Abstract
The exact role of inflammatory response in hemorrhagic contusions is not fully characterized. The present study quantified IL-6 plasmatic levels in patients with closed head trauma and hemorrhagic contusions during the first 6 to 12 hours postrauma. The association between the plasmatic IL-6 levels, severity of trauma according to the Glasgow Coma Scale, volume of intracerebral hemorrhage and patient's clinical evolution were investigated. Although inflammation is a multifactorial process, a strong correlation between IL-6 levels, volume of traumatic hemorrhage and in-hospital evolution could be observed. A correlation between the IL-6 levels quantified 6 hours postrauma and progression of lesion volume between admission and 12 hours postrauma is suggested. The present study reinforces the importance of IL-6 in influencing the clinical conditions of a patient with cerebral injuries, particularly hemorrhagic contusions.
Keywords: head injury, cerebral contusion, trauma, IL-6, clinical prognosis
INTRODUCTION
In almost every trauma, the most important factor related to the outcome is the kind of damage sustained by the brain[12. In an attempt to provide clinicopathological correlations in closed head injury, two categories of contusions have been emphasized: the ones occurring at the local site of the impact, called direct or coup-contusions, and those that occur in the brain diametrically opposite to the point of injury, called countercoup contusions[8]. They occur characteristically in the frontal and temporal lobes, more commonly on the inferior surfaces, where brain tissue comes in divece contact with bony protuberances and are related to the amount of energy transmitted by the impact in the skull to the underlying brain, worsened by the acceleration/deceleration processes and/or brain rotation[14].
The clinical course of these contusions is quite variable. Neurological worsening after trauma is common, as a consequence of the lesion progression, edema formation and intracranial hypertension. In some patients, the initial isolated contusion, as seen in brain CT, tends to disappear in a few days, in others, however, contusion evolves with an extensive area of neural injury[51].
The structural modifications presented by the encephalon immediately after traumatic insult characterise the primary injury2. Lacerations of the scalp, fractures of the skull, contusions on the surface of the brain and diffuse axonal injury are all included as the main primary damage in nonmissile head injuries[1,2]. The tissue injured at the primary lesion forms a necrotic region that does not functionally recover[22,42]. Around the necrotic tissue there is an area partially injured and eventually capable of recovery[25]. The cells present in this region can be further injured by physiological and biochemical alterations occurring subsequently, characterizing the secondary injury[2,22,26,49].
Patients with[43] closed head TBI (Traumatic Brain Injury) usually develop conditions that encourage secondary injury propagation, such as hypotension, hypoxemia, hyperglycemia, tachycardia and bradycardia[26,27,36]. However, the mechanisms responsible for worsening, death or recovery are not fully understood. The importance of the hypoxic/ischemic lesion is recognized[23] as well as of the inflammatory process developed after the primary lesion[34,44]. In inflammatory processes, the increase of pro-inflammatory cytokines synthesis is observed, among them, interleukin-6 (IL-6)[16,17,19,21,40,43,47]. The loss of local homeostasis leads to the activation of immunological cells - T lymphocytes and monocytes - and tissue cells -fibroblasts and endothelial cells, all of them capable of starting to secrete IL-6, which, in turn, induces maturation of B lymphocytes, activation of T lymphocytes and hematopoietic differentiation[15].
IL-6 is the cytokine found in the largest quantity in the circulation in response to inflammatory or traumatic insults, acting in both systemic and local responses[18,41,43,48]. IL-6 acts as a cellular mediator for immunological responses and can be produced through inflammatory events in the central nervous system (CNS)[9,16,17,19,21,24,31,35,47]. In cases of secondary cerebral damage, IL-6 plasmatic levels were increased[19,39,50].
This study analyzes IL-6 plasmatic levels in patients with closed head trauma and cerebral hemorrhage, correlating them to clinical evolution.
MATERIAL AND METHODS
The Ethical Committee on Research of the Center of Health and Biological Sciences of the Pontifícia Universidade Católica do Paraná (PUCPR) approved this study, according to the resolution 426/05/CEP-PUCPR, register number 790.
INCLUSION CRITERIA
All patients included in the research were admitted after TBI at Hospital Universitário Cajuru, from December 2005 to November 2006, and met the following criteria: a) Pre-hospital care given by Pre-Hospital Trauma Rescue System (SIATE); b) Initial tomographic diagnosis of intracerebral hemorrhage resulting from closed head trauma (hemorrhagic contusion); c) Diagnosis established up to 5 hours of the trauma; d) Absence of severe polytrauma, severe superficial wounds, coagulation disturbances, infections or need for any other emergency surgery upon admission to the hospital;
To put into effect their participation in this research it was necessary for the patients or next of kin to fully understand and sign the Informed Consent.
Thirty patients, average age 40 (± 18) years, were included. There were 25 men and 5 women. The patients were evaluated and classified at hospital admission using Glasgow Coma Scale (GCS). Four patients presented with GCS 3-8, four patients, with 9 – 12 and all other 22 patients had GCS between 13 and 15. Criteria registered on the first 72 hours of postrauma, were used to classify patients according to clinical evolution and included: a) Glasgow Coma Scale scores, b) Need for mechanical ventilation, c) Need for surgical measures to treat hemorrhagic contusions. Patients considered of favorable evolution did not present the following conditions: a) GCS scores equal or below 7; b) Need for mechanical ventilation; c) Need for a neurosurgical procedure. Similarly, patients who died during this period were not allocated to this group. According to these criteria, 21 patients presented favorable and 9 had poor evolution.
CALCULATION OF INTRACEREBRAL LESION VOLUME
A CT scan was performed at admission. Follow-up scans for assessment of lesion evolution were performed 12 hours post-trauma in all, but one patient, due to clinical instability. The volume (in cm3) of lesions was calculated using the software eFilm Workstation 2.1®-Merge Healthcare Software).
For determination of lesion volume, both, the hemorrhagic area and perihemorrhagic edema were included. If the patient presented multiple lesions, the volume of each lesion was calculated and added to obtain the total amount considered.
To determine the lesion volume, identification of the CT slice with the largest area of hemorrhage was done. The largest diameter was considered “A”, the largest diameter perpendicular to “A” on the same slice was “B” and for determination of C, the number of 10mm slices was counted: if the hemorrhage area was greater than 75% compared with the initial slice, it was considered as 1; if the hemorrhage was 25% to 75%, 0.5 was counted; if the hemorrhage was less than 25%, the slice was not counted. The ellipsoid method, in which the volume is the product of the diameters divided by two, as proposed by Bullock et al (2006)[6] was used.
IL-6 QUANTIFICATION
For IL-6 quantification, five blood samples from each patient were collected. The blood samples were collected at 6, 12, 24, 48 and 72 hours postrauma. Those who needed surgical interventions or died had the blood samples collected up to that occurrence.
For IL-6 quantitative analysis, the plasma was isolated immediately after sampled[28,29]. The material was centrifuged at 3000×g for 10 minutes and supernatant stored at -70°C.
Quantification was carried out using “human IL-6 quantikine ELISA kit, 2nd generation, HS600B” (R&D Systems) commercial kit. The results in absorbance (690 nm) of the ELISA test were analyzed using GraphPad Prism 5® (GraphPad Software).
STATISTICAL ANALYSIS
For analysis of correlation between IL-6 levels and volume of lesions, Spearman's non-parametric test was used. The significante analysis between defined groups (good and poor in-hospital evolution) in dichotomic variables, in relation to the quantitative variables employed Mann-Whitney U Test. The logistic regression test for the variables: clinical evolution, lesion volume and IL-6 levels were used. MedCalc® 9.2.0.0 – Windows Vista™ software was used for statistical analyses.
RESULTS
INTRACEREBRAL LESIONS VOLUME CALCULATIONS
Upon admission, two patients in the poor evolution group and three in the favorable evolution group presented multiple hemorrhagic lesions. A patient with good evolution presented a single lesion and perihemorrhagic edema. The same situation was observed in two patients with poor evolution. In the other cases, one single lesion without edema was observed.
The mean volume of lesions obtained from admission CT was 6.4 cm3 and 36.1 cm3 for patients in the good and poor evolution groups, respectively.
The analysis of the images obtained 12 hours postrauma showed that patients in good evolution group maintained the radiological pattern observed upon admission. However, one of these patients with the presence of multiple lesions and two, presenting a single lesion on admission CT, developed increasing perihemorrhagic edema.
Among the patients in the poor evolution group, two who initially presented multiple lesions and two others, presenting a single lesion, developed perihemorrhagic edema. In all cases of poor in-hospital evolution, increasing in lesion's volume on CT was observed.
The mean intracerebral lesion volume was of 10.35 cm3 and 69.05 cm3 respectively, for patients in favorable and poor evolution groups, 12 hours postrauma. The relationship of average intracerebral lesion volume and patients’ clinical evolution is presented in Graphic 1.
Graphic 1.
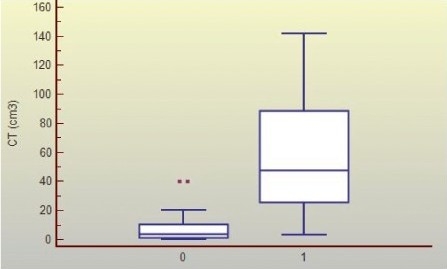
Distribution of Intracerebral Lesion Volume (cm3) in Good and Poor Evolution Groups Legend: Group of patients with a good (0) and poor (1) evolution; (p< 0.0001)
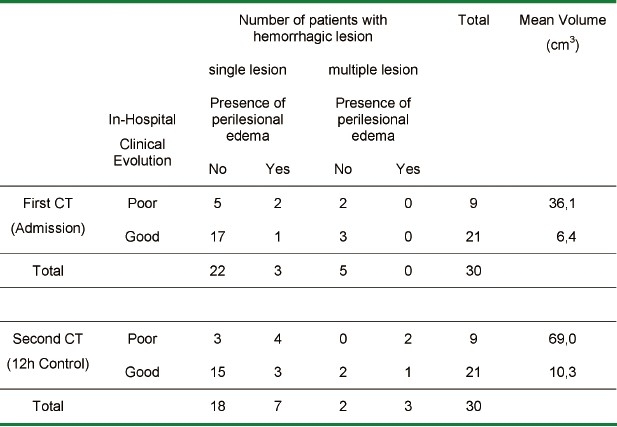
Comparing the CT scans performed at admission and at 12 hours the variation of lesion volume showed a non-homogeneous distribution. When analyzing all patients together, a mean value of 15.32 cm3 (initial CT) and 26.54 cm3 (control CT) was observed, with a standard deviation of ± 27.77 cm3 and ± 42.53 cm3, respectively. The heterogeneity was also observed when mean volumes in the different groups were analyzed: in the favorable evolution group, the standard deviation was of ± 11.48 cm3 and ± 12.92 cm3 and ± 42.13 cm3 and ± 62.50 cm3, for poor evolution group, in the first and second CT analyses, respectively. The values of intracerebral lesion volume are presented in Table 1.
Table 1.
Intracerebral Hemorrhagic Lesion: Volume and Distribution on Cranial CT
IL-6 QUANTIFICATION
Only samples collected 6 and 12 hours postrauma were analyzed, because of variability in the number of samples collected at other proposed times. The IL-6 mean levels being respectively 18.57 (± 4.89) and 15.98 (± 5.69) pg/ml.
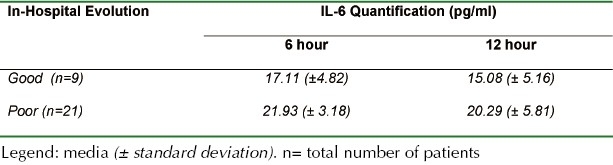
For the favorable evolution group (21 patients), the mean values were: 17.11 (± 4.82) and 15.08 (± 5.16) pg/ml in both periods (6 and 12 hours postrauma). The poor evolution group (9 patients) presented a mean value of 21.93 (± 3.18) and 18.97 (± 6.85) pg/ml, for the same intervals (Table 2 and Graphic 2).
Table 2.
IL-6 Quantification
Graphic 2.
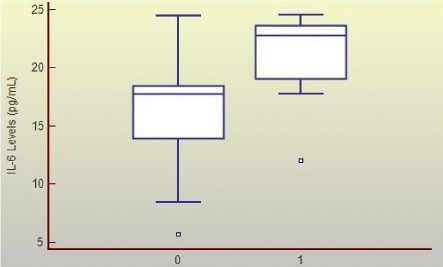
Mean Plasmatic Il-6 Levels (Pg/Ml) (6 and 12 Hours Post-Trauma) and Clinical Evolution of Patients with Cerebral Hemorrhagic Contusions Legend: Group of patients with a good (0) and poor (1) evolution; p< 0.0001.
STATISTICAL ANALYSIS
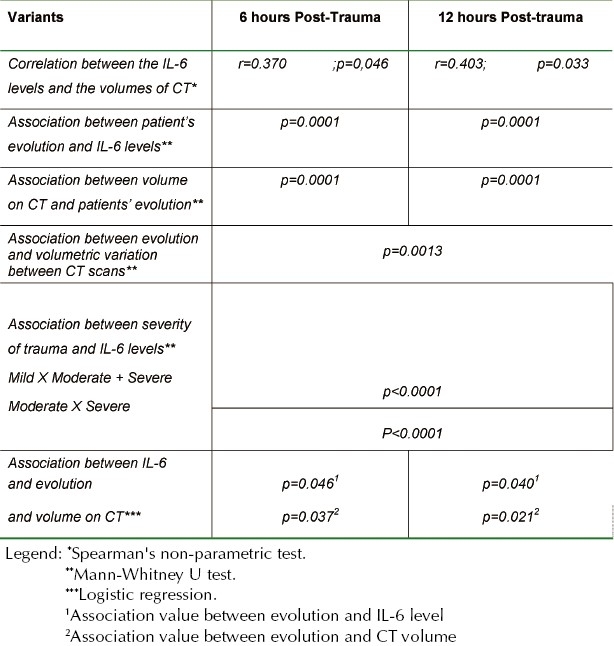
There was a correlation between the IL-6 levels 6 hours postrauma and the volumes of intracerebral lesions analyzed at the admission CT scan (r= 0.370; p= 0.047) and control CT, 12 hours post-trauma (r= 0.403; p= 0.033).
An association with patients’ evolution and IL-6 levels quantified 6 hours (p= 0.0001) and 12 hours (p= 0.0001) postrauma was observed. It was also possible to observe an association between intracerebral lesions volumes calculated from both, admission (p= 0.0001) and control (p= 0.0001) CT scans, and patients’ evolution. Similarly, there was an association between evolution and volumetric variation observed between the CT scans (p= 0.0013).
Considering the severity of trauma as classified by GCS – mild trauma as scores 13 – 15, moderate, 9 – 12 and severe TBI as GCS 3 – 8, there was an association between these groups and the quantified 6-hour postrauma IL-6. Initially, IL-6 levels were associated with two variables: the group with mild trauma versus moderate and severe trauma (p< 0.0001). In a subsequent analysis, the association between the quantified IL-6 levels and the moderate versus the severe trauma (p< 0.0001) was also noted.
Using logistic regression analysis, an association was found between in-hospital evolution, quantified IL-6 levels 6 hours postrauma (p= 0.046) and lesion volume calculated from admission CT scans (p= 0.037). Likewise, an association was found between evolution, IL-6 levels 12 hours after trauma (p= 0.040) and lesion volume on admission CT (p= 0.021).
These results are presented in Table 3.
Table 3.
Statistical Results: Analysis of Variants
DISCUSSION
The inflammatory process which develops in CNS is complex and involves cells from the immunological system and those residing in the brain[7]. However, different from what occurs in other tissues, the immunological response developing in CNS usually favours more damage, than tissue repair[7,37]. Several cerebral insults - like trauma, ischemia, infection and others - result in production of cell mediators such as cytokines[7,11,15,30,43,50]. In general, these inflammatory mediators are less expressed in the healthy CNS. Nevertheless, through injury or tissue infection, these mediators promote an immunological response and have their levels increased[13,37]. Pro-inflammatory cytokines have an essential role in inflammation occurring in the CNS, through the induction of adhesion molecules expression, chemokines, recruitment of immunological cells into the parenchyma and cellular activation[37].
The stimuli that increase the synthesis of these cytokines are several, but in the scenario of trauma, hypoxia and axoplasmatic exudation - resulting from cellular necrosis - are the main stimuli for production of these mediators[40,47].
Several injuries to the CNS result in an increase in IL-6 levels. The values of IL-6 levels found in this study, were quantitatively inferior to those of other studies that evaluated IL-6 synthesis[4,20,30,41,45,46], however there is no reference value for IL-6 levels in cerebral injuries.
In this study, the IL-6 average level found was of 18.56 (± 4.89) and 15.98 (± 5.69) pg/ml, quantified 6 and 12 hours postrauma, respectively. The values for patients in the good prognosis group were 17.11 (± 4.82) and 15.08 (± 5.16) pg/ml, and for the ones in the poor prognosis group, 21.93 (± 3.18) and 18.97 (± 6.85) pg/ml, for the same intervals.
IL-6 plasmatic levels inferior to those found in our study were described in ischemic stroke (4.6 ± 4.2 pg/ml)[4]. Higher values were found in studies considering IL-6 plasmatic levels in different surgical procedures, with an average of 30.83 pg/ml[30]. Values closer to the ones found in this study - and a mean value of 13.9 pg/ml -were observed after a surgical procedure (craniotomy)[15].
The inclusion criterion applied in our study prevented inclusion of patients with infectious processes. Absence of infectious processes was carefully observed in our patients from the beginning until the end of the study and this may help explain IL-6 lower values, once the presence of infection increases significantly the levels of this cytokine[4].
Quantified IL-6 level in the cerebrospinal fluid (CSF) probably reflects its production in the CNS[20]. Qureshi et al (2001)[35]in animal experimentation with spontaneous cerebral hemorrhage found similar values of IL-6 concentration in plasma and in regional CSF (< 5 pg/ml). Still, Kaplin et al (2005)[20] found IL-6 plasmatic levels (3.79 ± 2.03 pg/ml) inferior to those quantified in the CSF (654.3 ± 1239.6), in patients with neuroinflammatory disorders (transverse myelitis). In our study, IL-6 level quantification was carried out from plasma samples. The fact that cranioencephalic trauma is an acute process, as spontaneous hemorrhage, and not a chronic inflammatory process like myelitis, suggests that plasmatic levels might mirror satisfactorily the IL-6 production in the CNS in this scenario.
Control of Il-6 levels seem advisable in patients prone to complications after trauma[15], as higher plasmatic levels of IL6 were associated with smaller survival rates of patients with septic meningococcal shock[43] and in patients that suffered ischemic stroke[41].
Our findings indicate that IL-6 levels may also be important in patients harboring hemorrhagic contusions. The present study found an association between quantified IL-6 plasmatic levels 6 (p= 0.0001) and 12 hours (p= 0.0001) postrauma; the intracerebral lesion volumes calculated from admission (p= 0.0001) and control (p= 0.0001) CT scans and patients’ evolution. Furthermore, the variation in lesion volumes seen on CT scans corresponded to clinical evolution (p= 0.0013). These findings indicate that IL-6 may be an earlier indicator of volume progression of a cerebral contusion. Rising levels of IL- 6 may point the need for more aggressive monitoring/treatment of hemorrhagic contusion in this group, as volumetric expansion of this lesion directly correlates with prognosis.
When analyzing the average level of IL-6, 6 and 12 hours postrauma in patients in the poor and favorable evolution groups, a significant difference was observed (p< 0.0001), suggesting that the highest IL-6 levels are seen on patients with worse clinical course (Graphic 2). The same was observed for lesion mean volume (p< 0.0001) (Graphic 1), as patients showing a poor evolution presented larger intracerebral lesions. Furthermore, an association was found between IL-6 levels 6 hours postrauma and intracerebral lesion volume calculated from the admission (r= 0.370; p= 0.0466) and control (r= 0.403; p= 0.0328) CT scans.
No association between lesion volume on CT and patient classification according to the GCS was found, but Il-6 levels measured 6h post-trauma seem to accurately reflect the initial classification of TBI by severity, suggesting that the higher the IL-6 in patients harboring hemorrhagic contusions, the more prone this individual is to require surgical measures to treat the lesion and overall tend to follow a poor clinical course after trauma.
The average life span of the IL-6 in vivo is approximately one hour[30]. IL-6 production detected 6 and 12 hours, post-trauma, as observed in this study, suggests a continual inflammatory stimulus. Ayala et al (1991)[3] and Roumen et al (1993)[38] showed that an hemorrhagic process is stimuli enough to maintain systemic IL-6 levels increased - a reasonable explanation for our findings. Slight numerical reduction in IL-6 found in both groups at 12h might suggest that interleukin production begins to fade at this point. If this were the case, dosages performed at 24, 48 and 72h would certainly corroborate this finding. However, as a great variability in the number of samples collected at these times happened during this study, this observation remains to be proved.
The adjustment of the logistic regression model has shown a correlation between in-hospital evolution, IL-6 levels at 6 hours (p= 0.046) and lesion volume on admission CT (p=0,037), as well as the IL-6 levels quantified 12 hours postrauma (p= 0.040) and the volumes found on admission CT (p= 0.021) and evolution. Emsley et al (2003)[10] and Smith et al (2004)[41], in prospective studies of patients with ischemic stroke, found a strong association between inflammatory markers, including IL-6, with the severity of stroke and patient prognosis.
This study suggests that IL-6 levels as well as the initial hemorrhagic volumes observed on CT are important for defining the patient's initial evolution.
Since patients treated with steroids had inferior IL-6 plasmatic level values comparing to those without steroidal treatment[30], therapeutic use of steroids could be suggested as a measure to contain propagation cerebral damage resulting from the inflammatory process. This is a highly controversial matter, since there is Level I grade of evidence showing that the use of steroids is not recommended for improving outcome or reducing intracranial pressure, and even that high-dose methylprednisolone in patients with moderate or severe traumatic brain injury is associated with increased mortality and is contraindicated[5]. The question, which still remains, is if there is not a special group that could have benefitted from this therapy.
Monitoring and control of blood pressure, glycemia, heart frequency, temperature and levels of oxygen saturation are recommended as measures for containing leucocitary recruitment to the inflammatory site[47]. The leucocitary infiltration, one of the factors determining secondary lesion progression and cerebral damage, in experimental studies with mice, can be reduced by mild hypothermia (31°C – 33°C), locally applied[33]. The same author (2002)[32], in studies with rabbits, showed that the area of ischemic cerebral infarct can be reduced when treated with mild hypothermia.
CONCLUSION
This study indicates a) an association between IL-6 plasmatic levels quantified during the first 6 to 12 hours postrauma with initial course of patients harbouring closed head trauma and cerebral hemorrhagic contusions, b) an association between IL-6 levels with the severity of trauma, according to the GCS, c) a correlation between IL-6 levels quantified 6 hours postrauma with the lesion evolution as seen on CT.
These findings strengthen the importance of IL-6 in influencing the clinical condition of a patient with cerebral injury, particularly hemorrhagic contusions.
References
- 1.Adams JH, Graham DI, Gennarelli TA. Head injury in man and experimental animals: neuropathology. Acta Neurochir Suppl (Wien) 1983;32:15–30. doi: 10.1007/978-3-7091-4147-2_2. [DOI] [PubMed] [Google Scholar]
- 2.Adams JH, Graham DI, Scott G, Parker LS, Doyle D. Brain damage in fatal non-missile head injury. J Clin Pathol. 1980;33:1132–1145. doi: 10.1136/jcp.33.12.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ayala A, Wang P, Ba ZF, Perrin MM, Ertel W, Chaudry IH. Differential alterations in plasma IL-6 and TNF levels after trauma and hemorrhage. Am J Physiol. 1991;260:R167–171. doi: 10.1152/ajpregu.1991.260.1.R167. [DOI] [PubMed] [Google Scholar]
- 4.Beamer NB, Coull BM, Clark WM, Hazel JS, Silberger JR. Interleukin-6 and interleukin-1 receptor antagonist in acute stroke. Ann Neurol. 1995;37:800–805. doi: 10.1002/ana.410370614. [DOI] [PubMed] [Google Scholar]
- 5.Bratton SL, Chestnut RM, Ghajar J, McConnell Hammond FF, Harris OA, Hartl R, et al. Guidelines for the management of severe traumatic brain injury. XV. Steroids. J Neurotrauma. 2007;24(Suppl 1):S91–95. doi: 10.1089/neu.2007.9981. [DOI] [PubMed] [Google Scholar]
- 6.Bullock RM, Chesnut R, Ghajar J, Gordon D, Hartl R, Newell DW, et al. Guidelines for the Surgical Management of Traumatic Brain Injury. Appendix I: Post-traumatic Mass Volume Measurement in Traumatic Brain Injury Patients. Neurosurgery. 2006;58:S2–61. [Google Scholar]
- 7.Chavarria A, Alcocer-Varela J. Is damage in central nervous system due to inflammation? Autoimmun Rev. 2004;3:251–260. doi: 10.1016/j.autrev.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 8.Courville CB. Coup contre-coup mechanism of craniocerebral injuries. Arch Surg. 1942;45:19–43. [Google Scholar]
- 9.de Bandt JP, Chollet-Martin S, Hernvann A, Lioret N, du Roure LD, Lim SK, et al. Cytokine response to burn injury: relationship with protein metabolism. J Trauma. 1994;36:624–628. doi: 10.1097/00005373-199405000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Emsley HC, Smith CJ, Gavin CM, Georgiou RF, Vail A, Barberan EM, et al. An early and sustained peripheral inflammatory response in acute ischaemic stroke: relationships with infection and atherosclerosis. J Neuroimmunol. 2003;139:93–101. doi: 10.1016/s0165-5728(03)00134-6. [DOI] [PubMed] [Google Scholar]
- 11.Emsley HC, Smith CJ, Gavin CM, Georgiou RF, Vail A, Barberan EM, et al. Clinical outcome following acute ischaemic stroke relates to both activation and autoregulatory inhibition of cytokine production. BMC Neurol. 2007;7:5. doi: 10.1186/1471-2377-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Graham DI, Adams JH, Gennarelli TA. Pathology of brain damage in head injury. In: Cooper PR, editor. Head Injury. Baltimore: Williams & Wilkins; 1993. pp. 91–113. [Google Scholar]
- 13.Gruol DL, Nelson TE. Physiological and pathological roles of interleukin-6 in the central nervous system. Mol Neurobiol. 1997;15:307–339. doi: 10.1007/BF02740665. [DOI] [PubMed] [Google Scholar]
- 14.Gurdjian ES. Cerebral contusions: re-evaluation of the mechanism of their development. J Trauma. 1976;16:35–51. [PubMed] [Google Scholar]
- 15.Heesen M, Deinsberger W, Dietrich GV, Detsch O, Boldt J, Hempelmann G. Increase of interleukin-6 plasma levels after elective craniotomy: influence of interleukin-10 and catecholamines. Acta Neurochir (Wien) 1996;138:77–80. doi: 10.1007/BF01411728. [DOI] [PubMed] [Google Scholar]
- 16.Herx LM, Rivest S, Yong VW. Central nervous system-initiated inflammation and neurotrophism in trauma: IL-1 beta is required for the production of ciliary neurotrophic factor. J Immunol. 2000;165:2232–2239. doi: 10.4049/jimmunol.165.4.2232. [DOI] [PubMed] [Google Scholar]
- 17.Holmin S, Soderlund J, Biberfeld P, Mathiesen T. Intracerebral inflammation after human brain contusion. Neurosurgery. 1998;42:291–298. doi: 10.1097/00006123-199802000-00047. discussion 298-299. [DOI] [PubMed] [Google Scholar]
- 18.Hulkkonen J, Pertovaara M, Antonen J, Pasternack A, Hurme M. Elevated interleukin-6 plasma levels are regulated by the promoter region polymorphism of the IL6 gene in primary Sjogren's syndrome and correlate with the clinical manifestations of the disease. Rheumatology (Oxford) 2001;40:656–661. doi: 10.1093/rheumatology/40.6.656. [DOI] [PubMed] [Google Scholar]
- 19.Jean WC, Spellman SR, Nussbaum ES, Low WC. Reperfusion injury after focal cerebral ischemia: the role of inflammation and the therapeutic horizon. Neurosurgery. 1998;43:1382–1396. doi: 10.1097/00006123-199812000-00076. discussion 1396-1387. [DOI] [PubMed] [Google Scholar]
- 20.Kaplin AI, Deshpande DM, Scott E, Krishnan C, Carmen JS, Shats I, et al. IL-6 induces regionally selective spinal cord injury in patients with the neuroinflammatory disorder transverse myelitis. J Clin Invest. 2005;115:2731–2741. doi: 10.1172/JCI25141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kinoshita K, Chatzipanteli K, Vitarbo E, Truettner JS, Alonso OF, Dietrich WD. Interleukin-1beta messenger ribonucleic acid and protein levels after fluid-percussion brain injury in rats: importance of injury severity and brain temperature. Neurosurgery. 2002;51:195–203. doi: 10.1097/00006123-200207000-00027. discussion 203. [DOI] [PubMed] [Google Scholar]
- 22.Kipnis J, Yoles E, Schori H, Hauben E, Shaked I, Schwartz M. Neuronal survival after CNS insult is determined by a genetically encoded autoimmune response. J Neurosci. 2001;21:4564–4571. doi: 10.1523/JNEUROSCI.21-13-04564.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee JM, Grabb MC, Zipfel GJ, Choi DW. Brain tissue responses to ischemia. J Clin Invest. 2000;106:723–731. doi: 10.1172/JCI11003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lichtor T, Libermann TA. Coexpression of interleukin-1 beta and interleukin-6 in human brain tumors. Neurosurgery. 1994;34:669–672. doi: 10.1227/00006123-199404000-00015. discussion 672-663. [DOI] [PubMed] [Google Scholar]
- 25.Menon DK. Procrustes, the traumatic penumbra, and perfusion pressure targets in closed head injury. Anesthesiology. 2003;98:805–807. doi: 10.1097/00000542-200304000-00002. [DOI] [PubMed] [Google Scholar]
- 26.Miller JD, Corales RL. Brain edema as a result of head injury: fact and fallacy. In: de Vlieger M, de Lauge SA, Becks JWS, editors. Brain Edema. New York: John Wiley; 1981. pp. 99–115. [Google Scholar]
- 27.Miller JD, Sweet RC, Narayan R, Becker DP. Early insults to the injured brain. JAMA. 1978;240:439–442. [PubMed] [Google Scholar]
- 28.Muylle L, Joos M, Wouters E, De Bock R, Peetermans ME. Increased tumor necrosis factor alpha (TNF alpha), interleukin 1, and interleukin 6 (IL-6) levels in the plasma of stored platelet concentrates: relationship between TNF alpha and IL-6 levels and febrile transfusion reactions. Transfusion. 1993;33:195–199. doi: 10.1046/j.1537-2995.1993.33393174443.x. [DOI] [PubMed] [Google Scholar]
- 29.Muylle L, Peetermans ME. Effect of prestorage leukocyte removal on the cytokine levels in stored platelet concentrates. Vox Sang. 1994;66:14–17. doi: 10.1111/j.1423-0410.1994.tb00270.x. [DOI] [PubMed] [Google Scholar]
- 30.Osuka K, Suzuki Y, Saito K, Takayasu M, Shibuya M. Changes in serum cytokine concentrations after neurosurgical procedures. Acta Neurochir (Wien) 1996;138:970–976. doi: 10.1007/BF01411287. [DOI] [PubMed] [Google Scholar]
- 31.Pasquale MD, Cipolle MD, Monaco J, Simon N. Early inflammatory response correlates with the severity of injury. Crit Care Med. 1996;24:1238–1242. doi: 10.1097/00003246-199607000-00029. [DOI] [PubMed] [Google Scholar]
- 32.Prandini MN, Lacanna SN, Valente PR, Stavale JN. Regional mild hypothermia in the protection for isquemic brain. Acta Cir Bras. 2002;17:232–235. [Google Scholar]
- 33.Prandini MN, Neves Filho A, Lapa AJ, Stavale JN. Mild hypothermia reduces polymorphonuclear leukocytes infiltration in induced brain inflammation. Arq Neuropsiquiatr. 2005;63:779–784. doi: 10.1590/s0004-282x2005000500012. [DOI] [PubMed] [Google Scholar]
- 34.Prins RM, Liau LM. Immunology and immunotherapy in neurosurgical disease. Neurosurgery. 2003;53:144–152. doi: 10.1227/01.neu.0000068865.34216.3a. discussion 152-143. [DOI] [PubMed] [Google Scholar]
- 35.Qureshi AI, Suri MF, Ling GS, Khan J, Guterman LR, Hopkins LN. Absence of early proinflammatory cytokine expression in experimental intracerebral hemorrhage. Neurosurgery. 2001;49:416–420. doi: 10.1097/00006123-200108000-00027. discussion 421. [DOI] [PubMed] [Google Scholar]
- 36.Rose J, Valtonen S, Jennett B. Avoidable factors contributing to death after head injury. Br Med J. 1977;2:615–618. doi: 10.1136/bmj.2.6087.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rothwell NJ, Strijbos PJ. Cytokines in neurodegeneration and repair. Int J Dev Neurosci. 1995;13:179–185. doi: 10.1016/0736-5748(95)00018-c. [DOI] [PubMed] [Google Scholar]
- 38.Roumen RM, Hendriks T, van der Ven-Jongekrijg J, Nieuwenhuijzen GA, Sauerwein RW, van der Meer JW, et al. Cytokine patterns in patients after major vascular surgery, hemorrhagic shock, and severe blunt trauma. Relation with subsequent adult respiratory distress syndrome and multiple organ failure. Ann Surg. 1993;218:769–776. doi: 10.1097/00000658-199312000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sandhir R, Puri V, Klein RM, Berman NE. Differential expression of cytokines and chemokines during secondary neuron death following brain injury in old and young mice. Neurosci Lett. 2004;369:28–32. doi: 10.1016/j.neulet.2004.07.032. [DOI] [PubMed] [Google Scholar]
- 40.Schoning B, Elepfandt P, Lanksch WR, Volk HD, Woiciechowsky C. Continuous infusion of proinflammatory cytokines into the brain to study brain cytokine induced local and systemic immune effects. Brain Res Brain Res Protoc. 1999;4:217–222. doi: 10.1016/s1385-299x(99)00022-7. [DOI] [PubMed] [Google Scholar]
- 41.Smith CJ, Emsley HC, Gavin CM, Georgiou RF, Vail A, Barberan EM, et al. Peak plasma interleukin-6 and other peripheral markers of inflammation in the first week of ischaemic stroke correlate with brain infarct volume, stroke severity and long-term outcome. BMC Neurol. 2004;4:2. doi: 10.1186/1471-2377-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Teasdale GM, Graham DI. Craniocerebral trauma: protection and retrieval of the neuronal population after injury. Neurosurgery. 1998;43:723–737. doi: 10.1097/00006123-199810000-00001. discussion 737-728. [DOI] [PubMed] [Google Scholar]
- 43.Waage A, Brandtzaeg P, Halstensen A, Kierulf P, Espevik T. The complex pattern of cytokines in serum from patients with meningococcal septic shock. Association between interleukin 6, interleukin 1, and fatal outcome. J Exp Med. 1989;169:333–338. doi: 10.1084/jem.169.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang Q, Tang XN, Yenari MA. The inflammatory response in stroke. J Neuroimmunol. 2007;184:53–68. doi: 10.1016/j.jneuroim.2006.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Woiciechowsky C, Asadullah K, Nestler D, Glockner F, Robinson PN, Volk HD, et al. Different release of cytokines into the cerebrospinal fluid following surgery for intra- and extra-axial brain tumours. Acta Neurochir (Wien) 1997;139:619–624. doi: 10.1007/BF01411996. [DOI] [PubMed] [Google Scholar]
- 46.Woiciechowsky C, Schoning B, Cobanov J, Lanksch WR, Volk HD, Docke WD. Early IL-6 plasma concentrations correlate with severity of brain injury and pneumonia in brain-injured patients. J Trauma. 2002;52:339–345. doi: 10.1097/00005373-200202000-00021. [DOI] [PubMed] [Google Scholar]
- 47.Woiciechowsky C, Schoning B, Lanksch WR, Volk HD, Docke WD. Mechanisms of brain-mediated systemic anti-inflammatory syndrome causing immunodepression. J Mol Med. 1999;77:769–780. doi: 10.1007/s001099900051. [DOI] [PubMed] [Google Scholar]
- 48.Xing Z, Gauldie J, Cox G, Baumann H, Jordana M, Lei XF, et al. IL-6 is an antiinflammatory cytokine required for controlling local or systemic acute inflammatory responses. J Clin Invest. 1998;101:311–320. doi: 10.1172/JCI1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yoles E, Hauben E, Palgi O, Agranov E, Gothilf A, Cohen A, et al. Protective autoimmunity is a physiological response to CNS trauma. J Neurosci. 2001;21:3740–3748. doi: 10.1523/JNEUROSCI.21-11-03740.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhu T, Yao Z, Yuan HN, Lu BG, Yang SY. Changes of interleukin-1 beta, tumor necrosis factor alpha and interleukin-6 in brain and plasma after brain injury in rats. Chin J Traumatol. 2004;7:32–35. [PubMed] [Google Scholar]
- 51.Zimmerman RA, Bilaniuk LT. Computer tomography of traumatic intracerebral hemorrhagic lesions: the change in density and mass effect with time. Neuroradiology. 1978;16:320–321. doi: 10.1007/BF00395288. [DOI] [PubMed] [Google Scholar]


