Abstract
Background
Wnt/β-catenin signaling is involved in various aspects of skeletal muscle development and regeneration. In addition, Wnt3a and β-catenin are required for muscle-specific gene transcription in embryonic carcinoma cells and satellite-cell proliferation during adult skeletal muscle regeneration. Downstream targets of canonical Wnt signaling are cyclin D1 and c-myc. However both target genes are suppressed during differentiation of mouse myoblast cells, C2C12. Underlying molecular mechanisms of β-catenin signaling during myogenic differentiation remain unknown.
Results
Using C2C12 cells, we examined intracellular signaling and gene transcription during myoblast proliferation and differentiation. We confirmed that several Wnt signaling components, including Wnt9a, Sfrp2 and porcupine, were consistently upregulated in differentiating C2C12 cells. Troponin T-positive myotubes were decreased by Wnt3a overexpression, but not Wnt4. TOP/FOP reporter assays revealed that co-expression with Wnt4 reduced Wnt3a-induced luciferase activity, suggesting that Wnt4 signaling counteracted Wnt3a signaling in myoblasts. FH535, a small-molecule inhibitor of β-catenin/Tcf complex formation, reduced basal β-catenin in the cytoplasm and decreased myoblast proliferation. K252a, a protein kinase inhibitor, increased both cytosolic and membrane-bound β-catenin and enhanced myoblast fusion. Treatments with K252a or Wnt4 resulted in increased cytoplasmic vesicles containing phosphorylated β-catenin (Tyr654) during myogenic differentiation.
Conclusions
These results suggest that various Wnt ligands control subcellular β-catenin localization, which regulate myoblast proliferation and myotube formation. Wnt signaling via β-catenin likely acts as a molecular switch that regulates the transition from cell proliferation to myogenic differentiation.
Background
Wnt signaling plays key roles in stem cell maintenance and adult tissue homeostasis [1,2]. In addition, Wnt signaling controls cell proliferation and differentiation, as well as organized cell movements and tissue polarity establishment. Wnt signaling dysregulation can induce degenerative and cancerous disorders. The Wnt signaling pathway has gained attention as a potential therapeutic target for cancer treatment, as well as research interest in regenerative medicine and stem cell biology.
Members of the Wnt family are involved in various stages of skeletal muscle development and regeneration [3]. Wnt1 and Wnt3a expression in the developing neural tube initiate myogenic differentiation in dorsal and medial somites [4,5]. Wnt3a overexpression significantly decreases terminally differentiated myogenic cells and causes chick limb malformation by inhibiting chondrogenesis [6,7]. In chick embryos, Wnt4 is expressed in developing limbs, particularly in the central elbow region and joint interzones of the wrist-forming region [8]. Wnt4 overexpression induces muscle satellite cell markers Pax7 and MyoD, and increases skeletal muscle mass in chick embryos [9]. Wnt5a and Wnt11 have been implicated in varying the number of fast and/or slow myofiber types; Wnt5a increases and decreases, the number of slow and fast myofibers, respectively, whereas Wnt11 has a reversion activity on myofiber specification [6]. Compared to the characterization of these Wnt ligands, intracellular Wnt signaling cooperation during skeletal muscle development and homeostasis is not fully understood.
Wnt family proteins consist of two subfamilies based on downstream intracellular signaling. The canonical Wnt pathway stabilizes β-catenin and activates target genes via TCF/Lef transcription factors. Other Wnt pathways are independent of β-catenin signaling and known as non-canonical Wnt pathways that include stimulation of intracellular Ca2+ release and activation of phospholipase C and protein kinase C. Non-canonical signaling pathways also activate G proteins, RhoGTPases and c-Jun N-terminal kinase (JNK). A recent studies showed that the β-catenin pathway is inhibited by Ror that contains extracellular immunoglobulin (Ig)-like, frizzled-like cysteine-rich, kringle, cytoplasmic tyrosine kinase and proline-rich domains [10]. Ror2 negatively regulates the β-catenin pathway at the TCF-mediated transcription level and activates JNK [11]. The Wnt/Ror pathway is considered to be involved in non-canonical pathways.
Previously, we demonstrated that Wnt4 overexpression increases skeletal muscle mass in chick embryos [9]. Wnt4 signaling pathway involvement in skeletal muscle development has been debated, although the level of involvement is dependent on the cell type and context of other regulatory influences. Indeed, Wnt4 can function via the canonical Wnt/β-catenin signaling pathway [12], whereas Wnt4 is mediated by JNK in frog eye and human kidney development [13-15]. While Wnt4 functions are well defined, the underlying mechanisms that regulate expression remain largely unknown.
In this study, we investigate Wnt signaling during differentiation of C2C12 cells that can differentiate into contractile myotubes under a low-serum condition [16]. We use microarray analysis to identify the expression profile of Wnt signaling components during myoblast differentiation. In addition, several Wnt members were overexpressed in C2C12 cells to assess their roles in myoblast differentiation. We also examined small-molecule inhibitor effects on Wnt signaling to evaluate β-catenin/TCF complex and membrane-bound β-catenin involvement in regulating cell proliferation and myoblast differentiation.
Results
Expression of Wnt signaling molecules during myogenic differentiation of C2C12 cells
Mouse mesenchymal C2C12 cells can differentiate into muscle cells under a low-serum condition [16]. Using real-time PCR, we investigated the expression of endogenous Wnt signaling components before and after C2C12 cell differentiation. Expression profiles of cells cultured in proliferation medium were used as controls for comparisons to the second and fourth days of culture in differentiation medium.
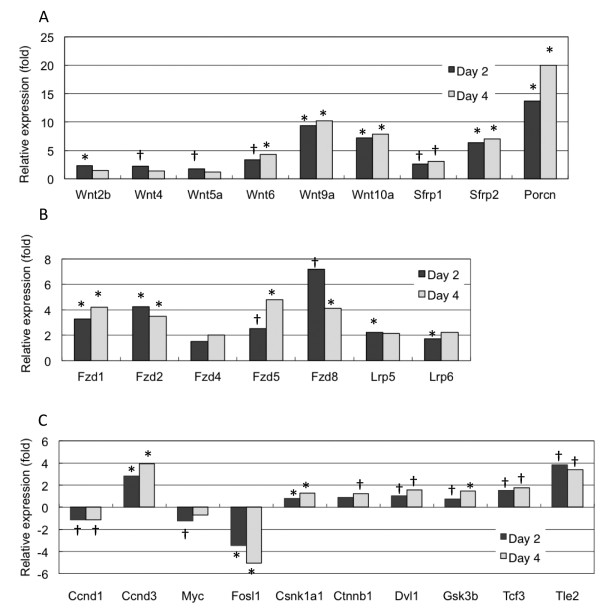
Wnt9a, Wnt10a and Wnt6 expression increased up to day 4, while Wnt2b, Wnt4 and Wnt5a expression reached a maximum at day 2 (Figure 1). Net expression levels of Wnt members were as follows in descending order: Wnt10a > Wnt9a > Wnt2b > Wnt4, Wnt6 > Wnt5a. This expression pattern was consistent with the expression of downstream signaling molecules involved in non-canonical Wnt signaling. Among transmembrane receptors, frizzled2 and frizzled8 were expressed during early differentiation stages compared with those of frizzled1, frizzled4 and frizzled5. For canonical Wnt signaling target genes, fosl1 and c-myc expression were decreased, while Tle2, a negative regulator of canonical signaling, was increased compared with those of β-catenin (Ctnnb1), TCF7 and Apc. Cyclin D1 and c-myc expression decreased, while cyclin D3 and Gsk3β increased during differentiation [17]. This was consistent with a report that describes Gsk3β regulation of cyclin D1 degradation and subcellular localization [18]. We also found that porcupine (Porcn) expression was 13-fold and 19-fold higher at days 2 and 4, respectively, compared with those of cells in a proliferative state (Additional files 1, 2, 3). Porcn encodes an O-acyltransferase involved in lipid modification of Wnt proteins in the endoplasmic reticulum. Thus, Porcn upregulation indicated that Wnt protein transport and secretion was stimulated during myoblast differentiation. Wnt signaling downstream also dynamically changed toward non-canonical pathways following myogenic differentiation.
Figure 1.
Endogenous Wnt signaling component expression during myogenic differentiation of C2C12 cells. Wnt signaling molecule expression was measured by quantitative RT-PCR. After 2 or 4 days culture in differentiation medium, cells were harvested, total RNA prepared and relative gene expression quantified by real-time PCR as follows: (A) wingless-related MMTV integration, Wnt; secreted frizzled-related protein, Sfrp; and porcupine, Porcn. (B) frizzled, Fzd; and low density lipoprotein receptor-related protein, Lrp. (C) cyclin D, Ccnd; myelocytomatosis oncogene, Myc; fos-like antigen 1, Fosl1; casein kinase 1, alpha 1, Csnk1a1; catenin, beta 1, Ctnnb1; dishevelled 1, Dvl1; glycogen synthase kinase 3 beta, Gsk3b; transcription factor 3, Tcf3; and transducin-like enhancer of split 2, Tle2. Day 2 and 4 time points were normalized to expression levels at day 0. Data are from three independent experiments, *P < 0.005 and † P < 0.05.
Wnt-4 enhances myogenic differentiation of C2C12 cells and antagonizes the canonical Wnt signaling pathway
Next, we evaluated myogenic differentiation induced by Wnt family members. Expression plasmids containing Wnt3a, Wnt4, Wnt5a, Wnt6, Wnt7a, Wnt9a and Wnt10a cDNAs were transfected into C2C12 cells. Differentiation was determined by troponin T immunostaining. Although transfection efficiency as indicated by HA-tag immunostaining was ~10%, troponin T expression in Wnt4-transfected cells was 3.5-fold higher compared with that of the control (Additional file 4). Troponin T expression was also significantly increased with Wnt6, Wnt7a and Wnt9a overexpression, but not Wnt3a, Wnt5a and Wnt10a. Similar results were also obtained with immunostaining, when skeletal muscle myosin heavy chain was used as a marker protein in place of troponin T (data not shown).
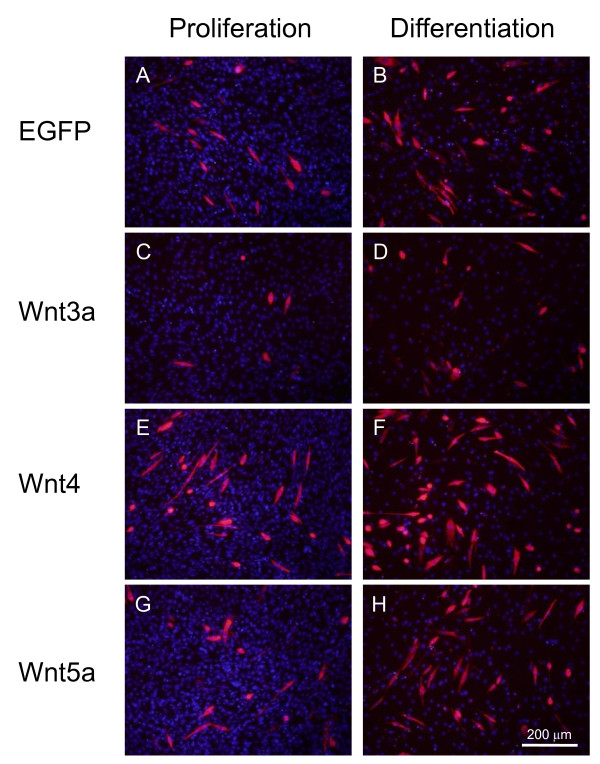
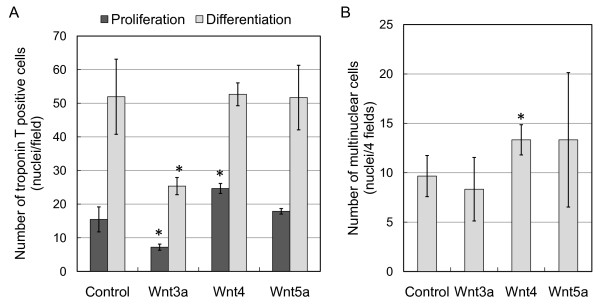
DNA transfection by cationic liposomes is generally less effective compared with that of recombinant virus infection concerning proportion of the cells expressing HA-tagged Wnt proteins. Therefore, we evaluated myogenic activity using a recombinant adenovirus encoding Wnt3a, Wnt4 and Wnt5a. Within the Wnt family, we selected Wnt3a, Wnt4 and Wnt5a, because these Wnts exhibit significantly varying biological activities in chick embryos [6-9], although endogenous expression levels of Wnt3a and Wnt5a in C2C12 cells was less than one-tenth of Wnt4 (Additional files 2, 3). C2C12 cells cultured in 24 well plates were infected by the recombinant adenovirus with a multiplicity of infection of 400, because C2C12 cells lack coxsackie and adenovirus receptor expression. Under these conditions, uniform weak expression of the transgene was usually detected at 48 h post-infection. Troponin T immunostaining showed that Wnt4-overexpressing cells expressed troponin T and differentiated into myofibers (Figure 2). However, cells that overexpressed Wnt3a showed decreased troponin T staining and poor differentiation compared with that of the control expressing eGFP. Wnt5a overexpression did not significantly affect myoblast differentiation and myofiber formation. Wnt3a overexpression significantly reduced troponin T-positive cells in proliferation and differentiation medium (Figure 3A). However, Wnt4 overexpression significantly increased troponin T-positive cells in proliferation medium. In differentiation medium, Wnt4 overexpression enhanced myotube formation as indicated by fused multinuclear troponin T-positive cells (Figure 3B). Wnt5a overexpression demonstrated no effect on C2C12 differentiation under identical conditions.
Figure 2.
Wnt4 promotes myogenic differentiation of C2C12 cells. Expression constructs of Wnt family members in adenovirus vector were transfected into C2C12 cells and myogenic differentiation was determined by troponin T immunostaining after 2 days culture in differentiation medium, which commenced 24 h post-transfection.
Figure 3.
Wnt4 promotes myogenic differentiation and induces fusion of C2C12 cells. (A) Troponin T expression after 3 day culture in proliferation or differentiation medium with recombinant adenoviruses expressing Wnt3a, Wnt4, Wnt5a or eGFP (control). Cell numbers are the means and standard deviations of three independent experiments performed in triplicate, *P < 0.01 vs. control. (B) Differentiated myotubes were evaluated by counting nuclei within fused multinucleated cells expressing troponin T. Four fields in triplicate cultures were used for counting fused cells cultured under a differentiation condition, *P < 0.04 vs. control.
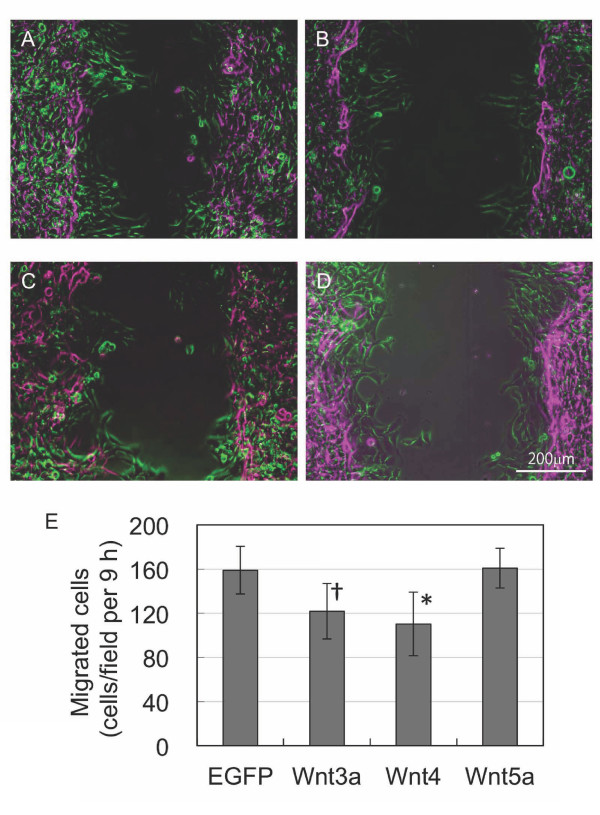
A scratch wound-healing assay was performed to examine proliferation and migration of cells overexpressing Wnt3a, Wnt4 and Wnt5a. After 48 h post-transfection, C2C12 cell monolayers were scratched to remove cells along the middle of the culture plate surface. Then, cell migration was monitored by time-lapse video microscopy (Additional files 5, 6, 7, 8). Cellular migration of Wnt5a-expressing cells was nearly identical to that of control cells expressing enhanced green florescent protein (eGFP), while Wnt4- and Wnt3a-expressing cells showed decreased migration to 69% and 77%, respectively (Figure 4). Although we observed cell migration in the scratch assay, we could not detect any differences between eGFP and Wnt5a transgenes during regeneration of the cells at the wound edges.
Figure 4.
Effect of Wnt expression on cell migration. A scratch test was used to determine C2C12 cell proliferation and migration. Cell numbers were counted over 9 h, and mobility was monitored by time-lapse microscopy. Phase contrast images at 0 h (purple) and 9 h (green) were merged to show the migration of C2C12 cells expressing eGFP (A), Wnt3a (B), Wnt4(C) and Wnt5a (D). (E) Summary of cell numbers counted over 9 h (cells/field) with n = 3 for control, Wnt3a and Wnt4, and n = 4 for Wnt5a. *P < 0.01; † P < 0.02 vs. eGFP.
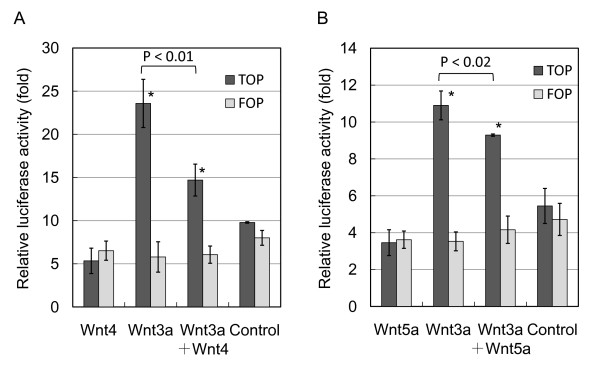
We then examined whether Wnt4 mediated myogenesis induction by canonical or non-canonical signaling using a TOP/FOP reporter assay in C2C12 cells under a proliferative condition. Wnt4 and Wnt3a reporter constructs were transfected into C2C12 cells and luciferase activity was determined at 24 h post-transfection. Although Wnt3a significantly elevated reporter activity, Wnt4 did not show increased activity above FOP (Figure 5A). However, Wnt4 coexpressed with Wnt3a showed 47% inhibition. Weak inhibition was observed when Wnt5a was coexpressed with Wnt3a (Figure 5B). These results indicated that Wnt4 suppressed canonical Wnt signaling mediated by the β-catenin/TCF complex and promoted myogenic differentiation. This observation was consistent with down-regulated cyclin D1 and c-myc in differentiating myoblasts, both of which are direct targets of the β-catenin/TCF complex.
Figure 5.
Wnt4 reduces Wnt-3a-induced transcriptional activity in myoblasts. Reporter assays were performed using TOP/FLASH and FOP/FLASH (luciferase assay) in C2C12 cells. Wnt3a significantly increases luciferase activity in TOP over FOP. (A) Wnt4 has no effect on luciferase activity, whereas Wnt4 inhibits Wnt3a activity. (B) Wnt5a poorly inhibits Wnt3a activity. Bars are the means of four independent experiments. *P < 0.01 between TOP and FOP; P < 0.01 between Wnt3a and Wnt3a + Wnt4 in TOP; P < 0.02 between Wnt3a and Wnt3a + Wnt5a in TOP.
β-Catenin is a molecular switch between C2C12 cell proliferation or differentiation
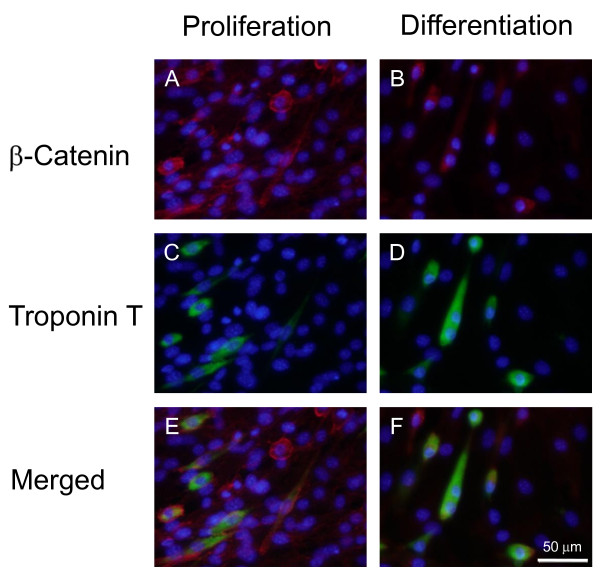
Wnt4, represented by luciferase activity, inhibited transcriptional activation via β-catenin/TCF. In HEK-293T cells, Wnt4 relocates β-catenin to the cell membrane [19]. We observed subcellular β-catenin localization by immunohistochemistry in C2C12 cells cultured in proliferation and differentiation medium (Figure 6). Under a proliferative condition, β-catenin was predominantly localized in the cytoplasm. β-Catenin was also localized within the nucleus of round proliferating cells and weak β-catenin was coexpressed with differentiation marker troponin T (Figure 6E). This observation coincided with changing morphology to flattened multinucleated cells. In differentiation medium, cellular β-catenin accumulated in the membrane and nuclei as differentiation proceeded into myotubes (Figure 6B,F).
Figure 6.
Localization of β-catenin and troponin T in proliferative and differentiating C2C12 cells. C2C12 cells cultured in proliferation and differentiation medium were double immunostained for β-catenin (A, B; red) and troponin T (C, D; green), and counterstained with DAPI (blue). Immunofluorescent images with various filters are merged (E, F).
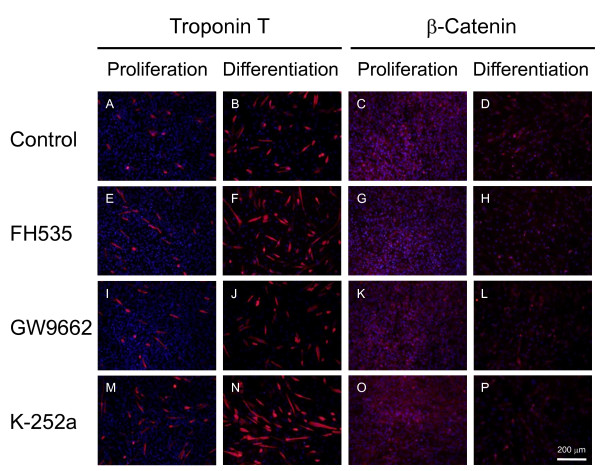
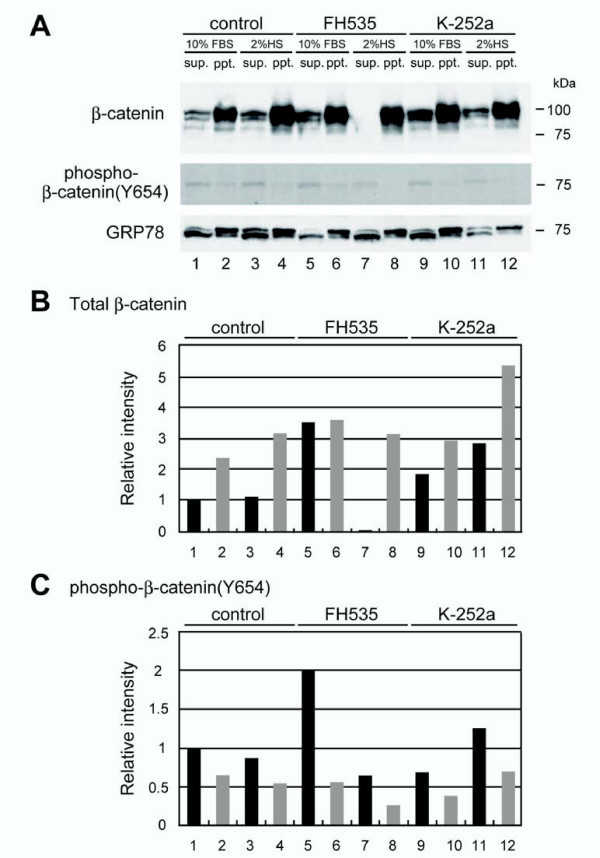
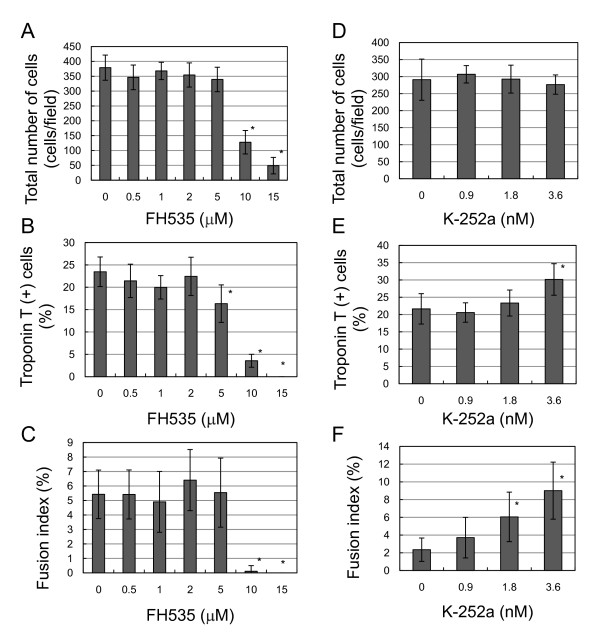
Small-molecule inhibitors of Wnt signaling have been developed for human cancer therapy and allow manipulation of intracellular signaling to decrease cell proliferation and induce differentiation or apoptosis [20]. To investigate Wnt signaling and β-catenin during C2C12 myoblast differentiation, we used chemical inhibitors FH535, GW9662 and K252a to observe troponin T expression and subcellular β-catenin localization by immunohistochemistry (Figure 7) and western blotting (Figure 8). FH535 interferes with β-catenin/Tcf complex formation, whereas GW9662 is structurally similar to FH535 but does not interfere with complex formation. K252a is a Trk kinase inhibitor, which phosphorylates β-catenin at tyrosine 654 [21,22]. FH535 and GW9662 did not affect myoblast differentiation at 1 μM, although FH535 exhibited cytotoxicity at concentrations > 10 μM (Figure 9). K252a dose-dependently increased troponin T-positive cells and promoted myoblast fusion, as reported elsewhere [21]. Subcellular β-catenin localization was determined by western blot analysis of cytoplasmic and membrane extracts from proliferative and differentiating C2C12 cells (Figure 9). FH535 increased the cytosolic level of β-catenin in proliferation medium, but decreased basal β-catenin in differentiation medium (Figure 8A, B). In contrast, K252a increased cytosolic and membrane-bound β-catenin during myogenic differentiation (Figure 9B).
Figure 7.
Effect of small-molecule inhibitors on C2C12 cell proliferation and differentiation. C2C12 cells were cultured without inhibitors (A-D) and with 1 μM FH535 (E-H), 1 μM GW9662 (I-L) and 3.6 nM K-252a (M-P), then immunostained with anti-troponin T (A, B, E, F, I, J, M, N) and anti-β-catenin (C, D, G, H, K, L, O, P) antibodies. FH535 interferes with β-catenin/Tcf complex formation, whereas GW9662 is structurally similar to FH535, but does not interfere with complex formation. K252a is a Trk kinase inhibitor, which phosphorylates β-catenin at tyrosine 654.
Figure 8.
FH535 and K252a alter total and phospho-β-catenin (Y654) in C2C12 cells. (A) Western blots of cytosolic and membrane fractions from C2C12 cells treated with FH535 and K-252a using antibodies against total cellular-β-catenin (upper panel) and phosphorylated β-catenin (lower panel). GRP78 indicates loading control. (B, C) Relative levels of total and phosphorylated β-catenin in cytosolic (black) and membrane (grey) fractions were calculated as the β-catenin/GRP78 ratio. Band intensities are relative to the control cell values in the cytosolic fraction and defined as 1.0. Similar results were obtained in three independent experiments.
Figure 9.
Effect of FH535 and K252a on C2C12 cell proliferation and differentiation. C2C12 cells were cultured with or without various FH535 and K252a concentrations followed by troponin T immunostaining. Total cell numbers are the counted DAPI-stained nuclei and the fusion index is the percentage of multinucleated cells among troponin T-positive cells. *P < 0.001 vs. absence of inhibitors.
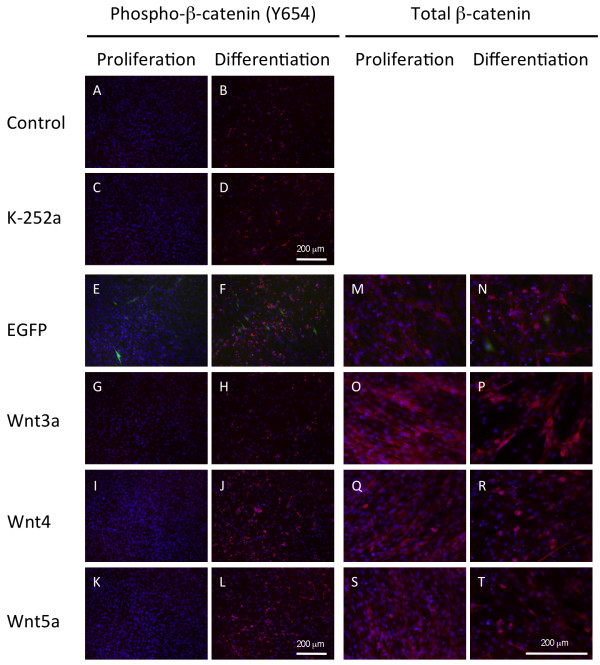
Next, we analyzed β-catenin phosphorylation at residue Y654 during myoblast proliferation and differentiation. Proliferation medium supplemented with FH535 increased phospho-β-catenin (Y654) by 2-fold, but decreased phospho-β-catenin to less than half in differentiation medium (Figure 8C). In contrast, K252a-supplemented proliferation medium decreased phospho-β-catenin (Y654) as anticipated, but increased phosphorylated β-catenin and total β-catenin in differentiation medium. This observation was consistent with the immunohistochemical analysis. K252a treatment in differentiation medium caused cytosolic accumulation of small vesicles containing phospho-β-catenin (Y654) (Figure 10A,B). Together with western blot data, these results suggested that K252a increases total β-catenin in C2C12 cells. Simultaneously, β-catenin may be phosphorylated at tyrosine 654 by a kinase that was not inhibited by K252a and induced in differentiation medium via serum starvation.
Figure 10.
Wnt family members differentially regulate β-catenin phosphorylation and subcellular localization in C2C12 cells. (A-D) C2C12 cells were cultured with or without K252a, and then phospho-β-catenin (Y654) was immunostained. K252a caused an accumulation of vesicles containing phospho-β-catenin (Y654) in C2C12 cells. (E-L) Wnt3a, Wnt4 and Wnt5a are overexpressed in C2C12 cells by adenovirus-mediated gene transfer. After 2 day culture in differentiation medium commencing 24 h post-transfection, subcellular phospho-β-catenin (Y654) localization was determined by immunostaining. (M-T) C2C12 cells expressing eGFP, Wnt3a, Wnt4 and Wnt5a were immunostained with anti-pan-β-catenin antibodies. Cytoplasmic β-catenin is elevated with Wnt3a expression in proliferation medium and maintained at a higher level in differentiation medium, whereas nuclear and membrane-bound β-catenin is increased with Wnt4 expression.
C2C12 cells overexpressing Wnt3a, Wnt4 and Wnt5a were stained with the same monoclonal antibody to determine cellular phospho-β-catenin in differentiation medium. Consistent with troponin T expression, decreased vesicles containing phospho-β-catenin (Y654) were observed in Wnt3a-overexpressing cells cultured in differentiation medium, whereas Wnt4- and Wnt5a-overexpressing cells contained increased vesicles compared with that of the control cells expressing eGFP (Figure 10F, H, J, L).
Lastly, we analyzed localization of β-catenin within cells overexpressing Wnt3a, Wnt4 and Wnt5a. Wnt3a overexpression resulted in an elevated cytoplasmic β-catenin level in both proliferation and differentiation mediums (Figure 10O, P). In contrast, Wnt4 and Wnt5a overexpression showed an increase in nuclear and membrane-bound β-catenin levels (Figure 10Q, R, S, T). Collectively, our results suggested that Wnt family members differentially regulate β-catenin phosphorylation and subcellular localization. Moreover, β-catenin is required to maintain cell proliferation and acts as a molecular switch that regulates myogenic differentiation.
Discussion
Wnt/β-catenin signaling is essential for skeletal muscle development and regeneration. Wnt signals via β-catenin are necessary to induce muscle-specific gene transcription in P19 embryonic carcinoma cells [23]. Canonical Wnt signaling also promotes muscle satellite-cell proliferation in response to skeletal muscle injury [24]. The β-catenin/TCF complex binds cyclin D1 and c-myc promoters, and increases expression in carcinoma cells. However, β-catenin/TCF target gene expression is decreased in differentiating C2C12 myoblast cells. Cyclin D1, c-myc and fosl1 down-regulation as well as a compensating cyclin D3 elevation suggest pathway switching from β-catenin dominant signaling to other pathways. We demonstrated that Wnt family members, including Wnt2b, Wnt4, Wnt6, Wnt9a, and Wnt10a, are significantly elevated following myogenic differentiation of C2C12 cells. Within the Wnt family, Wnt4, Wnt6, Wnt7a, and Wnt9a induce myogenic differentiation following transient overexpression in C2C12 cells, whereas Wnt3a, Wnt5a, and Wnt10a do not affect troponin T expression. Wnt4 overexpression optimally promotes myogenesis and antagonizes transcription mediated by β-catenin/TCF. We therefore selected Wnt3a, Wnt4 and Wnt5a to analyze canonical and/or non-canonical signaling during myogenic differentiation of C2C12 cells.
We found that Wnt4 suppresses canonical Wnt signaling mediated by the β-catenin/TCF complex and promotes myogenic differentiation. However, numerous reports indicate that activation of the Wnt/β-catenin signaling pathway increases myogenic differentiation directly or indirectly [24-30]. One possible explanation may be that canonical Wnt signaling maintains proliferation of myoblast and progenitors, such as satellite cells and reserve cells, and subsequently induces myogenic differentiation. Indeed, canonical Wnt signaling downstream targets cyclin D1 and c-myc are suppressed during myoblast differentiation [17] (Figure 1). This observation is consistent with c-myc overexpression in C2C12 cells resulting in inhibition of myoblast fusion and myotube formation [31]. Moreover, increased canonical Wnt signaling is related to skeletal muscle aging [32,33]. Further analyses of the cell cycle and β-catenin are required to characterize canonical Wnt signaling during myogenic differentiation.
Recent studies have shown that Wnt4 activates the canonical β-catenin pathway in C2C12 cells [30]. This difference of results may be partly due to cell culture conditions and C2C12 cell characteristics. First, the C2C12 cell cycle is not synchronized with medium replacement and various cell cycle phases are observed, as shown in Figure 6. Second, serum components in the medium may affect the results, because proliferation and differentiation of C2C12 cells is controlled by lysophosphatidic acid and cholesterol [34,35]. Third, C2C12 cell characteristics may substantially change during serial passaging. Therefore, Wnt4 could mediate either canonical or non-canonical signaling pathways and overexpression may promote myogenic differentiation of C2C12 cells. During the transition from cell proliferation to differentiation in C2C12 cells, β-catenin expression is down-regulated and troponin T expression is predominantly observed in β-catenin-negative cells (Figure 6). Therefore, β-catenin signaling may be required during early myogenic differentiation stages and unnecessary or inhibitory toward myotube formation after myogenic determination.
Functional switching from cell proliferation to myogenic differentiation is accompanied by changes in cell migration, as revealed by a scratch test analysis (Figure 4). Maintenance of an undifferentiated state with Wnt3a overexpression results in decreased migration, while transition toward myogenic differentiation further reduces migration possibly via non-canonical pathways. Wnt3a activity via the canonical pathway affects cell survival and proliferation. Additionally, Wnt3a is required for maintenance of the myoblast undifferentiated state as well as differentiation induced by Wnt4 signaling.
Our results suggest that β-catenin acts as a molecular switch between proliferation and differentiation of C2C12 cells. The β-catenin/Tcf complex is essential for C2C12 cell proliferation, and inhibition of complex formation by FH535 promotes cell death, although the detailed mechanism of FH535 inhibition is unknown at present. β-Catenin is phosphorylated by various kinases, which causes β-catenin degradation or signaling activity. K252a inhibition of Y654 phosphorylation shifts β-catenin localization from the cytoplasm to the cell membrane, which stimulates myotube formation (Figure 10). Wnt4 induces β-catenin shuttling from the nucleus to the cytoplasm and the regulatory mechanism is not known.
Conclusions
We found that various Wnt ligands regulate differentially subcellular β-catenin localization as well as myoblast proliferation and myotube formation. These results implicate β-catenin in functional switching from cell proliferation to myogenic differentiation.
Methods
Plasmid construction, cell culture and transfection assay
eGFP cDNA was purchased from Wako Chemicals as GFP pQBI-polII. Wnt4 and eGFP cDNAs were subcloned into pcDNA3.2DEST (Invitrogen, Carlsbad, CA) for overexpression in C2C12 cells. A HA-tag was added to the Wnt4 C-terminal using mouse Wnt3a cDNA in pUSEamp (Upstate Biotechnology Inc., Temecula, CA) that contained the HA-tag sequence at the C-terminal end, after subcloning to replace the Wnt3a sequence with Wnt4. All Wnt cDNAs used in the present studies contained HA-tag sequence at the C-terminal end. Wnt3aHA, Wnt4HA, Wnt5aHA, Wnt6HA, Wnt7aHA, Wnt9aHA, Wnt10aHA and eGFP (Wako Chemicals, Osaka, Japan) cDNAs were subcloned into pcDNA3.2 (Invitrogen) for transfection and transient expression.
The C2C12 cell line (myoblast-like cell line from the C3H mouse) was purchased from the RIKEN Cell Bank (RIKEN, Wako, Japan) [16,36] and cultured in Dulbecco's modified Eagle's medium (D-MEM) supplemented with 10% fetal bovine serum (FBS). At 12-24 h after subculture, transfection was performed using Lipofectamine 2000 according to the manufacturer's instructions (Invitrogen). Transfected C2C12 cells were cultured in differentiation medium consisting of 2% horse serum in D-MEM to induce myogenesis.
Real-time quantitative PCR
C2C12 cells were harvested at days 2 (n = 3) and 4 (n = 3) after replacement of proliferation medium with differentiation medium. Total RNA was extracted from C2C12 cells using ISOGEN (NIPPON GENE CO., Tokyo, Japan) according to the manufacturer's instructions. RNA was reverse transcribed into cDNA (RT2 First Strand Kit, SABiosciences) and the cDNA used for quantitative PCR analysis. Wnt signaling component expression in C2C12 cells was analyzed by quantitative Real-Time RT-PCR using a SA Biosciences RT2Profiler™ PCR Array (http://www.sabiosciences.com/rt_pcr_product/HTML/PAMM-043A.html) and RT2 SYBR Green Master Mixes. The PCR condition was 95°C for 10 min followed by 40 cycles of 95°C for 15 sec and 60°C for 1 min. Analyses were performed on an ABI Real-Time PCR System 7500 (Applied Biosystems, Foster City, CA). Data were evaluated by the ΔΔCt method against control cells cultured in proliferation medium (n = 4), using an on-line template (http://pcrdataanalysis.sabiosciences.com/pcr/arrayanalysis.php). ΔCt values were calculated by subtracting average cycle threshold (Ct) values of housekeeping genes (glucuronidase, beta, Gusb; Heat shock protein 90 alpha (cytosolic), class B member 1, Hsp90ab1; glyceraldehyde-3-phosphate dehydrogenase, Gapdh; actin, beta, Actb; and hypoxanthine guanine phosphoribosyl transferase 1, Hprt1). MIAME (Minimum Information About a Microarray Experiment) compliant array data including raw data is deposited in GEO (Gene Expression Omnibus) at NCBI [GSE30077].
Viral vector production and treatment
Adenoviruses carrying Wnt cDNAs were prepared using a ViraPower adenovirus expression system (Invitrogen). Human Wnt3a, Wnt4 and Wnt5a cDNAs (Origene, Rockville, MD) were PCR-amplified and subcloned into a pAd/CMV/V5-DEST vector (Invitrogen) using a Gateway system with LR clonase (Invitrogen). eGFP cDNA was used as a control after subcloning into a pAd/CMV/V5-DEST vector. Plasmids were purified and digested with PacI (New England Biolabs Japan Inc., Tokyo, Japan). Linearized plasmids (1-2 μg) were then mixed with 3 μl Lipofectamine 2000 in 200 μl Opti-MEM medium (Invitrogen) and transfected into subconfluent 293A cells (Invitrogen) cultured in 1 ml Opti-MEM in 35 mm plates (Nunc, Thermo Fisher Scientific, Yokohama, Japan). 293A cells were cultured in 100 mm plates for 2 weeks in proliferation medium that was exchanged every 3 days. Cells and medium were harvested upon cell detachment from culture plates, freeze-thawed four times and then centrifuged to obtain adenovirus-enriched supernatants. Aliquots of supernatant were added to fresh 293A cells and cultured for 2-3 days to propagate the adenovirus. After 2-fold amplification, adenovirus-containing medium was used as the virus stock. Viral titers were determined by a plaque-forming assay using 293A cells.
Reporter assays
C2C12 cells were seeded into 24 well plates at 2.5 × 104 cells/well and cultured for 24 h. Cells were then cotransfected with 0.2 μg DNA constructs (Wnt3aHA + pcDNA3.2, Wnt4HA + pcDNA3.2, Wnt3aHA + Wnt4HA), 0.2 μg reporter plasmid (TOPFLASH, FOPFLASH) and 0.2 μg internal control pRG-TK, then cultured for a further 24 h. Luciferase activity was measured and normalized for transfection efficiency using a Dual-Glo luciferase assay system (Promega, Madison, WI). Graphs show the average of three independent experiments with normalized transfection efficiency using Rluc.
Scratch test analysis
Scratch testing was performed using C2C12 cells transfected with Wnt3a, Wnt4, Wnt5a and eGFP in pcDNA3.2, which were cultured in 35 mm dishes. Two days after transfection, the cell monolayer was scratched using a toothpick. Cell proliferation and migration were recorded at 5 min intervals and observed for 16 h using an AQUACOSMOS image acquisition and analysis system (Hamamatsu, Japan). Initial cells counts were subtracted from the 9 h counting.
Immunofluorescence assays
Cells were fixed in a solution of ethanol: formalin: acetic acid: H2O (14:2:1:6, v/v) for 10 min. After three PBS washes, cells were treated with a blocking buffer consisting of 2% goat serum, 2% skim milk, 0.2% Tween20 in Tris-buffered saline (TBS; 50 mM Tris-HCl, pH 7.5, 150 mM NaCl) for 30 min. Then, cells were incubated with anti-troponin T (MAB1487, clone TT-98, Abnova, CA), anti-β-catenin (C2206, Sigma-Aldrich) and anti-phospho-β-catenin (Y654) (ab24925, Abcam, Tokyo, Japan) antibodies at 1:40, 1:200 and 1:50 dilutions, respectively, in blocking buffer at 4°C overnight. After three TBS washes, cells were incubated with a 1:200 dilution of secondary Alexa Fluor 594 or 488-conjugated goat anti-mouse or rabbit IgG antibodies (A11032, A11037, A11029, Molecular Probes, Invitrogen) for 1 h. After a TBS wash, cell nuclei were stained with 1 μg/ml 4', 6'-diamino-2-phenylindole solution (DAPI, DOJINDO, Kumamoto, Japan). Fluorescent images were taken using All-in-One Fluorescence Microscope BZ-9000 (Keyence Japan, Osaka, Japan).
Inhibitor assays
C2C12 cells were seeded into 24 well plates at 2.5 × 104 cells/well and cultured for 24 h. Cells were incubated in proliferation or differentiation medium with or without chemical inhibitors FH535 (1 μM), GW9662 (1 μM) and K-252a (3.6 nM).
Western blot analysis
At 24 h post-subculture, C2C12 cell culture medium was changed to differentiation medium supplemented with FH535 (1 μM) or K-252a (3.6 nM). After 72 h, cells were harvested to prepare total protein extracts.
Cytosolic β-catenin accumulation was assayed as described elsewhere [37,38]. Protein extracts were resolved by electrophoresis in 5-20% sodium dodecyl sulfate-polyacrylamide gels. Anti-β-catenin (BD Transduction Laboratory, San Diego, CA), anti-phospho-β-catenin (Y654) (ab24925, Abcam), and anti-GPR78 (Santa Cruz) antibodies were used at 1:2000, 1:500, and 1:5000 dilutions, respectively. Proteins were detected using an ECL Plus System (Amersham Pharmacia Biotech, Uppsala, Sweden) with a 1:10 reagent dilution. Band intensities were determined using Image J software (NIH USA).
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
TN conceived and designed the experiments; ST and KT performed the experiments and analyzed the data; ST and TN prepared the manuscript. All authors read and approved the final manuscript.
Supplementary Material
Real-time PCR analysis. (A) Array layout of 96 well RT-PCR kit. (B) Heat map of PCR results between day 2 and 0 samples in differentiation medium. (C) Heat map of PCR results between day 4 and 0 samples in differentiation medium. Note: the scales are not identical in panels B and C.
Real-time PCR results (Day 2 vs control). Array results of real-time PCR analysis. n = 3 for the Day 2 group and n = 4 for the control group.
Real-time PCR results (Day 4 vs control). Array results of real-time PCR analysis. n = 3 for the Day 4 group and n = 4 for the control group.
Effect of Wnt expression on cell differentiation. C2C12 cells were transfected with Wnt cDNAs in pcDNA3.2 and cultured in proliferation medium for 2 days. Cells were fixed, troponin T immunostained and positive cell nuclei were counted. *P < 0.03 vs. control.
Time-lapse movie (control). The movie consists of micrographs taken at 5 min intervals for 16 h. Cells were transfected with an eGFP-expressing plasmid.
Time-lapse movie (Wnt4). The movie consists of micrographs taken at 5 min intervals for 16 h. Cells were transfected with a Wnt4-expressing plasmid.
Time-lapse movie (Wnt5a). The movie consists of micrographs taken at 5 min intervals for 16 h. Cells were transfected with a Wnt5a-expressing plasmid.
Time-lapse movie (Wnt3a). The movie consists of micrographs taken at 5 min intervals for 16 h. Cells were transfected with a Wnt3a-expressing plasmid.
Contributor Information
Shingo Tanaka, Email: prstanaka@med.kawasaki-m.ac.jp.
Kumiko Terada, Email: terarin@med.kawasaki-m.ac.jp.
Tsutomu Nohno, Email: nohno@bcc.kawasaki-m.ac.jp.
Acknowledgements and Funding
We thank Dr. Shin-ichiro Nishimatsu for helpful discussion about results and the manuscript, Dr. Tomohiro Narita for technical support and helpful advice, and Keiko Fujioka and Satomi Misao for excellent technical assistance. This work was supported by a research grant for Comprehensive Research on Disability Health and Welfare from the Ministry of Health, Labour and Welfare of Japan, by a Grant-in-Aid for Scientific Research (C) from the Japan Society for the Promotion of Science (23590227), and by Research Project grants from Kawasaki Medical School (21-102, 22-A17).
References
- Nusse R. Wnt signaling and stem cell control. Cell Res. 2008;18:523–527. doi: 10.1038/cr.2008.47. [DOI] [PubMed] [Google Scholar]
- van Amerongen R, Nusse R. Towards an integrated view of Wnt signaling in development. Development. 2009;136:3205–3214. doi: 10.1242/dev.033910. [DOI] [PubMed] [Google Scholar]
- Shi X, Garry DJ. Muscle stem cells in development, regeneration, and disease. Genes Dev. 2006;20:1692–1708. doi: 10.1101/gad.1419406. [DOI] [PubMed] [Google Scholar]
- Ikeya M, Takada S. Wnt signaling from the dorsal neural tube is required for the formation of the medial dermomyotome. Development. 1998;125:4969–4976. doi: 10.1242/dev.125.24.4969. [DOI] [PubMed] [Google Scholar]
- Tajbakhsh S, Borello U, Vivarelli E, Kelly R, Papkoff J, Duprez D, Buckingham M, Cossu G. Differential activation of Myf5 and MyoD by different Wnts in explants of mouse paraxial mesoderm and the later activation of myogenesis in the absence of Myf5. Development. 1998;125:4155–4162. doi: 10.1242/dev.125.21.4155. [DOI] [PubMed] [Google Scholar]
- Anakwe K, Robson L, Hadley J, Buxton P, Church V, Allen S, Hartmann C, Harfe B, Nohno T, Brown AM, Evans DJ, Francis-West P. Wnt signalling regulates myogenic differentiation in the developing avian wing. Development. 2003;130:3503–3514. doi: 10.1242/dev.00538. [DOI] [PubMed] [Google Scholar]
- Narita T, Nishimatsu S, Wada N, Nohno T. A Wnt3a variant participates in chick apical ectodermal ridge formation: distinct biological activities of Wnt3a splice variants in chick limb development. Dev Growth Differ. 2007;49:493–501. doi: 10.1111/j.1440-169X.2007.00938.x. [DOI] [PubMed] [Google Scholar]
- Kawakami Y, Wada N, Nishimatsu SI, Ishikawa T, Noji S, Nohno T. Involvement of Wnt-5a in chondrogenic pattern formation in the chick limb bud. Dev Growth Differ. 1999;41:29–40. doi: 10.1046/j.1440-169x.1999.00402.x. [DOI] [PubMed] [Google Scholar]
- Takata H, Terada K, Oka H, Sunada Y, Moriguchi T, Nohno T. Involvement of Wnt4 signaling during myogenic proliferation and differentiation of skeletal muscle. Dev Dyn. 2007;236:2800–2807. doi: 10.1002/dvdy.21327. [DOI] [PubMed] [Google Scholar]
- Nishita M, Yoo SK, Nomachi A, Kani S, Sougawa N, Ohta Y, Takada S, Kikuchi A, Minami Y. Filopodia formation mediated by receptor tyrosine kinase Ror2 is required for Wnt5a-induced cell migration. J Cell Biol. 2006;175:555–562. doi: 10.1083/jcb.200607127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikels AJ, Nusse R. Purified Wnt5a protein activates or inhibits beta-catenin-TCF signaling depending on receptor context. PLoS Biol. 2006;4:e115. doi: 10.1371/journal.pbio.0040115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons JP, Mueller UW, Ji H, Everett C, Fang X, Hsieh JC, Barth AM, McCrea PD. Wnt-4 activates the canonical beta-catenin-mediated Wnt pathway and binds Frizzled-6 CRD: functional implications of Wnt/beta-catenin activity in kidney epithelial cells. Exp Cell Res. 2004;298:369–387. doi: 10.1016/j.yexcr.2004.04.036. [DOI] [PubMed] [Google Scholar]
- Maurus D, Héligon C, Bürger-Schwärzler A, Brändli AW, Kühl M. Noncanonical Wnt-4 signaling and EAF2 are required for eye development in Xenopus laevis. EMBO J. 2005;24:1181–1191. doi: 10.1038/sj.emboj.7600603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J, Sonoyama W, Wang Z, Jin Q, Zhang C, Krebsbach PH, Giannobile W, Shi S, Wang CY. Noncanonical Wnt-4 signaling enhances bone regeneration of mesenchymal stem cells in craniofacial defects through activation of p38 MAPK. J Biol Chem. 2007;282:30938–30948. doi: 10.1074/jbc.M702391200. [DOI] [PubMed] [Google Scholar]
- Torban E, Dziarmaga A, Iglesias D, Chu LL, Vassilieva T, Little M, Eccles M, Discenza M, Pelletier J, Goodyer P. PAX2 activates WNT4 expression during mammalian kidney development. J Biol Chem. 2006;281:12705–12712. doi: 10.1074/jbc.M513181200. [DOI] [PubMed] [Google Scholar]
- Yaffe D, Saxel O. Serial passaging and differentiation of myogenic cells isolated from dystrophic mouse muscle. Nature. 1977;270:725–727. doi: 10.1038/270725a0. [DOI] [PubMed] [Google Scholar]
- Skapek SX, Rhee J, Spicer DB, Lassar AB. Inhibition of myogenic differentiation in proliferating myoblasts by cyclin D1-dependent kinase. Science. 1995;267:1022–1024. doi: 10.1126/science.7863328. [DOI] [PubMed] [Google Scholar]
- Diehl JA, Cheng M, Roussel MF, Sherr CJ. Glycogen synthase kinase-3beta regulates cyclin D1 proteolysis and subcellular localization. Genes Dev. 1998;12:3499–3511. doi: 10.1101/gad.12.22.3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard P, Fleming A, Lacombe A, Harley VR, Vilain E. Wnt4 inhibits β-catenin/TCF signalling by redirecting β-catenin to the cell membrane. Biol Cell. 2008;100:167–177. doi: 10.1042/BC20070072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker N, Clevers H. Tracking down the stem cells of the intestine: strategies to identify adult stem cells. Gastroenterology. 2007;133:1755–1760. doi: 10.1053/j.gastro.2007.10.029. [DOI] [PubMed] [Google Scholar]
- Lee KH, Lee SH, Kim D, Rhee S, Kim C, Chung CH, Kwon H, Kang MS. Promotion of skeletal muscle differentiation by K252a with tyrosine phosphorylation of focal adhesion: A possible involvement of small GTPase Rho. Exp Cell Res. 1999;252:401–415. doi: 10.1006/excr.1999.4648. [DOI] [PubMed] [Google Scholar]
- David MD, Yeramian A, Duñach M, Llovera M, Cantí C, de Herreros AG, Comella JX, Herreros J. Signalling by neurotrophins and hepatocyte growth factor regulates axon morphogenesis by differential beta-catenin phosphorylation. J Cell Sci. 2008;121:2718–2730. doi: 10.1242/jcs.029660. [DOI] [PubMed] [Google Scholar]
- Petropoulos H, Skerjanc IS. Beta-catenin is essential and sufficient for skeletal myogenesis in P19 cells. J Biol Chem. 2002;277:15393–15399. doi: 10.1074/jbc.M112141200. [DOI] [PubMed] [Google Scholar]
- Otto A, Schmidt C, Luke G, Allen S, Valasek P, Muntoni F, Lawrence-Watt D, Patel K. Canonical Wnt signalling induces satellite-cell proliferation during adult skeletal muscle regeneration. J Cell Sci. 2008;121:2939–2950. doi: 10.1242/jcs.026534. [DOI] [PubMed] [Google Scholar]
- Vyas DR, Spangenburg EE, Abraha TW, Childs TE, Booth FW. GSK-3beta negatively regulates skeletal myotube hypertrophy. Am J Physiol Cell Physiol. 2002;283:C545–C551. doi: 10.1152/ajpcell.00049.2002. [DOI] [PubMed] [Google Scholar]
- Rochat A, Fernandez A, Vandromme M, Molès JP, Bouschet T, Carnac G, Lamb NJ. Insulin and wnt1 pathways cooperate to induce reserve cell activation in differentiation and myotube hypertrophy. Mol Biol Cell. 2004;10:4544–4555. doi: 10.1091/mbc.E03-11-0816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Velden JL, Schols AM, Willems J, Kelders MC, Langen RC. Glycogen synthase kinase 3 suppresses myogenic differentiation through negative regulation of NFATc3. J Biol Chem. 2008;283:358–366. doi: 10.1074/jbc.M707812200. [DOI] [PubMed] [Google Scholar]
- Abiola M, Favier M, Christodoulou-Vafeiadou E, Pichard AL, Martelly I, Guillet-Deniau I. Activation of Wnt/beta-catenin signaling increases insulin sensitivity through a reciprocal regulation of Wnt10b and SREBP-1c in skeletal muscle cells. PLoS One. 2009;4:e8509. doi: 10.1371/journal.pone.0008509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Yang J, Liu R, Li H, Luo X, Yang G. Accumulation of β-catenin by lithium chloride in porcine myoblast cultures accelerates cell differentiation. Mol Biol Rep. 2011;38:2043–2049. doi: 10.1007/s11033-010-0328-3. [DOI] [PubMed] [Google Scholar]
- Bernardi H, Gay S, Fedon Y, Vernus B, Bonnieu A, Bacou F. Wnt4 activates the canonical β-catenin pathway and regulates negatively myostatin: functional implication in myogenesis. Am J Physiol Cell Physiol. 2011;300:C1122–C1138. doi: 10.1152/ajpcell.00214.2010. [DOI] [PubMed] [Google Scholar]
- Crescenzi M, Crouch DH, Tatò F. Transformation by myc prevents fusion but not biochemical differentiation of C2C12 myoblasts: mechanisms of phenotypic correction in mixed culture with normal cells. J Cell Biol. 1994;125:1137–1145. doi: 10.1083/jcb.125.5.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Fergusson MM, Castilho RM, Liu J, Cao L, Chen J, Malide D, Rovira II, Schimel D, Kuo CJ, Gutkind JS, Hwang PM, Finkel T. Augmented Wnt signaling in a mammalian model of accelerated aging. Science. 2007;317:803–806. doi: 10.1126/science.1143578. [DOI] [PubMed] [Google Scholar]
- Brack AS, Conboy MJ, Roy S, Lee M, Kuo CJ, Keller C, Rando TA. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science. 2007;317:807–810. doi: 10.1126/science.1144090. [DOI] [PubMed] [Google Scholar]
- Jean-Baptiste G, Yang Z, Khoury C, Greenwood MT. Lysophosphatidic acid mediates pleiotropic responses in skeletal muscle cells. Biochem Biophys Res Commun. 2005;335:1155–1162. doi: 10.1016/j.bbrc.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Mermelstein CS, Portilho DM, Mendes FA, Costa ML, Abreu JG. Wnt/β-catenin pathway activation and myogenic differentiation are induced by cholesterol depletion. Differentiation. 2007;75:184–192. doi: 10.1111/j.1432-0436.2006.00129.x. [DOI] [PubMed] [Google Scholar]
- Blau HM, Chiu C, Webster C. Cytoplasmic activation of human nuclear genes in stable heterocaryons. Cell. 1983;32:1171–1180. doi: 10.1016/0092-8674(83)90300-8. [DOI] [PubMed] [Google Scholar]
- Shimizu H, Julius MA, Giarré M, Zheng Z, Brown AM, Kitajewski J. Transformation by Wnt family proteins correlates with regulation of beta-catenin. Cell Growth Differ. 1997;8:1349–1358. [PubMed] [Google Scholar]
- Narita T, Sasaoka S, Udagawa K, Ohyama T, Wada N, Nishimatsu S, Takada S, Nohno T. Wnt10a is involved in AER formation during chick limb development. Dev Dyn. 2005;233:282–287. doi: 10.1002/dvdy.20321. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Real-time PCR analysis. (A) Array layout of 96 well RT-PCR kit. (B) Heat map of PCR results between day 2 and 0 samples in differentiation medium. (C) Heat map of PCR results between day 4 and 0 samples in differentiation medium. Note: the scales are not identical in panels B and C.
Real-time PCR results (Day 2 vs control). Array results of real-time PCR analysis. n = 3 for the Day 2 group and n = 4 for the control group.
Real-time PCR results (Day 4 vs control). Array results of real-time PCR analysis. n = 3 for the Day 4 group and n = 4 for the control group.
Effect of Wnt expression on cell differentiation. C2C12 cells were transfected with Wnt cDNAs in pcDNA3.2 and cultured in proliferation medium for 2 days. Cells were fixed, troponin T immunostained and positive cell nuclei were counted. *P < 0.03 vs. control.
Time-lapse movie (control). The movie consists of micrographs taken at 5 min intervals for 16 h. Cells were transfected with an eGFP-expressing plasmid.
Time-lapse movie (Wnt4). The movie consists of micrographs taken at 5 min intervals for 16 h. Cells were transfected with a Wnt4-expressing plasmid.
Time-lapse movie (Wnt5a). The movie consists of micrographs taken at 5 min intervals for 16 h. Cells were transfected with a Wnt5a-expressing plasmid.
Time-lapse movie (Wnt3a). The movie consists of micrographs taken at 5 min intervals for 16 h. Cells were transfected with a Wnt3a-expressing plasmid.