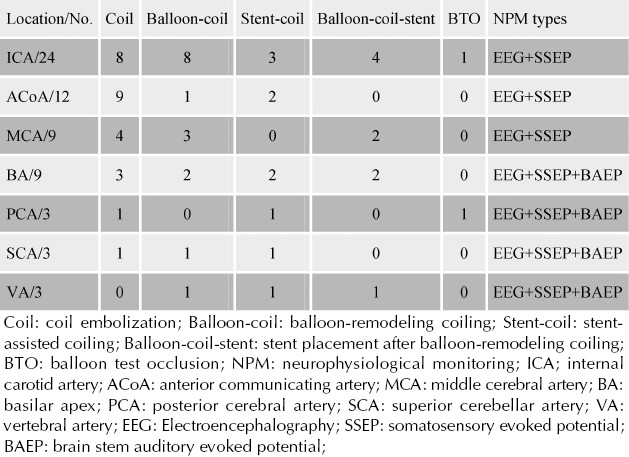
The aneurysm location, treatment strategies and NPM types of these patients are summarized in Table 1.
Table 1.
Aneurysm location, treatment strategies and NPM types in 63 patients
Electrophysiological Monitoring
NPM included EEG, somatosensory evoked potentials (SSEPs), and brain stem auditory evoked potentials (BAEPs), based on the location of the aneurysm and vascular territory at risk (Table 1). In cases in which the internal carotid artery, anterior cerebral artery or middle cerebral artery vascular territory was at risk, NPM was accomplished by using a combination of EEG and SSEPs testing. Vertebral and basilar artery vascular supply required a combination of EEG, SSEPs and BAEPs.
In elective or emergency cases, preoperative NPM baseline monitoring was performed in addition to a thorough or limited neurological examination while the patient was clear on the angiography table; for unconscious patients, only NPM was done preoperatively.
A Nicolet Viking IV Electrodiagnostic System (Nicolet Instrument Corporation, Madison, WI) was used to obtain the evoked potentials. (Table1). Abnormal (scalp) EEG changes were defined as a 50% decline in amplitude or burst suppression patterns. Critical SSEP changes were defined as an amplitude reduction of the cerebral evoked potential of >50% or a latency delay (N19/P24) of >10%. BAEP analysis was accomplished by using the latency and amplitude of all peaks (I–V). Changes in BAEPs that were thought to be significant included >50% amplitude reduction of waves III or V and/or an increase in latency of the fifth peak or of the interpeak latency difference (PV-PI) >1 ms. Changes were further classified as permanent if the changes persisted to the end of the procedure and transient if the changes resolved to >50% of baseline before completion of endovascular treatment.
Results
Among 63 patients, 26 underwent coil embolization, 16 balloon-remodeling coiling, 10 stent-assisted coiling, 9 balloon-stent-assisted coiling, and 2 balloon test occlusion. The average balloon inflation time for cases with balloon remodeling or assisted coiling was 4.4 min.
Table 2 lists the patients for whom NPM changes were identified and describes when these changes happened and were resolved. NPM changes were seen in 3 (4.8%) patients with balloon-remodeling coil embolization, and the procedures were altered immediately (balloons were deflated). The coil embolizations were completed or near completed before NPM changes took place. Accompanying with NPM changes, there was only 1 patient displaying coil protrusion angiographically. No neurological changes were detected postoperatively. The angiographic images and NPM changes of these 3 patients were shown in Figures 1–3.
Table 2.
NPM changes and resolution during endovascular procedures in 3 cases
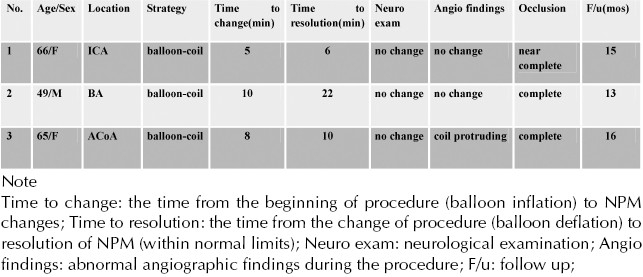
Figure 1.
Images from the case of a 66-year-old female who presented with headache. A, Angiography showing a left internal carotid artery aneurysm packed with coils (white arrow). Balloon remodeling was attempted (green arrow). B, Three minutes after balloon inflation, a >50% decrease in amplitude of the right median nerve SSEP was noted (red and white arrow). Considering the change in potentials, it was decided to quickly deflate the balloon. Seven minutes later, the evoked potential returned to baseline levels.
Figure 3.
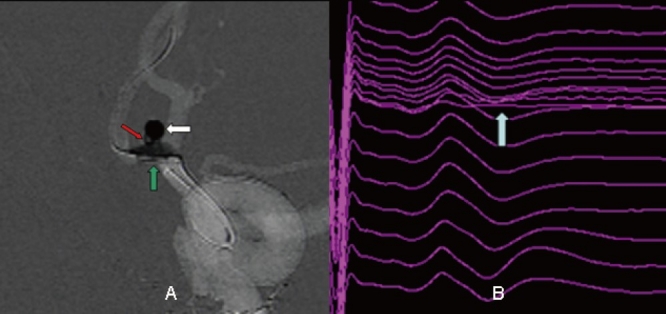
Images from the case of a 65-year-old female who was admitted incidently. A, Angiography showing an anterior communicating artery aneurysm packed with coils (white arrow). A loop of coil was noted to invaginate into the anterior communicating artery. Balloon remodeling was attempted (green arrow). B, Eight minutes after balloon inflation, a >50% decrease in amplitude of the left tibial nerve SSEP was noted (white arrow). Considering the change in potentials, it was decided to quickly deflate the balloon. Four minutes later, the evoked potential returned to baseline levels.
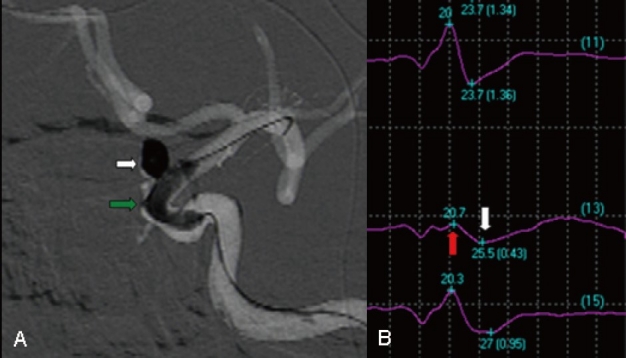
Figure 2.
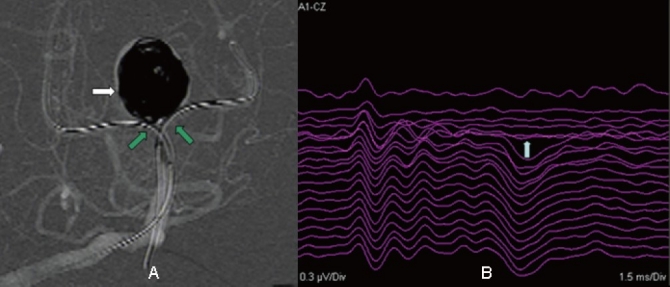
Images from the case of a 49-year-old male who presented with headache also. A, Angiography showing a basilar apex aneurysm packed with coils (white arrow). Double balloon remodeling was attempted (green arrow). B, Ten minutes after balloon inflation, a >50% decrease in amplitude of BAEP wave IV-V was noted (white arrow). Considering the change in potentials, it was decided to quickly deflate the balloon. Twenty two minutes later, the evoked potential returned to baseline levels.
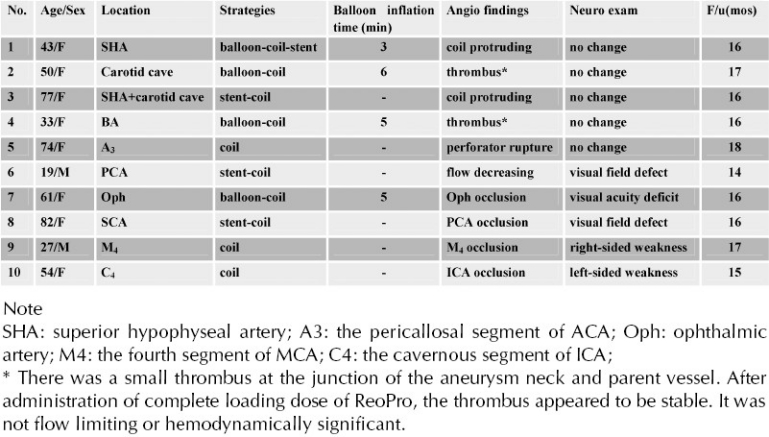
Ten patients demonstrated abnormal angiographic findings without concurrent NPM changes, of which 5 patients developed postoperative permanent neurological deficits (Table 3). The neurological deficits without concurrent NPM changes at the time of the procedure were: 1) visual acuity deficit or visual field defect in 3 patients with BTO, balloon-remodeling coiling or stent-assisted coiling respectively; and 2) limb weakness in 2 patients with coil embolization. The causes of immediate neurological deficits were parent vessel occlusion or flow slowing during the procedure. However, we did not observe delayed ischemic complications in our cases with serial imaging studies. In the other 5 patients the abnormal angiographic findings (Table 3) did not result in neurological deficits, probably because neither true flow slowing nor other haemodynamically significant changes occurred in these cases.
Table 3.
Abnormal findings in angiogram or neurological examination in 10 cases (without NPM changes)
Discussion
Interventional neuroradiologists have been entering the era of neurophysiological monitoring (NPM) for endovascular treatment of cerebral aneurysms. There were a few articles discussing NPM application in endovascular procedures [2,13,23,27,29,30]. NPM including EEG and evoked potentials is particularly valuable when the patient must be placed under general anesthesia for coil embolization because concurrent neurological examination is not possible.
Neurophysiological techniques can directly assess the functional state of specific cerebral regions and thus detect local ischemia, which can be continuously monitored during endovascular aneurysm treatment. The correlation between the NPM changes and cerebral ischemia has been well documented. In patients undergoing carotid endarterectomy, major EEG changes occurred with rCBF <10 mL/100 g/min [31]. SSEPs are maintained at levels of rCBF >16 mL/100 g/min but are absent at levels of rCBF <12 mL/100 g/min, with sharp reductions in the cortical SSEP amplitude (50% of baseline level) at rCBF levels between 14 and 16 mL/100 g/min [3,17]. Central conduction time is also prolonged with cerebral ischemia at a rCBF threshold of approximately 15 mL/100 g/min [10]. Cerebral infarction occurred at a rCBF threshold of 12 mL/100 g/min maintained for >2 hr [14]. These findings suggest that a 50% reduction in amplitude of the SSEP or a prolonged central conduction time >10 ms corresponds to a rCBF of 14 to 16 mL/100 g/min, is indicative of ischemia and possible progression to infarction. The usefulness of NPM has also been shown in the operating room [7,9,12,15,28] and during surgical treatment of cerebral aneurysms [19].
EEG provides a global assessment of cerebral ischemia but cannot be used to monitor the posterior fossa. Also, its sensitivity in the cerebrum is limited. In addition, only a limited number of electrodes can be placed on the patient's head because more electrodes would obscure optimal working projections for endovascular treatment. Martin CJ et al [20] recommended that recording of EEG from strip electrodes placed on the cortical surface detects changes more frequently than either scalp EEG or SSEPs during vascular occlusion. Also, Debatisse D et al [6], suggested that, by detecting earlier and more focal changes than scalp EEG, mEcoG may favor during surgery and increase in interactive strategies and reduction of deleterious event. SSEPs that use median and posterior tibial nerve stimulation are useful in assessing the functional state of the middle and anterior cerebral artery territories. However, SSEPs may be relatively insensitive in detecting ischemic changes in the cerebellum or posterior cerebral artery territories, because SSEPs monitor the integrity of only specific sensory pathways and is dependent on somatosensory cortex and afferent pathways to generate the evoked response. BAEP monitoring detects functional changes along the auditory brain stem pathways. BAEP changes are most often caused by a brain stem insult, which could result from vertebrobasilar ischemia. However, ischemia in the cerebellum or posterior cerebral artery territories could still be missed. Other technical limitations include confounding anesthesia-related effects, which may mimic cerebral ischemia. These effects can generally be distinguished from true ischemia by recognizing typical waveform characteristics (bilateral rather than unilateral waveform changes) and by recognizing changes either in the type or dose of anesthetic agent used.
This study evaluates the effectiveness and efficacy of NPM to detect ischemia and alter treatment decisions during endovascular treatment of cerebral aneurysms; as such, it does not focus on clinical outcomes (complete or incomplete occlusion of aneurysms). In our series, we use a combination of EEG, SSEPs, and BAEPs in the angiography suite. The type of NPM is determined by the location of the aneurysm and its associated vascular territory, and the setup is critical. Actually, focal EEG slowing was not found, while SSEP or BAEP changes altered treatment decisions for 4.8 % of our patients (3 of 63 patients). In these 3 patients with balloon-remodeling coil embolization, the evoked potentials finally returned to baseline after immediate deflation of balloons. None of these patients developed delayed neurological deficits due to ischemia or infarction. It can not be concluded, however, that these 3 patients would have developed neurological deficits if the procedures of balloon inflation had not been altered. As a matter of fact, the false positive rate of NPM changes can not be obtained in clinical practice.
It was disappointed that ischemic events that resulted in moderate or mild neurological deficits (hemiparesis or visual disturbance) in the study population occurred in 5 cases before NPM changes were detected. However, it can not be concluded that these ischemic events represent false-negative NPM test results, though there was a reported significant incidence of false negative NPM results [16,18,20,21]. In our series, motor evoked potentials (MEPs) were not actually employed for monitoring of motor function. SSEP may be unchanged whereas MEPs’ changes are detected and a motor deficit occurs. In addition, the flash visual evoked potentials (VEPs) had proven to be of little use for intraoperative monitoring [4] and thus was denied for NPM in our series. Therefore, it was emphasized that a combination of SSEP, EEG, BAEP as well as MEP, based on vascular territories and possible ischemic regions, should be considered individually as a supplement to angiographic findings or concurrent clinical neurological monitoring for endovascular treatment of cerebral aneurysms.
In our study, 3 cases showed neurophysiological changes after 5 to 10 minutes’ balloon inflation and coil deployment. So far, there has not yet a definitive answer regarding how long NPM changes can persist before ischemia proceeds to infarction. One report evaluated temporary occlusion in aneurysm surgery [22]. The mean occlusion time was 20.3 min for the patients with middle cerebral artery aneurysms and 15.8 min for the patients with internal carotid artery aneurysms. The SSEP disappeared during parent artery occlusion. All except 3 eventually returned to baseline after recirculation, none of which experienced postoperative sequelae. The time period from the start of occlusion till the complete loss of the SSEP averaged 8.6 min, and the time period from total SSEP loss till recirculation averaged 12 min.
In conclusion, NPM is a valuable monitoring tool for endovascular treatment of cerebral aneurysms. A combination of SSEP, EEG, BAEP or MEP, may be particularly useful in situations in which neurological examination is not possible (such as when the patient is under general anesthesia) or when a patient's condition (such as obtunded subarachnoid hemorrhage) precludes neurological examination. It should also be emphasized that the decision-making and changing of endovascular procedures would not only be based on NPM changes, but also the real-time abnormal angiographic findings.
References
- 1.Anderson LC, Hemler DE, Luethke JM, Latchaw RE. Transcranial magnetic evoked potentials used to monitor the spinal cord during neuroradiologic angiography of the spine. Spine. 1994;19:613–616. doi: 10.1097/00007632-199403000-00019. [DOI] [PubMed] [Google Scholar]
- 2.Berenstein A, Young W, Ransohoff J, Benjamin V, Merkin H. Somatosensory evoked potentials during spinal angiography and therapeutic transvascular embolization. J Neurosurg. 1984;60(4):777–785. doi: 10.3171/jns.1984.60.4.0777. [DOI] [PubMed] [Google Scholar]
- 3.Branston NM, Ladds A, Symon L, Wang AD. Comparison of the effects of ischaemia on early components of the somatosensory evoked potential in brainstem, thalamus, and cerebral cortex. J Cereb Blood Flow Metab. 1984;4:68–81. doi: 10.1038/jcbfm.1984.9. [DOI] [PubMed] [Google Scholar]
- 4.Cedzich C, Schramm J, Mengedoht CF, Fahlbusch R. Factors that limit the use of flash visual evoked potentials for surgical monitoring. Electroencephalogr Clin Neurophysiol. 1988;71(2):142–145. doi: 10.1016/0168-5597(88)90072-x. [DOI] [PubMed] [Google Scholar]
- 5.Cloughesy TF, Nuwer MR, Hoch D, Viñuela F, Duckwiler G, Martin N. Monitoring carotid test occlusions with continuous EEG and clinical examination. J Clin Neurophysiol. 1993;10:363–369. doi: 10.1097/00004691-199307000-00010. [DOI] [PubMed] [Google Scholar]
- 6.Debatisse D, Pralong E, Dehdashti AR, Regli L. simultaneous multilobar electrocorticography (mEcoG) and scalp electroencephalography (scalp EEG) during intracranial vascular surgery: a new approach in neuromonitoring. Clin Neurophysiol. 2005;116:2734–2740. doi: 10.1016/j.clinph.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 7.Deletis V, Sala F. Intraoperative neurophysiological monitoring of the spinal cord during spinal cord and spine surgery: a review focus on the corticospinal tracts. Clin Neurophysiol. 2008;119(2):248–264. doi: 10.1016/j.clinph.2007.09.135. [DOI] [PubMed] [Google Scholar]
- 8.Ferbert A, Buchner H, Bruckmann H, Zeumer H, Hacke W. Evoked potentials in basilar artery thrombosis: correlation with clinical and angiographic findings. Electroencephalogr Clin Neurophysiol. 1988;69:136–147. doi: 10.1016/0013-4694(88)90209-x. [DOI] [PubMed] [Google Scholar]
- 9.Florence G, Guerit JM, Gueguen B. Electroencephalography (EEG) and somatosensory evoked potentials (SEP) to prevent cerebral ischaemia in the operating room. Neurophysiol Clin. 2004;34:17–32. doi: 10.1016/j.neucli.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 10.Hargadine JR, Branston NM, Symon L. Central conduction time in primate brain ischemia: a study in baboons. Stroke. 1980;11:637–642. doi: 10.1161/01.str.11.6.637. [DOI] [PubMed] [Google Scholar]
- 11.Huotari AM, Koskinen M, Suominen K, Alahuhta S, Remes R, Hartikainen KM, Jantti V. Evoked EEG patterns during burst suppression with propofol. Br J Anaesth. 2004;92:18–24. doi: 10.1093/bja/aeh022. [DOI] [PubMed] [Google Scholar]
- 12.Hyun SJ, Rhim SC. Combined motor and somatosensory evoked potential monitoring for intramedullary spinal cord tumor surgery: correlation of clinical and neurophysiological data in 17 consecutive procedures. Br J Neurosurg. 2009;23(4):393–400. doi: 10.1080/02688690902964744. [DOI] [PubMed] [Google Scholar]
- 13.John ER, Chabot RJ, Prichep LS, Ransohoff J, Epstein F, Berenstein A. Real-time intraoperative monitoring during neurosurgical and neuroradiological procedures. J Clin Neurophysiol. 1989;6(2):125–158. doi: 10.1097/00004691-198904000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Jones TH, Morawetz RB, Crowell RM. Thresholds of focal cerebral ischemia in awake monkeys. J Neurosurg. 1981;54:773–782. doi: 10.3171/jns.1981.54.6.0773. [DOI] [PubMed] [Google Scholar]
- 15.Kothbauer KF. Intraoperative neurophysiologic monitoring for intramedullary spinal-cord tumor surgery. Neurophysiol Clin. 2007;37(6):407–414. doi: 10.1016/j.neucli.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 16.Krieger D, Adams HP, Albert DIMF, Haken MV, Hacke W. Pure motor hemiparesis with stable somatosensory evoked potential monitoring during aneurysm surgery: case report. Neurosurgery. 1992;31:145–150. doi: 10.1227/00006123-199207000-00024. [DOI] [PubMed] [Google Scholar]
- 17.Lesnick JE, Michele JJ, Simeone FA, DeFeo S, Welsh FA. Alteration of somatosensory evoked potentials in response to global ischemia. J Neurosurg. 1984;60:490–494. doi: 10.3171/jns.1984.60.3.0490. [DOI] [PubMed] [Google Scholar]
- 18.Little JR, Lesser RP, Luders H. Electrophysiological monitoring during basilar aneurysm operation. Neurosurgery. 1987:421–426. doi: 10.1227/00006123-198703000-00011. [DOI] [PubMed] [Google Scholar]
- 19.Lopez JR, Chang SD, Steinberg GK. The use of electrophysiological monitoring in the intraoperative management of intracranial aneurysms. J Neurol Neurosurg Psychiatry. 1999;66:189–196. doi: 10.1136/jnnp.66.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martin CJ, Sinson G, Patterson T, Zager EL, Strecker MM. Sensitivity of scalp EEG, cortical EEG, and somatosensory evoked responses during surgery for intracranial aneurysms. Surg Neurol. 2002;58:317–320. doi: 10.1016/s0090-3019(02)00881-9. [DOI] [PubMed] [Google Scholar]
- 21.Min KT, Kim JH, Shin YS, Kwon SY, Nam YT. The monitoring of somatosensory evoked potentials and neurologic complications in aneurysm surgery. Yonsei Med J. 2001;42(2):227–232. doi: 10.3349/ymj.2001.42.2.227. [DOI] [PubMed] [Google Scholar]
- 22.Mizoi K, Yoshimoto T. Permissible temporary occlusion time in aneurysm surgery as evaluated by evoked potential monitoring. Neurosurgery. 1993;33:434–440. doi: 10.1227/00006123-199309000-00013. [DOI] [PubMed] [Google Scholar]
- 23.Niimi Y, Sala F, Deletis V, Setton A, de Camargo AB, Berenstein A. Neurophysiologic monitoring and pharmacologic provocative testing for embolization of spinal cord arteriovenous malformations. AJNR Am J Neuroradiol. 2004;25(7):1131–1138. [PMC free article] [PubMed] [Google Scholar]
- 24.Paiva T, Campos J, Baeta E, Gomes LB, Martins IP, Parreira E. EEG monitoring during endovascular embolization of cerebral arteriovenous malformations. Electroencephalogr Clin Neurophysio. 1995;195:3–13. doi: 10.1016/0013-4694(95)00016-r. [DOI] [PubMed] [Google Scholar]
- 25.Paulsen RD, Steinberg GK, Norbash AM, Marcellus ML, Lopez JR, Marks MP. Embolization of rolandic cortex arteriovenous malformations. Neurosurgery. 1999;44:479–486. doi: 10.1097/00006123-199903000-00022. [DOI] [PubMed] [Google Scholar]
- 26.Paulsen RD, Steinberg GK, Norbash AM, Marcellus ML, Marks MP. Embolization of basal ganglia and thalamic arteriovenous malformations. Neurosurgery. 1999;44:991–997. doi: 10.1097/00006123-199905000-00031. [DOI] [PubMed] [Google Scholar]
- 27.Sala F, Beltramello A, Gerosa M. Neuroprotective role of neurophysiological monitoring during endovascular procedures in the brain and spinal cord. Neurophysiol Clin. 2007;37(6):415–421. doi: 10.1016/j.neucli.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 28.Sala F, Bricolo A, Faccioli F, Lanteri P, Gerosa M. Surgery for intramedullary spinal cord tumors: the role of intraoperative (neurophysiological) monitoring. Eur Spine J. 2007;16(S2:S1):30–39. doi: 10.1007/s00586-007-0423-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sala F, Niimi Y, Berenstein A, Deletis V. Neuroprotective role of neurophysiological monitoring during endovascular procedures in the spinal cord. Ann N Y Acad Sci. 2001;939:126–136. doi: 10.1111/j.1749-6632.2001.tb03619.x. [DOI] [PubMed] [Google Scholar]
- 30.Sala F, Niimi Y, Krzan MJ, Berenstein A, Deletis V. Embolization of a spinal arteriovenous malformation: correlation between motor evoked potentials and angiographic findings: technical case report. Neurosurgery. 1999;45(4):932–937. doi: 10.1097/00006123-199910000-00045. [DOI] [PubMed] [Google Scholar]
- 31.Sundt TM, Jr, Sharbrough FW, Piepgras DG, Kearns TP, Messick JM, Jr, O’Fallon WM. Correlation of cerebral blood flow and electroencephalographic changes during carotid endarterectomy: with results of surgery and hemodynamics of cerebral ischemia. Mayo Clin Proc. 1981;56:533–543. [PubMed] [Google Scholar]
- 32.Zentner J, Schumacher M, Bien S. Motor evoked potentials during interventional neuroradiology. Neuroradiology. 1988;30:252–255. doi: 10.1007/BF00341837. [DOI] [PubMed] [Google Scholar]


