Abstract
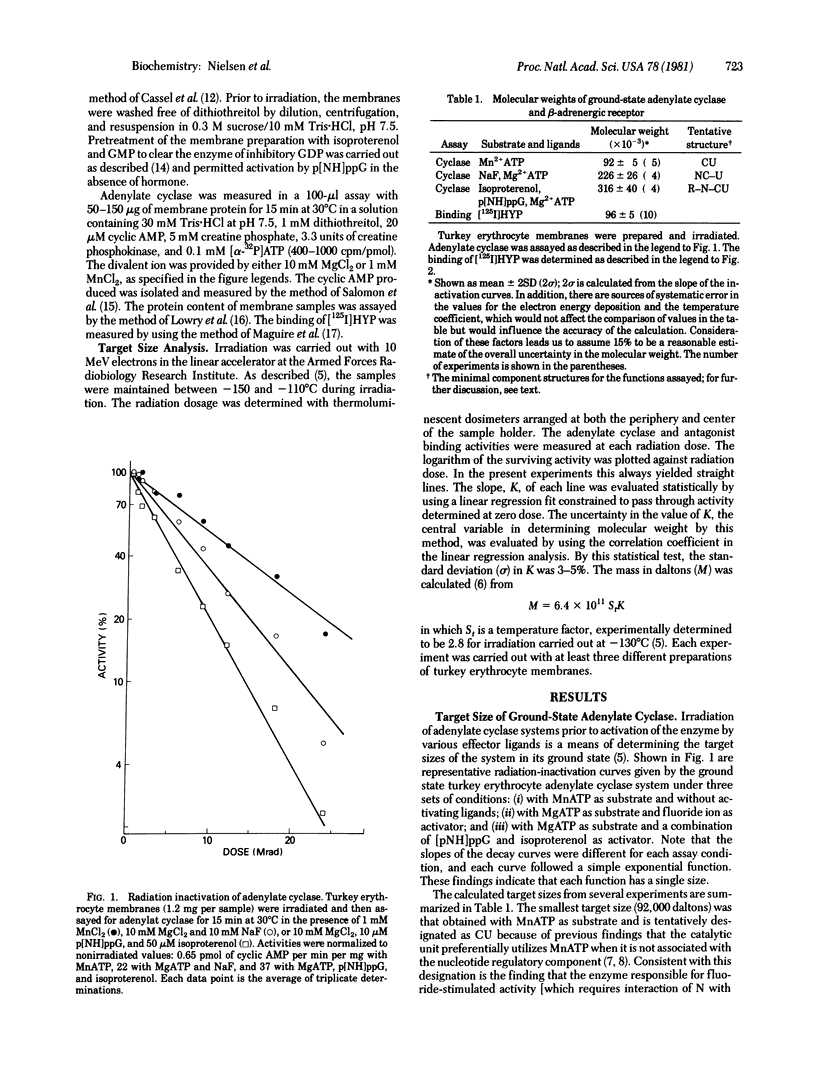
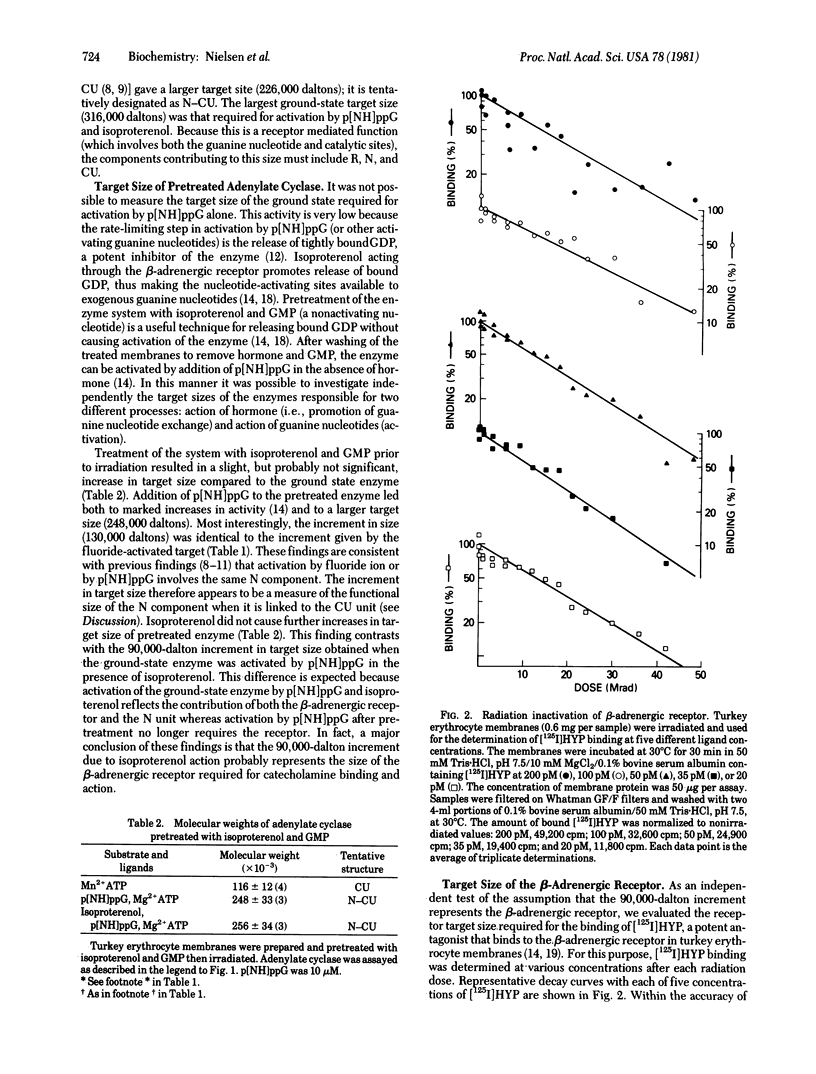
Target analysis of the turkey erythrocyte adenylate cyclase [ATP pyrophosphate-lyase (cyclizing), EC 4.6.1.1] system showed that the molecular weight of the ground state enzyme increases from 92,000 with MnATP as substrate and no stimulatory ligands to 226,000 when activated by fluoride ion or by 5'-guanyl imidodiphosphate (p[NH]ppG) subsequent to clearance of previously bound GDP. The identical increment in size (130,000) suggests that the same regulatory unit is involved in the activation by both effectors. When assayed with isoproterenol and p[NH]ppG, the enzyme system displayed a further increment in size of 90,000 daltons. Based on binding of the antagonist 125I-labeled hydroxybenzylpindolol, the beta-adrenergic receptor is about 90,000 daltons or the same as that seen for activation of the enzyme by isoproterenol through the beta-adrenergic-receptor. Because single targets were seen for the ground state enzyme system under all conditions, it would appear that the various regulatory and catalytic components are structurally linked prior to activation by hormone, guanine nucleotides, and fluoride ion. Furthermore, based on reported subunit sizes of the nucleotide regulatory and receptor components are composed of multiple subunits, either homologous or heterologous in structure.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atlas D., Levitzki A. Tentative identification of beta-adrenoreceptor subunts. Nature. 1978 Mar 23;272(5651):370–371. doi: 10.1038/272370a0. [DOI] [PubMed] [Google Scholar]
- Brown E. M., Aurbach G. D. Beta-Adrenergic receptor interactions. Characterization of iodohydroxybenzylpindolol as a specific ligand. J Biol Chem. 1976 Mar 10;251(5):1232–1238. [PubMed] [Google Scholar]
- Cassel D., Levkovitz H., Selinger Z. The regulatory GTPase cycle of turkey erythrocyte adenylate cyclase. J Cyclic Nucleotide Res. 1977 Dec;3(6):393–406. [PubMed] [Google Scholar]
- Cassel D., Pfeuffer T. Mechanism of cholera toxin action: covalent modification of the guanyl nucleotide-binding protein of the adenylate cyclase system. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2669–2673. doi: 10.1073/pnas.75.6.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill D. M., Meren R. ADP-ribosylation of membrane proteins catalyzed by cholera toxin: basis of the activation of adenylate cyclase. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3050–3054. doi: 10.1073/pnas.75.7.3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haga T., Haga K., Gilman A. G. Hydrodynamic properties of the beta-adrenergic receptor and adenylate cyclase from wild type and varient S49 lymphoma cells. J Biol Chem. 1977 Aug 25;252(16):5776–5782. [PubMed] [Google Scholar]
- Howlett A. C., Gilman A. G. Hydrodynamic properties of the regulatory component of adenylate cyclase. J Biol Chem. 1980 Apr 10;255(7):2861–2866. [PubMed] [Google Scholar]
- Kaslow H. R., Johnson G. L., Brothers V. M., Bourne H. R. A regulatory component of adenylate cyclase from human erythrocyte membranes. J Biol Chem. 1980 Apr 25;255(8):3736–3741. [PubMed] [Google Scholar]
- Kempner E. S., Schlegel W. Size determination of enzymes by radiation inactivation. Anal Biochem. 1979 Jan 1;92(1):2–10. doi: 10.1016/0003-2697(79)90617-1. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lad P. M., Nielsen T. B., Preston M. S., Rodbell M. The role of the guanine nucleotide exchange reaction in the regulation of the beta-adrenergic receptor and in the actions of catecholamines and cholera toxin on adenylate cyclase in turkey erythrocyte membranes. J Biol Chem. 1980 Feb 10;255(3):988–995. [PubMed] [Google Scholar]
- Lad P. M., Welton A. F., Rodbell M. Evidence for distinct guanine nucleotide sites in the regulation of the glucagon receptor and of adenylate cyclase activity. J Biol Chem. 1977 Sep 10;252(17):5942–5946. [PubMed] [Google Scholar]
- Lefkowitz R. J., Williams L. T. Molecular mechanisms of activation and desensitization of adenylate cyclase coupled beta-adrenergic receptors. Adv Cyclic Nucleotide Res. 1978;9:1–17. [PubMed] [Google Scholar]
- Limbird L. E., Gill D. M., Lefkowitz R. J. Agonist-promoted coupling of the beta-adrenergic receptor with the guanine nucleotide regulatory protein of the adenylate cyclase system. Proc Natl Acad Sci U S A. 1980 Feb;77(2):775–779. doi: 10.1073/pnas.77.2.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limbird L. E., Lefkowitz R. J. Agonist-induced increase in apparent beta-adrenergic receptor size. Proc Natl Acad Sci U S A. 1978 Jan;75(1):228–232. doi: 10.1073/pnas.75.1.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire M. E., Ross E. M., Gilman A. G. beta-Adrenergic receptor: ligand binding properties and the interaction with adenylyl cyclase. Adv Cyclic Nucleotide Res. 1977;8:1–83. [PubMed] [Google Scholar]
- Maguire M. E., Wiklund R. A., Anderson H. J., Gilman A. G. Binding of (125I)iodohydroxybenzylpindolol to putative beta-adrenergic receptors of rat glioma cells and other cell clones. J Biol Chem. 1976 Mar 10;251(5):1221–1231. [PubMed] [Google Scholar]
- Nielsen T. B., Lad P. M., Preston M. S., Rodbell M. Characteristics of the guanine nucleotide regulatory component of adenylate cyclase in human erythrocyte membranes. Biochim Biophys Acta. 1980 Apr 17;629(1):143–155. doi: 10.1016/0304-4165(80)90273-1. [DOI] [PubMed] [Google Scholar]
- Pfeuffer T. GTP-binding proteins in membranes and the control of adenylate cyclase activity. J Biol Chem. 1977 Oct 25;252(20):7224–7234. [PubMed] [Google Scholar]
- Pfeuffer T., Helmreich E. J. Activation of pigeon erythrocyte membrane adenylate cyclase by guanylnucleotide analogues and separation of a nucleotide binding protein. J Biol Chem. 1975 Feb 10;250(3):867–876. [PubMed] [Google Scholar]
- Rodbell M., Lin M. C., Salomon Y. Evidence for interdependent action of glucagon and nucleotides on the hepatic adenylate cyclase system. J Biol Chem. 1974 Jan 10;249(1):59–65. [PubMed] [Google Scholar]
- Rodbell M. The role of hormone receptors and GTP-regulatory proteins in membrane transduction. Nature. 1980 Mar 6;284(5751):17–22. doi: 10.1038/284017a0. [DOI] [PubMed] [Google Scholar]
- Salomon Y., Londos C., Rodbell M. A highly sensitive adenylate cyclase assay. Anal Biochem. 1974 Apr;58(2):541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- Schlegel W., Cooper D. M., Rodbell M. Inhibition and activation of fat cell adenylate cyclase by GTP is mediated by structures of different size. Arch Biochem Biophys. 1980 May;201(2):678–682. doi: 10.1016/0003-9861(80)90559-7. [DOI] [PubMed] [Google Scholar]
- Schlegel W., Kempner E. S., Rodbell M. Activation of adenylate cyclase in hepatic membranes involves interactions of the catalytic unit with multimeric complexes of regulatory proteins. J Biol Chem. 1979 Jun 25;254(12):5168–5176. [PubMed] [Google Scholar]
- Stadel J. M., DeLean A., Lefkowitz R. J. A high affinity agonist . beta-adrenergic receptor complex is an intermediate for catecholamine stimulation of adenylate cyclase in turkey and frog erythrocyte membranes. J Biol Chem. 1980 Feb 25;255(4):1436–1441. [PubMed] [Google Scholar]
- Tolkovsky A. M., Levitzki A. Coupling of a single adenylate cyclase to two receptors: adenosine and catecholamine. Biochemistry. 1978 Sep 5;17(18):3811–3817. doi: 10.1021/bi00611a021. [DOI] [PubMed] [Google Scholar]
- Welton A. F., Lad P. M., Newby A. C., Yamamura H., Nicosia S., Rodbell M. Solubilization and separation of the glucagon receptor and adenylate cyclase in guanine nucleotide-sensitive states. J Biol Chem. 1977 Sep 10;252(17):5947–5950. [PubMed] [Google Scholar]



