Abstract
Epidermoids are generally recognized as benign tumors; however, total resection is often difficult. The recurrence from the residual capsule, dissemination of the tumor, and aseptic meningitis are common problems. The aim of the present study was to analyze and report on the clinical characteristics of intracranial epidermoids, particularly complications and cases with a poor clinical outcome. 24 patients with intracranial epidermoids who were treated surgically at Tokyo Women's Medical University Hospital between 1997 and 2007 were examined. The location and size of the tumor, pre-and postoperative symptoms, adherence of the tumor to cranial nerves, and proliferative capacity were determined. The most frequent site of the tumor was the cerebello-pontine (C-P) angle (16/24); eight of these patients presented with hearing loss and six presented with trigeminal neuralgia. In many cases, hearing loss and diplopia persisted after surgery. All epidermoids located in the C-P angle were attached to and/or compressed the trigeminal nerves, therefore, the origin is suggested to be the dura mafer of petrous bone around the trigeminal nerve. Of all 24 patients, the tumor recurred in four (after 3, 5, 10 and 20 years). One patient had a poor prognosis, with dissemination and brain stem infarction. Epidermoids can recur from residual capsule adhering to the brain stem or cranial nerves up to 10-20 years after the initial surgery. Long-term follow-up imaging studies are required when complete resection of the tumor capsule is not possible. In rare cases, spontaneous cyst rupture, dissemination, and brain stem infarction result in a poor prognosis.
Keywords: epidermoid, cerebello-pontine angle, recurrence, malignant transformation, brain stem infarction
Introduction
An epidermoid is a rare type of tumor, accounting for approximately 1% of all intracranial tumors[11]. Epidermoids consist of aberrated ectodermal cells that develop into epithelium-like cells between the 3rd and 5th weeks of fetal development, when the neural tube forms. Epidermoids most commonly develop in the cerebello-pontine (C-P) angle and often cause neurological symptoms, including hearing loss, vertigo, and trigeminal neuralgia. These tumors strongly adhere to the brain stem or cranial nerves, and residual capsule often remains after surgery, which contributes to the risk of recurrence.
In the present study, we examined the clinical symptoms, neuroradiological findings, and histopathology, in 24 patients with intracranial epidermoids who underwent surgery at Tokyo Women's Medical University Hospital between 1997 and 2007. We also determined any postoperative complications and recorded cases with a poor outcome. Comparative analysis between initial and recurrent cases was used to determine whether the MIB-1 index is a useful tool to predict recurrence.
Materials and Methods
The medical records of 24 patients diagnosed with intracranial epidermoids who underwent surgery and treatment at Tokyo Women's Medical University Hospital between 1997 and 2007 were examined (Table 1). The subjects consisted of 20 primary cases and four recurrent cases. The four recurrent patients were referred to our hospital after undergoing surgery at other hospitals. Of the 24 patients, 13 were men and 11 were women; patient age ranged from 13 to 68 years (mean age 44 years). The site and size of the tumor, pre- and postoperative symptoms, the time interval between the onset of symptoms and surgery, adherence of the tumor to surrounding cranial nerves, and the proliferative capacity (scored using the MIB-1 index) were recorded for each patient.
Table 1.
Summary of 24 patients who underwent surgical treatment for intracranial epidermoid
Results
Location of tumors and duration of symptoms
In 16 of 24 patients, the tumors were located in the C-P angle. In three patients, the epidermoids were located in the lateral ventricle and extended into the cerebral parenchyma. Of the five remaining patients, two had tumors that originated in the fourth ventricle and compressed the brain stem, two had a tumor in the occipito-cerebellar region, and one had a tumor in the parasellar region. In the primary cases, the mean interval between the onset of symptoms and surgery was 4.5 years (range 2 weeks-15 years). The patient in whom there was a 15-year interval between the onset of symptoms and surgery had a tumor located in the C-P angle and reported experiencing persistent dental and mandibular pain.
Symptoms associated with epidermoids located in the C-P angle
Preoperative symptoms of the 16 patients with tumors located in the C-P angle included hearing loss (8/16), trigeminal neuralgia (6/16), headaches (3/16), gait disturbance (3/16), diplopia (2/16), and hemifacial spasm (1/16). Unlike typical neuralgia, patients with trigeminal neuralgia experienced persistent dull pain. Two cases of diplopia were attributed to trochlear nerve palsy. All epidermoids located in the C-P angle that were larger than 3 cm presented with brain stem compression, involving the VII/VIII and lower cranial nerves to some extent. However, no extension of the tumor into the internal auditory canal was observed. During surgical procedures to remove the tumors, it was found that most of the epidermoids located in the C-P angle originated from the petrous bone apex and were attached to or compressed the trigeminal nerve. Five small tumors (<3cm) located in the C-P angle were found around the trigeminal nerve.
There was no improvement in hearing loss after surgery in six of eight cases. However, trigeminal neuralgia improved markedly in all six patients with this symptom, despite the fact that an offending artery had not been observed initially around the trigeminal nerves. In the patient exhibiting hemifacial spasms, the anterior inferior cerebellar artery was found to be the cause, and the patient's symptoms resolved following microvascular decompression. There was no improvement in diplopia in both of the patients with this symptom. After surgery, diplopia developed in three patients, dysphagia developed in two patients, hoarseness developed in one patient, dysarthria developed in one patient, and facial sensory impairment developed in another patient. However, these symptoms improved in each of the patients over a period of several days to weeks.
Hydrocephalus was a complication in two cases. This was treated with a ventriculo-peritoneal shunt before tumor removal in one patient and after tumor removal in the other patient.
Symptoms of patients with epidermoids in the ventricular system
Three patients had tumors located in the lateral ventricle that extended into the thalamus in one patient, into the temporal lobe in another patient and into the corpus callosum in the remaining patient. The former two patients had diplopia, which did not resolve after surgery; the remaining patient experienced behavioral changes (violent behavior), which did improve after surgery.
Of the two patients with tumors located in the fourth ventricle, one tumor was located primarily in the fourth ventricle, whereas the other was found to be compressing the medulla oblongata. The former patient had paraparesis and nystagmus, whereas the latter reported experiencing dizziness and gait disturbance. After surgery, although there was a temporary disturbance to swallowing reported by the second patient, both patients experienced improvement in all preoperative symptoms.
Symptoms of patients with occipito-cerebellar epidermoids
Of the two patients with a tumor located in the dura mater of the occipito-cerebellar region, one experienced tinnitus, which did not improve after surgery. However, in the second patient, the headache experienced prior to surgery subsided after tumor removal.
Symptoms associated with parasellar epidermoids
The patient who had a tumor in the parasellar region had visual field defects due to compression of the optic nerve. The narrowing of the visual field did not change after surgery.
Neuroradiological findings
All patients underwent brain magnetic resonance imaging (MRI). For 22 patients (92%), the tumors showed low-intensity signals on T1-weighted images (T1-WI) and high-intensity signals on T2-weighted images (T2-WI). In the case of the remaining two patients, one tumor appeared as a high-intensity signal on T1-WI and as a mixed-intensity signal on T2-WI, whereas the other tumor appeared as a mixed-intensity signal on T1-WI and as a high-intensity signal on T2-WI. Both tumors consisted largely of keratin, with some granulation tissue. Diffusion-weighted images (DWI), which were obtained for 21 patients, showed high-intensity signals for the keratin content. Cerebral angiography or magnetic resonance angiography/venography (MRA/V) were performed for some patients, revealing vascular compression without tumor staining.
Histopathology
The proliferative activity of the pathological tissue (determined using the MIB-1 index) was 0–22%. The MIB-1 index was 3% or higher in 15 cases. In the primary cases, the mean MIB-1 index was 6.5% (range 0%-22%), whereas in the four recurrent cases, the mean MIB-1 index was 3.6% (range 1.9%-5.3%). Areas positive for epithelial cells demonstrated a higher MIB-1 index and, in most of the cases, there were no findings indicating malignancy, including mitoses.
Recurrent cases
The four patients with recurrent disease were referred to our hospital after undergoing surgery (partial–subtotal resection of the tumor) at other hospitals. One patient had undergone three surgeries at a previous hospital and the tumor had recurred 3 years later (MIB-1 index = 1.9% at the time of recurrence). The other three patients experienced recurrence 5, 10 and 20 years after the initial surgery (MIB-1 index=3.9%, 5.3%, and 3.2%, respectively, at the time of recurrence). None of these patients experienced a second recurrence. In addition, no recurrences were identified among the 19 of 20 patients who underwent surgery at our hospital between 1997 and 2007 and who were followed up for between 6 months and 11 years.
Total-subtotal removal of the tumor, where the cyst wall remained adhered to the brain stem or cranial nerves, was achieved in 18 patients and partial tumor removal was performed in one patient.
A poor clinical course was observed for one patient. This patient had multiple tumors, some of which ruptured spontaneously before hospitalization and developed aseptic meningitis. Moreover, the patient presented with infarction in the brain stem and cerebellar hemisphere. Postoperative meningitis did not occur in any of the other 23 cases, including those with ventricular epidermoids. A more detailed description of the patient with a poor clinical course is given below.
Case presentation of a patient with a poor clinical outcome
In April 2006, a 65-year-old man developed diplopia, gait disturbance, and hearing loss in the right ear. At a community hospital, infarction in the brain stem and right cerebellum was found and a tumor was identified in the right C-P angle. The patient underwent rehabilitation, but his gait and swallowing disturbances continued to worsen. He was then sent to our department for surgical treatment. Brain MRI revealed a tumor in the right C-P angle and cysts in the left frontal and bilateral temporal lobes, which were thought to reflect dissemination (Fig. 1–3). In addition, a right cerebellar infarction was found in the region of the right anterior inferior cerebellar artery; preoperative MRA did not show the artery clearly (Fig. 4). The cerebrospinal fluid (CSF) cell count was <3 /mm3 and the CSF protein concentration was 270-300 mg/dl. Surgical resection of the tumor was performed because of progression of brain stem compression. Subtotal resection of the tumor was achieved using a right lateral suboccipital approach. Part of the tumor capsule adhered tightly to the brain stem and was not resected. The basilar artery was compressed by the tumor and the right anterior inferior cerebellar artery was encased by the tumor, leading to spasms. Histopathology showed an epidermoid with a stratified squamous epithelium and an MIB-1 index of 22%. None of the findings indicated a malignant transformation. Hydrocephalus also progressed and a ventriculo-peritoneal shunt surgery was performed. Postoperataive MRA showed diffuse narrowing of the basilar trunk due to inflammation of the adherent epidermoid. Five months after the surgery, brain stem infarction had extended (Fig. 5, 6). The patient died in February of 2007.
Fig. 1A, B.
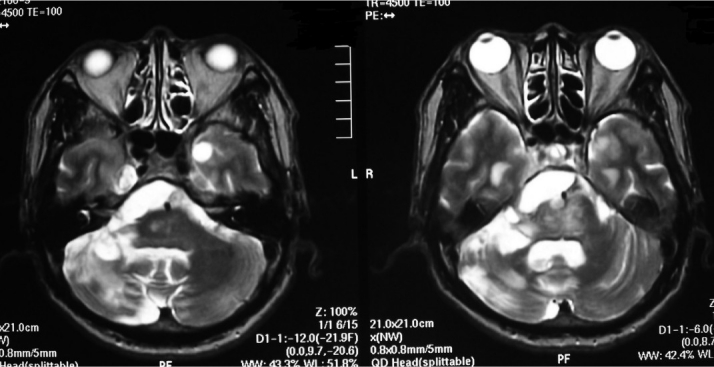
T2-weighted preoperative brain magnetic resonance imaging revealed an infarction in the brain stem and right cerebellum, as well as cysts in the right cerebellopontine angle and bilateral temporal lobes.
Fig. 3A,B.
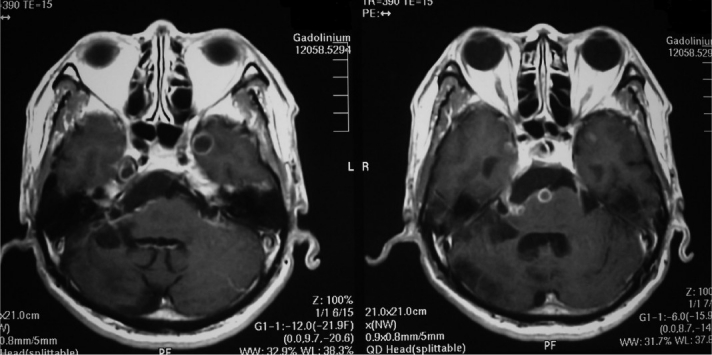
On a gadolinium magnetic resonance image, a tumor was visualized in the right cerebello-pontine angle, with a cyst wall in the bilateral temporal lobes.
Fig. 4.
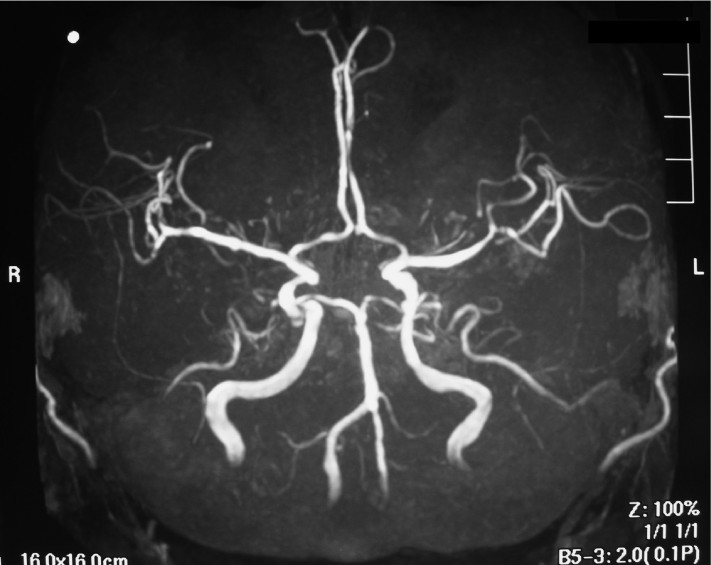
Preoperative brain magnetic resonance angiography did not show the right anterior inferior cerebellar artery clearly.
Fig. 5A,B.
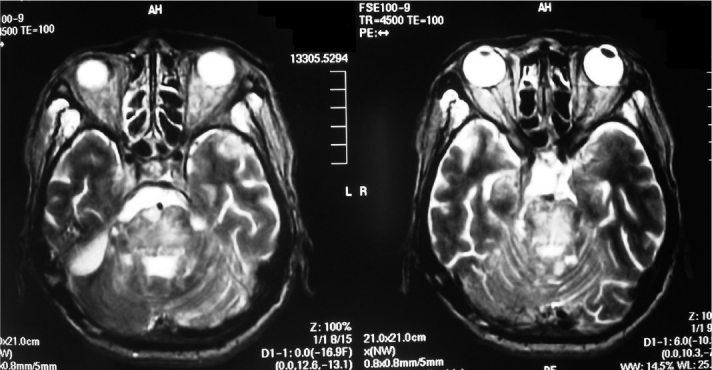
T2-weighted magnetic resonance image taken 5 months after surgery revealed progression of brain stem infarction.
Fig. 6A,B.
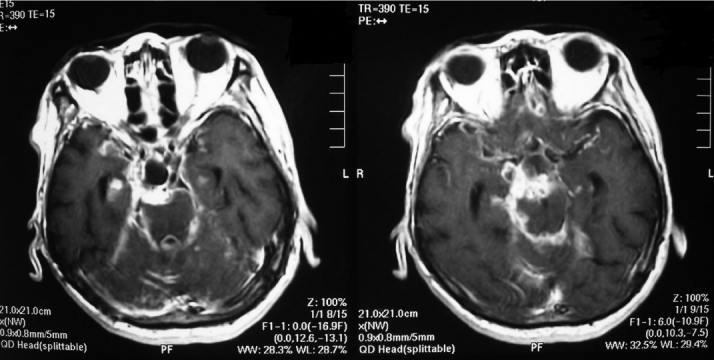
On a gadolinium magnetic resonance image taken 5 months after surgery, recurrent tumors were visualized in quadrigeminal-interpeduncular cistern and in bilateral temporal lobes.
Fig. 2.
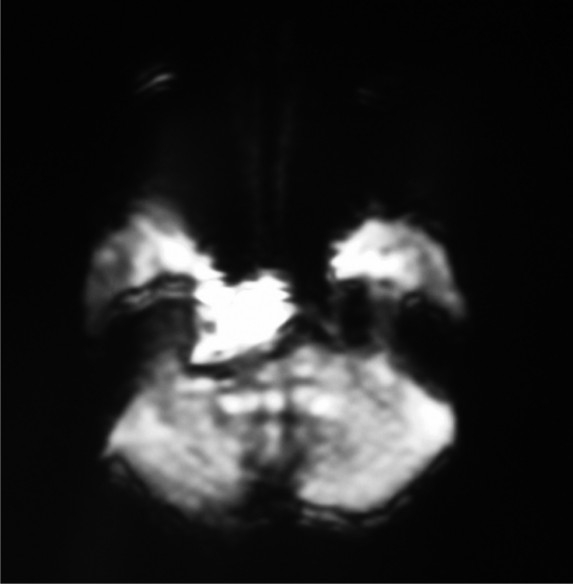
On the diffusion-weighted image, the area from the right cerebello-pontine angle to the right temporal lobe showed a high-intensity signal.
Discussion
Epidermoid tumors grow slowly and compress surrounding cerebral tissue, resulting in different symptoms depending on the location of the tumor. In the present study, epidermoids were most commonly found in the C-P angle (in 16 of 24 patients). All epidermoids located in the C-P angle were attached to the trigeminal nerve and small tumors were found when patients presented with trigeminal neuralgia. Tumors >3cm in diameter compressed the brain stem and extended into the prepontine cistern and/or caudal side, compressing the VII/VIII and lower cranial nerves. These observations suggest that epidermoids located in the C-P angle originate from the dura mater around the trigeminal nerve (petrous bone apex), and extend into surrounding cisterns.
Trigeminal neuralgia, which is caused by hyperactivity of the cranial nerve, was observed in six patients with epidermoids located in the C-P angle. Removal of the epidermoid around the trigeminal nerve led to the resolution of trigeminal neuralgia in all six patients. This is despite the fact that we did not find any artery responsible for compression of the trigeminal nerve in the first instance. Kubota et al. examined 30 patients with C-P angle epidermoids and trigeminal neuralgia or hemifacial spasm who underwent surgery and found that the symptoms were resolved in all patients after surgery[7]. These authors suggested that when the trigeminal nerve is compressed by the tumor or by displaced arteries, resulting in symptoms of trigeminal neuralgia or hemifacial spasm, careful resection of the tumor capsule is required. In the present series, the anterior inferior cerebellar artery was confirmed to be compressing the facial nerve in the patient with hemifacial spasms. Microvascular decompression was performed, alleviating the symptoms.
Epidermoids developed in (or extended to) the cerebral ventricles in four patients in the present series. Meng et al. reported that of 12 patients with intraventricular epidermoids, three developed aseptic meningitis after partial removal of the tumor, which improved following repeated lumbar puncture and treatment with steroids[8]. Patients with intraventricular epidermoids manifest intracranial hypertension, mental disturbances and diplopia, and aseptic meningitis can occur repeatedly owing to spontaneous rupture of the cysts. In the present study, we did not observe meningitis, suggestive of cysts rupture, in patients with ventricular epidermoids.
On DWI, epidermoids appear as a clearly hyperintense lesion. According to Chen et al., this is because of the T2 shine-through effect rather than diffusion restriction[3]. In the present study, the keratin content of epidermoids resulted in high-intensity signals on DWI, low-intensity signals on T1-WI, and high-intensity signals on T2-WI. In addition to keratin and cholesterol found in epidermoids, it has been suggested that protein and debris can change the signal intensity from a homogeneous to a non-homogeneous pattern[6].
Examination of four cases in which the tumor recurred 3-20 years after initial surgery revealed that the mean MIB-1 index in these cases was 3.6%, which is lower than the mean MIB-1 index of primary cases (6.5%). This indicates that residual epidermoids may recur in the long term, even though the MIB-1 index is not high. Moreover, clinical symptoms and pathological findings did not indicate a malignant transformation in any of the recurrent cases.
In the case with a poor clinical outcome, brain stem and cerebellar infarctions were present at the time of hospitalization due to encasement of the basilar artery and its perforating branches by the epidermoid. This was confirmed during surgery. Stenosis or occlusion of cerebral blood vessels due to epidermoids is rarely observed. Only two cases have been reported of brain stem infarction caused by compression of the branches of the basilar artery by an epidermoid located in the C-P angle[12]. In addition to compression or encasement by an epidermoid, chemical meningitis may contribute to stenosis of blood vessels in the brain stem. Using DWI alone, brain stem ischemia may not be detected. Thus, it is important to compare the results obtained with other MRI findings prior to surgery to detect any associated brain infarction. In the patient with a poor outcome in the present series, on the basis of MRI findings, we believe that malignant transformation or basilar trunk occlusion had occurred. Although the mechanism leading to malignant transformations is not completely understood, chronic inflammatory responses caused by cyst rupture or the remaining cyst wall have been suggested to be involved[1]. Malignant transformation of epidermoids into squamous cell carcinomas or poorly differentiated carcinomas has been previously reported[2,9]. The time until malignant transformation of benign epidermoids has been reported to range from 3 months to 33 years[10]. Garcia et al. defined the malignant transformation of epidermoids by stating that ‘…the tumor must be restricted to the intracranial, intradural compartment without extension beyond the dura, cranial orifices, or connection with the middle ear, air sinuses, or sella turcica, and a nasopharyngeal tumor should be ruled out’[4]. Hamlet et al. reviewed 52 cases that met these criteria and found that many cases were associated with hydrocephalus and intracranial hypertension[5]. The malignant area characteristically produces a contrast effect on computed tomography (CT)/MRI and the overall median survival time in the series of Hamlet et al. was 9 months. Cases of malignant transformation have a poor prognosis and there is, as yet, no effective treatment. Although gamma knife surgery inhibits tumor growth for a short period of time, it is difficult to obtain any beneficial long-term effects[10]. However, the cause of the poor clinical outcome for our patient was thought to be stenosis (spasm) of the basilar artery and its branches. This was induced not by malignant transformation of the epidermoid, but may have been due to chronic inflammation.
Epidermoids strongly adhere to surrounding brain tissue and nerves and, in some cases, the tumor capsule has to be left after surgery to ensure that vital structures are not damaged. Regular imaging studies are necessary because, in a small number of cases, recurrence or malignant transformation may originate from the residual tumor capsule. In particular, when the residual tumor shows a contrast effect on MRI with gadolinium, follow-up MRI is indicated.
Conclusion
Of the 24 cases of intracranial epidermoid in the present series, most tumors (16) developed in the C-P angle and were often accompanied by hearing loss, trigeminal neuralgia and diplopia. In many cases, hearing loss and diplopia persisted after surgery, whereas marked improvement was observed in the trigeminal neuralgia. In some patients, the tumor recurred 10-20 years after the initial surgery, which suggests that long-term follow-up imaging studies are necessary when the capsule adhering to the brain stem or cranial nerves has not been removed completely. The MIB-1 index does not appear to be a useful predictor of tumor recurrence because, in the present series, tumors with a low MIB-1 index also recurred in the long term. We have described one case with a poor clinical outcome in whom aseptic meningitis due to ruptured cysts and compression by the tumor caused a cerebrovascular spasm, leading to cerebral infarction and dissemination of the tumor. Although epidermoids are benign, patients should be followed closely after the tumor has been removed surgically because these tumors are associated with different clinical symptoms and malignant transformation does occur in rare cases.
References
- 1.Abramson RC, Morawetz RB, Schlitt M. Multiple complications from an intracranial epidermoid cyst: case report and literature review. Neurosurgery. 1989;24:574–578. doi: 10.1227/00006123-198904000-00014. [DOI] [PubMed] [Google Scholar]
- 2.Asahi T, Kurimoto M, Endo S, Monma F, Ohi M, Tamaki M. Malignant transformation of cerebello-pontine angle epidermoid. J Clin Neurosci. 2001;8:572–574. doi: 10.1054/jocn.2000.0856. [DOI] [PubMed] [Google Scholar]
- 3.Chen S, Ikawa F, Kurisu K, Arita K, Takaba J, Kanou Y. Quantitative MR evaluation of intracranial epidermoid tumors by fast fluid-attenuated inversion recovery imaging and echo-planar diffusion-weighted imaging. AJNR Am J Neuroradiol. 2001;22:1089–1096. [PMC free article] [PubMed] [Google Scholar]
- 4.Garcia AC, McGarry PA, Rodriguez F. Primary intracranial s quamous cell carcinoma of the right cerebellopontine angle. J Neurosurg. 1981;54:824–828. doi: 10.3171/jns.1981.54.6.0824. [DOI] [PubMed] [Google Scholar]
- 5.Hamlet A, Hua ZF, Saikali S, Laurent JF, Gedouin D, Ben-Hassel M, Guegan Y. Malignant transformation of intra-cranial epithelial cysts: systematic article review. J Neurooncol. 2005;74:187–194. doi: 10.1007/s11060-004-5175-4. [DOI] [PubMed] [Google Scholar]
- 6.Kallmes DF, Provenzale JM, Cloft HJ, McClendon RE. Typical and atypical MR imaging features of intracranial epidermoid tumors. AJR. 1997;169:883–887. doi: 10.2214/ajr.169.3.9275916. [DOI] [PubMed] [Google Scholar]
- 7.Kubota H, Kondo A, Iwasaki K. Cerebellopontine angle epidermoids presenting with cranial nerve hyperactive dysfunction: Pathogenesis and long-term surgical results in 30 patients. Neurosurgery. 2002;50:276–286. doi: 10.1097/00006123-200202000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Meng L, Yuguang L, Shugan Z, Xingang L, Chengyuan W. Intraventricular epidermoids. J Clin Neurosci. 2006;13:428–430. doi: 10.1016/j.jocn.2005.04.022. [DOI] [PubMed] [Google Scholar]
- 9.Michael LM, II, Moss T, Madhu T, Coakham HB. Malignant transformation of posterior fossa epidermoid cyst. Br L Neurosurg. 2005;19:505–510. doi: 10.1080/02688690500495356. [DOI] [PubMed] [Google Scholar]
- 10.Tamura K, Aoyagi M, Wakimoto H, Tamaki M, Yamamoto K, Yamamoto M, Ohno K. Malignant transformation eight years after removal of a benign epidermoid cyst: a case report. J Neurooncol. 2006;79:67–72. doi: 10.1007/s11060-005-9117-6. [DOI] [PubMed] [Google Scholar]
- 11.Ulrich J. Intracranial epidermoids.A study on their distribution and spread. J Neurosurg. 1964;21:1051–1058. doi: 10.3171/jns.1964.21.12.1051. [DOI] [PubMed] [Google Scholar]
- 12.Yilmazlar S, Kocaeli H, Cordan T. Brain stem stroke associated with epidermoid tumours: report of two cases. J Neurol Neurosurg Psychiatry. 2004;75:1340–1342. doi: 10.1136/jnnp.2003.029520. [DOI] [PMC free article] [PubMed] [Google Scholar]