Abstract
Depression in patients with mastocytosis is often reported but its prevalence and characteristics are not precisely described. In addition, the impact of therapies targeting mast cells proliferation, differentiation and degranulation on psychic symptoms of depression have never been investigated. Our objective was to determine the prevalence and to describe features of depression in a large cohort of mastocytosis patients (n = 288) and to investigate the therapeutic impact of the protein kinase inhibitor masitinib in depression symptoms. The description of depression was based on the analysis of a database with Hamilton scores using Principal Component Analysis (PCA). Efficacy of masitinib therapy was evaluated using non parametric Wilcoxon test for paired data within a three months period (n = 35). Our results show that patients with indolent mastocytosis present an elevated prevalence of depression (64%). Depression was moderate in 56% but severe in 8% of cases. Core symptoms (such as psychic anxiety, depressed mood, work and interests) characterized depression in mastocytosis patients. Masitinib therapy was associated with significant improvement (67% of the cases) of overall depression, with 75% of recovery cases. Global Quality of Life slightly improved after masitinib therapy and did not predicted depression improvement. In conclusion, depression is very frequent in mastocytosis patients and masitinib therapy is associated with the reduction its psychic experiences. We conclude that depression in mastocytosis may originate from processes related to mast cells activation. Masitinib could therefore be a useful treatment for mastocytosis patients with depression and anxiety symptoms.
Introduction
Mastocytosis is a rare disease characterized by mast cells (MC) accumulation in one or several organs [1]–[3]. Based on organ dysfunction, systemic mastocytosis is divided into indolent and aggressive disease [1], [4], [5]. In the majority of cases (>90%) mastocytosis presents as an indolent disease [6]. Even though mastocytosis is usually not a life threatening disease, indolent forms are associated with significant disability in more than 60% of patients [7]. As such, it can have a significant negative impact on quality of life.
Stem cell factor (SCF) is a growth factor that stimulates the proliferation, the survival and the development of mast cells [8]. The biological activity of SCF is induced following its binding to its receptor (SCF-R) encoded by the c-kit proto-oncogene. SCF-R/KIT (also called CD117) is a member of the type III receptor protein-tyrosine kinase family (TKR) [9]. Type III TKRs consist of a glycosylated extra-cellular ligand-binding domain followed by a transmembrane and a cytoplasmic domain which provides docking sites for adaptor proteins following receptor activation allowing cell signalling [10]. Gain of function mutations in c-kit result in constitutive KIT activation and are related to mastocytosis but also other neoplastic transformations including leukaemias, melanoma and others types of cancer [11], [12]. These mutations induce constitutive receptor autophosphorylation and its ligand-independent activity [13]. In most of adult forms of indolent systemic mastocytosis, KIT is constitutively activated as a consequence of D816V mutation, whereas in pediatric forms of mastocytosis extracellular and juxtamembrane mutations are more common [14], [15].
Treatment of mastocytosis is mainly symptomatic, except in aggressive and systemic forms with severe disabling symptoms. Masitinib is a phenylaminothiazole-type tyrosine kinase inhibitor that selectively targets KIT, platelet-derived growth factor receptor (PDGFR) and Lyn kinase [16]. Masitinib's inhibitory effect results in cell cycle arrest and apoptosis of cell lines dependent on KIT signaling [17]. In addition, masitinib inhibits constitutive activation of KIT as a result of juxtamembrane and extracellular mutations found in pediatric forms of mastocytosis. In contrast, activation of KIT as a consequence of D816V mutation is not inhibited by masitinib. However, by blocking Lyn masitinib may block mast cell degranulation and therefore improve symptoms related to mast cell degranulation. Thus, masitinib may have a cytotoxic effect and may reduce tumor burden in patients bearing pediatric's type mutations or wild type c-kit [16].
Although some reported cases of systemic mastocytosis with neurologic manifestations (neurosensory deafness, loss of consciousness, encephalopathy, hypoxic lesions leading to Parkinsonism), suggest central nervous system involvement, it is unsure whether it can be attributed to mast cell infiltration and/or mediators releasing [18]–[21]. Masitinib is a tyrosine kinase inhibitor (TKI) with exhibiting high affinity and selectivity in-vitro for KIT receptor and efficiency in vivo, because this molecule has been successfully tested in symptomatic patients with systemic mastocytosis [16], [22]–[24]. A phase 2a, multicenter open-label trial over 12 weeks using masitinib showed a strong impact reducing depression by 43% as compared with baseline [24]. This unexpected effect conveyed physician's attention to the links between physiopathological mechanisms inhibited by masitinib and depression in mastocytosis. Masitinib decreases mast cells burden and tryptase levels and may influence depression through mechanisms associated to one of these aspects.
Patients with mastocytosis present many psychopathological manifestations. These manifestations are mainly characterized by cognitive impairment (low attention span, difficulty concentrating and forgetfulness) and negative emotionality (depression, poor motivation, susceptibility to stress, irritability and anxiety). Depression seems to be the most common (sometimes the major) complaint among patients with mastocytosis and it has a profound impact in current professional, social and emotional life. Prevalence rates of depression among these patients range from 40% to 70% [7], [25], whereas prevalence rate is around 7% in the general population and 10%–50% in clinical samples with advanced chronic condition such as cancer or 14%–25% in advanced diabetes [26]–[30]. In addition, recent research in mastocytosis has found dissociation between the objective illness physical impact and depression suggesting that core aspects of depression in this condition are not secondary to the consequence of physical involvement but more a primitive feature of the disease [7].
One of the instruments commonly used to identify depression in patients in clinical trials (including those with mastocytosis) is the 17 items Hamilton Depression Scale (Ham-D17). However, the difficulty in using Ham-D17 to assess depression in mastocytosis may reside in the fact that patients frequently present a vast number of somatic symptoms including insomnia, muscular pain, headache, flush or gastro-intestinal disorders which could in some cases match with Ham-D17 items. Thus, mastocytosis patients presenting a large amount of somatic manifestations could have a high score in Ham-D17 without actually presenting core symptoms of depression such as depressive mood, psychic anxiety, psychomotor retardation or guilt. This means that when exploring depression and treatment effects in mastocytosis we first need to consider the core components of depressive symptoms. That is the reason why we first wanted to describe depression, its structure and symptoms in a large cohort of patients with mastocytosis. Secondarily, we intended to explore changes in depression components and symptoms following a masitinib treatment used to reduce clinical symptoms of mastocytosis.
Results
Features and prevalence of depression in mastocytosis patients (N = 288)
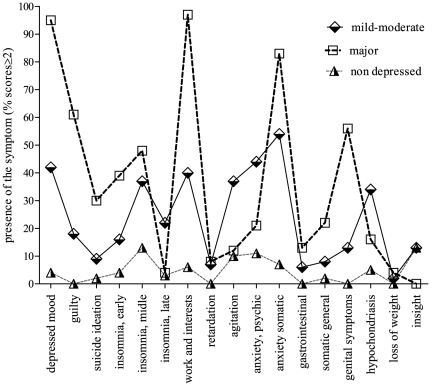
The prevalence rate of mild-moderate depression (Ham-D17 scores ≥ 8 and ≤22) in our sample (67% females; mean age = 47; standard deviation (S.D.) = 13.58) was 56% and severe depression (Ham-D17 scores ≥23) was observed in 8% of cases (n = 23). In order to know which Ham-D symptoms were mostly prevalent in the sample we analyzed the frequency of patients reporting scores ≥ 2 according to the severity of depression (Fig. 1). Patients presenting severe depression differed significantly from those presenting mild-moderate depression in all items scored ≥ 2 (p≤0.005) except the following: middle insomnia which was very prevalent in both groups (37%–48% respectively), psychomotor retardation, gastrointestinal symptoms and loss of weight which were rare in both groups (2%–3%). Severe depression was characterized by a very high prevalence of impairment in work and activities (97%), depressed mood (95%), somatic anxiety (83%) and guilt (61%). Genital symptoms (56%), early insomnia (39%) and suicide ideation (30%) were also very regular. General somatic symptoms (22%), psychic anxiety (21%) and hypochondriasis (16%) were less common in this group. Others symptoms were rare or only slightly present (less than 13%). When comparing these groups, patients with mild-moderate depression presented significantly (p≤0.0005) more late insomnia (22%), agitation (37%), psychic anxiety (44%) and hypochondriasis (34%).
Figure 1. Symptoms of depression from the Ham-D17 in non depressed, mild-moderate and severe depressed patients with mastocytosis.
Chi square test was performed to compare groups item by item. Severe depression was characterized by a huge prevalence of impairment in work and activities (97%), depressed mood (95%), somatic anxiety (83%), guilt (61%) and genital symptoms (56%). Mild-moderate depression was characterized by a higher prevalence of late insomnia (22%), agitation (37%), psychic anxiety (44%) and hypochondriasis (34%).
Depression in mastocytosis is composed by psychic depression symptoms and sleep disturbances
The Ham-D17 items of the final sample (N = 288) were submitted to a first Principal Component Analyses (PCA) and a promax rotation with a power of 4. Two stable components were found explaining 36.4% of the overall variance (27.96% and 8.38% respectively). Items with loadings under 0.40 were considered lacking sufficient explanation and were excluded from the dimensions. These two components were moderately correlated (r = 0.48). Based on this first PCA and excluding for items suicide and insight which had insufficient loadings, a two-factor solution appeared with a first dimension (items: depressed mood, feelings of guilt, work and activities impairment, psychomotor retardation, agitation, psychic anxiety, somatic anxiety, general somatic symptoms, genital symptoms and hypochondriasis) including most of the core symptoms of depression along with a dimension representing sleep and gastrointestinal disturbances (items: early, middle and late insomnia, gastrointestinal symptoms and loss of weight). To ascertain the consistency of these dimensions, we analyzed inter-item correlations and item-total correlations in each. In the first dimension most inter-item correlations ranged between 0.41 and 0.53. In the second dimension, only middle and late insomnia were correlated with correlations superior to 0.40 (r = 0.45; p<0.001). The following items had correlations under 0.40: early insomnia, psychomotor retardation, agitation, genital and gastrointestinal symptoms, hypochondriasis, loss of weight and loss of insight.
Based on the study of these correlations, we performed a second PCA with a promax rotation and a power of 4 including only the items with inter-item correlations exceeding 0.40 (depressed mood, guilt, middle and late insomnia, psychic and somatic anxiety, work and interests impairment and general somatic symptoms). This PCA resulted in a two components solution explaining 54.55% (41.4% and 13.15% respectively) of the overall variance and moderately correlated (r = 0.42). The first dimension included the psychic symptoms of depression: depressed mood, guilt, work and activities impairment and anxiety (psychic and somatic). We called this dimension “Anxious-depression”. The second dimension included the items middle and late insomnia and was called “Sleep disturbances” (Table 1). The correlation between this version of the scale and the 17 items version was 0.95 (p<0.001). Based on these results further investigations were done on these two components in addition to the traditional Ham-D17 items and score.
Table 1. Factor loadings after promax rotation.
| Hamilton depression items | Dimensions | |
| anxious-depression | Sleep disturbances | |
| depressed mood | 0.755 | 0.121 |
| Guilt | 0.657 | 0.151 |
| work and activities | 0.677 | 0.248 |
| psychic anxiety | 0.681 | 0.235 |
| somatic anxiety | 0.686 | 0.070 |
| general somatic symptoms | 0.610 | 0.121 |
| middle insomnia | 0.191 | 0.819 |
| late insomnia | 0.158 | 0.832 |
Two components solution explaining 54% of the overall variance and moderately correlated (r = .46) after exclusion of items with correlation loadings under .40. “Anxious-depression” dimension included the following items: depressed mood, guilt, work and activities and anxiety (psychic and somatic). “Sleep disturbances” dimension included the items middle and late insomnia. The Keiser-Meyer-Olkin measure of sampling adequacy revealed a score of 0.85 confirming the adequacy of the data for factor analysis.
Depression improved following masitinib therapy
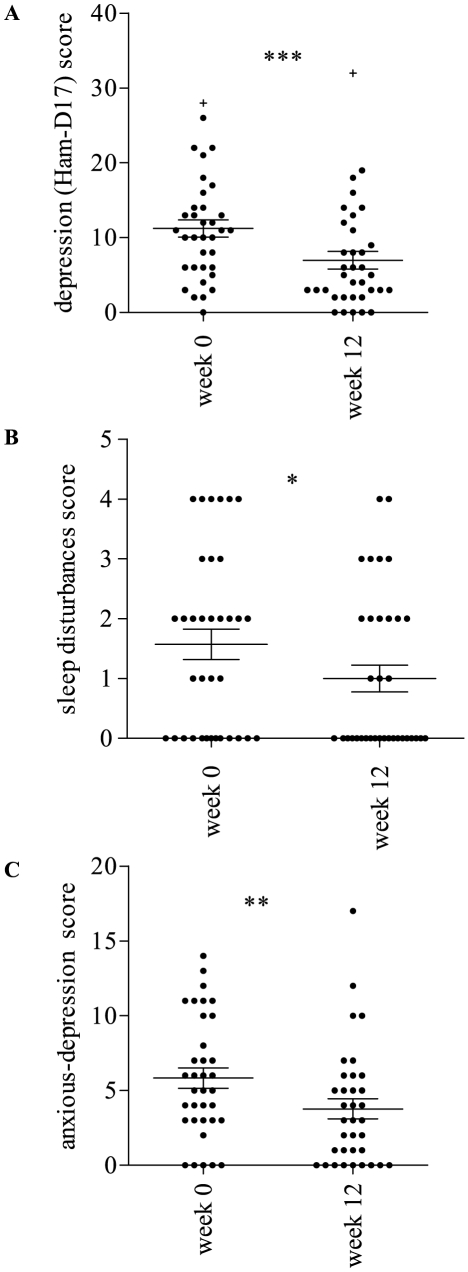
At the beginning of the trial (week 0) among 35 patients, 69% presented mild-moderate (n = 22, 92%) or severe (n = 2, 8%) depression. At the end (week 12), only one initially non depressed patient presented a mild depression score (Ham-D17 = 14) and 8 (33%) of the baseline depressed patients did not present any improvement (Table S1). Depression improvement was considered as a reduction of at least 20% of the initial score [31]. Changes in depression scores were significant (p = 0.0001, d = 0.63) and depression improvement concerned 67% (n = 16) of the cases (Fig. 2A). Among improved patients, 75% (n = 12) presented remission as defined by a score ≤7 [32]. Mean scores in anxious depression and sleep disturbances dimensions were also significantly reduced after masitinib therapy (p = 0.0004, d = 0.52 and p = 0.0112, d = 0.41 respectively) (Fig. 2B/C). In order to provide data comparable with pharmacological studies analyzing classical depression treatment response as with fluoxetine we tested response rate using similar depression baseline cut-point of Ham-D17≥16 and a decrease of at least 50% in final score to consider response to masitinib. Using these criteria, 8 patients (23%) were included in the depression group. The response rate was 50% and 25% displayed a remission score of HAM-D ≤7 (2 patients) (Table S1).
Figure 2. Depression improvement following masitinib therapy.
A. Mean total score in depression reduced significantly at the end of the trial (p = .0001); pre-mean Ham-D17 = 11.23 (S.D. = 6.8); post-mean Ham-D17 = 6.97 (S.D. = 6.9). B. Mean score in sleep disturbances dimension reduced significantly at the end of the trial (p = .0112); pre-mean Ham-D17 = 1.57 (S.D. = 1.5); post-mean Ham-D17 = 1.00 (S.D. = 1.3). C. Mean score in anxious depression dimension reduced significantly at the end of the trial (p = .0004); pre-mean Ham-D17 = 5.83 (S.D. = 4.0); post-mean Ham-D17 = 3.77 (S.D. = 3.9). + Exclusion of this patient from the analysis did not change the results. * (p≤0.05); ** (p≤0.0005); *** (p≤0.0001).
Depression improvement after masitinib therapy was not related to improvements in global quality of life
In order to know whether improvement of depression after masitinib therapy could be attributed to global quality of life (gQoL) improvement we investigated the relations between these evolutions. Global quality of life levels (mean week0 = 47.20; S.D. = 21.64, mean week12 = 58.35; S.D. = 20.73) slightly improved after mastinib therapy (p = 0.043, d = 0.53). Improvement in gQoL (defined as an increase of at least 20% in score) concerned 43% (n = 15) of patients; 17% (n = 6) were worsened and 40% (n = 14) did not display any changes. Among improved patients, only 4 presented an increase of at least 50% in gQoL scores. Baseline gQoL scores were negatively correlated to baseline scores in depression showing that a better global quality of life was related to lower depression scores at baseline (r = −0.471; p = 0.004). At the end of the trial, global gQoL was not correlated to depression scores (total and dimensions) (r = 0.077 to −0.287; ns). We computed relative change indices by dividing absolute raw changes by baseline raw values. Relative changes in gQoL were unrelated to relative changes in depression scores (r = −0.041; p = 0.815).
Discussion
In the present study we found in a sample of 288 patients with mastocytosis a high prevalence of depression (64%) and a significant improvement of depression following masitinib therapy.
Depression is a frequent comorbidity among patients with physical illness and it is associated to increased disability [33]. In this work we showed that patients with systemic indolent mastocytosis present an elevated prevalence of depression (64%) confirming and extending results from previous studies [7], [25]. However, this study was the first to evaluate the severity of depression in these patients. Our results show that depression in mastocytosis is mainly (56% of the cases) moderate whereas severe depression concerned 8% of depressed patients. This high prevalence is surprising because indolent forms of mastocytosis are not life threatening as is cancer and yet, depression prevalence rates are comparable or even higher than in this latter condition [26]–[28], [30].
We showed that depression among mastocytosis patients was predominantly characterized by anxious and depressive symptoms. Physical symptoms as gastrointestinal complaints, general symptoms and loss of weight were only slightly present in mastocytosis depressed patients. This suggests that depression among patients presenting indolent forms of the disease is rarely accompanied by appetite loss or anorexia. Consequently, ‘loss of weight’ was rarely scored. More surprising was the low prevalence of general somatic symptoms as they reflect pain (cephalic, muscular or articular) suggesting that these symptoms are often related to mastocytosis but not as much to depression among these patients. Psychomotor retardation and loss of insight were rare and should be considered as atypical symptoms in this population. Interestingly, hypochondriasis was more prevalent among mild-moderate depressed patients while genital complaints were more prevalent among severe depressed patients. This result suggests that severe depressed patients with mastocytosis are less concerned or at least less apprehensive about their bodily sensations and health state than mild-moderate depressed patients but are more impaired in sexuality.
Only 8% of patients presented a severe depression score. However, as might be expected, these severely depressed patients displayed a high frequency (30%–95%) of guilt, suicidal ideation and depressed mood. The high frequency of this group of symptoms is a warning for a risk of suicidal attempt that should be more seriously taken into account in severe depressed patients with mastocytosis as it is in people with other conditions. Our results suggest that depression in mastocytosis has a specific pattern different from those in other physical conditions. For example, in advanced diabetes, depression is characterized by cognitive/affective symptoms [34]. These very same symptoms were also associated with cardiovascular prognosis in patients with post-myocardial infarction [35]. In mastocytosis, depression does not seem to be related to physical related symptoms or severity and therefore should be considered as an endogenous manifestation.
In this study, we evaluated the response to masitinib without placebo condition among a cohort from an open-label trial. Indeed, masitinib is a tyrosine kinase inhibitor with a specific action on MC [16], [22]–[24]. Masitinib has already showed efficacy in the treatment of cutaneous mastocytosis [24]. Although in this study a placebo control was absent, our results showed impressive response rates for masitinib therapy on depression associated with mastocytosis. Masitinib treatment was associated with significant improvement in depression (67% of responders and 75% of remitted patients among responders). We also used different criteria for depression (Ham-D17≥16) and response (a decrease of at least 50% of scores in Ham-D17) and found a response rate of 50% and a remission rate of 25%. As a comparison, fluoxetine, a widely used antidepressant drug, has been associated with a response rate of 60% and a remission rate of 58% after 10 weeks of treatment in a sample of patients without mastocytosis, but with comparable baseline depression levels and same criteria for response [36]. These results bring evidence to anti-depressant effects of masitinib in the context of mastocytosis. However, more research is needed to confirm masitinib efficacy on depression using controlled trials and larger samples.
Quality of life in mastocytosis is often impaired because of the chronicity of some symptoms but most patients do not experience severe functional disabling. In our previous work we have shown that the impact of mastocytosis symptoms in quality of life was related to the patients' subjective perception of their own symptoms [7]. The masitinib efficacy in bringing relief to physical and clinical symptoms of mastocytosis has been demonstrated [24]. It has been shown in cancer or diabetic patients that poor quality of life is related to symptoms of depression and anxiety [29]. Therefore, we wanted to explore effects of masitinib in depression and if they were independent of gQoL. We showed that despite a relation between a deteriorated quality of life and depression at baseline, improvements in gQoL did not explain improvements in depression after masitinib therapy. This last result suggests that depression in mastocytosis does not improves in relation to a better QoL. This result is in line with our hypothesis that depression in mastocytosis represents a systemic neurological manifestation of the disease besides other physical symptoms. Instead, it suggests that improvements in depression could be influenced by the inhibitory effect of masitinib on mast cells activation and cerebral dysfunction underlining the systemic nature of depression in this condition. This strongly suggest that depression in mastocytosis is a primary systemic symptom of the disease rather than only a secondary psychological distress reaction and that masitinib could reduce neuropsychological symptoms by specifically targeting MC degranulation and migration.
We suggest that the configuration of depression in mastocytosis could be articulated into two dimensions: i. an “anxious-depression dimension” including the symptoms reflecting core cognitive, affective and somatic aspects of depression (depressed mood, guilt, work and interests impairments) and anxiety (somatic and psychic anxiety), and ii. a “sleep disturbances dimension” grouping sleep disturbances related to depression (middle and late insomnia). When exploring depression in mastocytosis, symptoms usually considered as the core of depression (psychic anxiety, depressed mood, work and interests) characterized depressed patients. In a subsample of 35 patients, Masitinib therapy was associated with significant improvement (50%–67% of the cases) of overall depression, with 25%–75% of recovery cases according to criteria for baseline depression and improvement. Response in depression symptoms after masitinib therapy was demonstrated through improvement of the anxious-depression dimension as well as of total scores. Global quality of life slightly improved after masitinib therapy but these changes did not predict depression improvement. These results suggest a specific MC involvement in psychological symptoms present in this disease.
This huge prevalence of depression suggests a systemic brain involvement probably through MC mediators such as serotonin, substance P or cytokines. Recent research suggested that mast cells are involved in mechanisms related to emotion regulation [37]–[39]. Mast cells are often located around vessels and it has been shown that, even if mast cells do not cross the blood-brain-barrier (BBB), these cells can be implicated in inflammatory process through mediators releasing that will increase permeability of the BBB [40]–[44]. Indeed, MC are involved in many other diseases including neuroinflammatory conditions such as irritable bowel syndrome, multiple sclerosis, asthma, atopic dermatitis, fibromyalgia, migraine, rheumatoid arthritis, scleroderma, etc [45]. In these conditions, negative emotionality such as anxiety is also common among patients [37]. This relation between MC and negative emotions (i.e. anxiety) has also been demonstrated in rodents suggesting a conservation of this relation through evolution and an involvement of MC in aspects of emotionality [39]. The presence of MC in brain regions; at the proximity of nerves and vessels, could sign a primary MC involvement in neurobiological determinants of depression [39], [46]–[48]. In the brain, many MC reside also in the thalamus [49]–[55]. Lesions or stimulation of the thalamus (medial dorsal and anterior nuclei) are associated with changes in emotional reactivity [53]–[55]. In addition, the regulation of emotional behavior is due to the connections of the nuclei of thalamus with other limbic system structures [56], [57]. We could hypothesize that masitinib could reduce depression symptoms in mastocytosis by two pathways: i. by blocking mediators secreted by mast cells involved in increasing permeability of the BBB and brain inflammation; ii. by reducing mast cells numbers in the thalamus or other structures of the limbic system through its direct effect on KIT and Lyn.
In this study we have analysed data from cohort of patients derived from a multicenter, open-label phase 1a and phase 2a trial. Therefore there was no group of patients with mastocytosis displaying depression which were not treated by masitinib to show that in this case depression scores remains stable between baseline and the end of the study. Secondly, although we used a validated measure of depression, this is not a structured diagnostic interview, which is usually considered as the gold standard to detect clinical depression. Further research should address these limitations and explore the link between MC and psychological manifestations, such as depression and anxiety in mast cell related diseases.
In conclusion, our results suggest that depression in mastocytosis may share common underlying processes related to the disease and/or MC Our results suggest that a pharmacological intervention, specifically targeting MC, alone brought a satisfactory improvement of depression. As it has been evidenced in other conditions such as obesity and diabetes, a systemic origin common to mastocytosis and depression could be searched for. In diabetes for example, a large part of depression has been suggested to be systemic rather than secondary because both conditions have been related to common underlying biological processes including inflammation processes [58], [59]. This may also be applicable to mastocytosis and would explain why we observe such a dramatic decrease of depression following masitinib. Furthermore, brain mast cells have been shown to evoke hypothalamic-pituitary-adrenal responses via centrally released histamine and corticotrophin-releasing factor [51]. Also, the hypersecretion of cortisol and the dysfunction of the hypothalamo-pituitary-adrenal axis seem to be implicated in medical co-morbidities associated with mood disorder [60]. The results underline the fact that masitinib not only affect mastocytosis physical symptoms as it has been shown [24], but also that it seem to favourably impact depression and anxiety symptoms, by targeting specifically MC.
Methods
Sample description (Table 2)
Table 2. Sample description.
| Afirmm protocol (N = 288) | |
| Gender | 98 men/163 women |
| Age in years (S.D.) – (range) | 47 (13.58) – (20–82) |
| Ham-D17 total score (S.D.) – (range) | 11.4 (7.7) – (0–35) |
S.D: standard deviation; Ham-D17: Hamilton depression scale-17 items.
We analyzed a database containing Ham-D17 scores for 288 patients identified by the Association Française pour les Initiatives et Recherche sur les Mastocytes et la Mastocytose (AFIRMM) and tested between 2003 and 2007. All patients had a proven diagnosis of mastocytosis following the WHO criteria [61]. From this large sample, 35 patients were included in a pharmacotherapeutical phase 1a (n = 25) and phase 2a (n = 25) multicenter, open-label trial to evaluate the response to masitinib in indolent mastocytosis with handicap, with a 3-month interval between the baseline (week 0) and final (week 12) tests [24]. We retained for our study only patients with complete measures in week 0 and week 12, thus 15 patients were excluded.
Measures
In the whole sample (N = 288), the following measures were available: age, gender, Hamilton scores (Table 2). With the exception of age, missing data represented less than 10% in this sample and an imputation was not necessary. In the follow-up study (masitinib protocols), additional measures of quality of life were taken (measured with the cancer quality of life questionnaire (EORTC-QLQ-C30 version 3).
The Hamilton Depression Scale
The Ham-D17 has been mainly used to assess depression in clinical trials and remains a reference measure to evaluate depression in research concerning somatic patients [62]. Ham-D17 is composed of 17 items scored 0-4 (depressed mood, guilt, suicide, psychic and somatic anxiety, psychomotor retardation, agitation, hypochondriasis, work and interests impairment) or 0–2 (early, middle and late insomnia, gastrointestinal, somatic general, genital, loss of weight and loss of insight items) according to the absence, presence and seriousness of the symptom. These items reflect two groups of symptoms: core symptoms of depression (depressed mood, guilt, work and interests impairment, psychic anxiety, psychomotor retardation and general somatic symptoms) and symptoms reflecting secondary aspects and related to cognitive (hypochondriasis, suicide thoughts, loss of insight, agitation), and somatic disturbances (somatic anxiety, gastrointestinal symptoms, genital symptoms, loss of weight, early, middle and late insomnia) [63], [64]. The total score of Ham-D17 is usually used as criterion to appreciate effect of treatment in clinical trials. However, the primary efficacy criterion should focus on the total score of core symptoms since they reflect the clinical response related to more typical aspects of depression [65]. Although the Ham-D17 was not initially designed as a self-report, Reynolds and Kobak developed a paper and pencil version with 17 items corresponding in contents and scoring to the standard Ham-D17 version and which showed excellent reliability [66].
Quality of life questionnaire
The European Organisation for Research and Treatment of Cancer Quality of Life Core Questionnaire (EORTC-QLQC30) is a cancer specific questionnaire, reporting health related quality of life in these patients [67]. It is a 30 item self-report instrument with Likert response scales whereby increasing scores indicate increasing burden for the functional and symptom scales. The questionnaire provides a quality of life “global score” (gQoL)” were an increased score indicates a good level of quality of life in addition to the functional and the symptoms scales.
Procedures
The study was approved by the local ethical committee of Necker hospital, patients were given informed consents and the study was carried out in compliance with the precepts of the Helsinki protocol. Data were collected through a mail-back procedure. Patients were sent questionnaires and had to mail back filled questionnaires within a week. For the follow-up subsample (protocols), patients were interviewed at baseline and at week 12 by a trained clinician.
Statistical analysis
SPSS version 17.0 0 (IBM SPSS Inc., Chicago, IL, USA) was used for most analysis. Wilcoxon and Chi-squared tests were performed using GraphPad Prism software version 5.01 (GraphPad Software Inc., San Diego, CA). To describe depression, we explored the structure of the Ham-D17 using Principal Component Analyses (PCA) and Chi-square tests. To explore the effect of therapy on depression symptoms, we compared pre-post scores and items using non parametric Wilcoxon test for paired data. To investigate the effects of quality of life on depression improvement after masitinib, we used correlation and linear regression. All reported p values are two tailed with a significance level of .05.
Supporting Information
Improvements in depression following masitinib therapy according to depression and improvement criteria used.
(DOCX)
Acknowledgments
The authors wish to thank Isabelle Hirsch, Anne-Florence Bellais, Dr. Marie-Olivia Chandesris, from the Centre de Référence des Mastocytoses and Cédric Baude from the Association Française pour les initiatives et la recherche sur les mastocytes et les mastocytoses for their help in collecting clinical data.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This research was funded by grants from the Cancéropôle Ile-de-France (Appel d'offre SHS 2009), the AFIRMM and the Fondation de la recherche médicale. In addition, part of this study was supported by AB Science, by grants from the Institut National du Cancer (2009, 2010 and 2011-SHS program) and start-up budgets from the CHU Sainte-Justine (Montréal, Qc). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Valent P, Horny HP, Escribano L, Longley BJ, Li CY, et al. Diagnostic criteria and classification of mastocytosis: a consensus proposal. Leuk Res. 2001;25:603–625. doi: 10.1016/s0145-2126(01)00038-8. [DOI] [PubMed] [Google Scholar]
- 2.Lennert K, Parwaresch MR. Mast cells and mast cell neoplasia: a review. Histopathology. 1979;3:349–365. doi: 10.1111/j.1365-2559.1979.tb03017.x. [DOI] [PubMed] [Google Scholar]
- 3.Metcalfe DD. Classification and diagnosis of mastocytosis: current status. J Invest Dermatol. 1991;96:2S–4S. [PubMed] [Google Scholar]
- 4.Valent P, Akin C, Escribano L, Fodinger M, Hartmann K, et al. Standards and standardization in mastocytosis: consensus statements on diagnostics, treatment recommendations and response criteria. Eur J Clin Invest. 2007;37:435–453. doi: 10.1111/j.1365-2362.2007.01807.x. [DOI] [PubMed] [Google Scholar]
- 5.Valent P, Akin C, Sperr WR, Horny HP, Metcalfe DD. Mast cell proliferative disorders: current view on variants recognized by the World Health Organization. Hematol Oncol Clin North Am. 2003;17:1227–1241. doi: 10.1016/s0889-8588(03)00089-3. [DOI] [PubMed] [Google Scholar]
- 6.Pardanani A, Akin C, Valent P. Pathogenesis, clinical features, and treatment advances in mastocytosis. Best Pract Res Clin Haematol. 2006;19:595–615. doi: 10.1016/j.beha.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 7.Hermine O, Lortholary O, Leventhal PS, Catteau A, Soppelsa F, et al. Case-control cohort study of patients' perceptions of disability in mastocytosis. PLoS ONE. 2008;3:e2266. doi: 10.1371/journal.pone.0002266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roskoski R., Jr Structure and regulation of Kit protein-tyrosine kinase—the stem cell factor receptor. Biochem Biophys Res Commun. 2005;338:1307–1315. doi: 10.1016/j.bbrc.2005.09.150. [DOI] [PubMed] [Google Scholar]
- 9.Roskoski R., Jr Signaling by Kit protein-tyrosine kinase—the stem cell factor receptor. Biochem Biophys Res Commun. 2005;337:1–13. doi: 10.1016/j.bbrc.2005.08.055. [DOI] [PubMed] [Google Scholar]
- 10.Tamborini E, Virdis E, Negri T, Orsenigo M, Brich S, et al. Analysis of receptor tyrosine kinases (RTKs) and downstream pathways in chordomas. Neuro Oncol. 2010;12:776–789. doi: 10.1093/neuonc/noq003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tests U. Kit mutations in cancer and their treatment with protein kinase inhibitors. Drugs Fut. 2008;33:161–174. [Google Scholar]
- 12.Lennarttson J, Jelacic T, Linnekin D, Shivakrupa R. Normal and Oncogenic Forms of the Receptor Tyrosine Kinase Kit. Stem Cells Dev. 2005;23:16–43. doi: 10.1634/stemcells.2004-0117. [DOI] [PubMed] [Google Scholar]
- 13.Letard S, Yang Y, Hanssens K, Palmerini F, Leventhal PS, et al. Gain-of-function mutations in the extracellular domain of KIT are common in canine mast cell tumors. Mol Cancer Res. 2008;6:1137–1145. doi: 10.1158/1541-7786.MCR-08-0067. [DOI] [PubMed] [Google Scholar]
- 14.Bodemer C, Hermine O, Palmerini F, Yang Y, Grandpeix-Guyodo C, et al. Pediatric mastocytosis is a clonal disease associated with D816V and other activating c-KIT mutations. J Invest Dermatol. 130:804–815. doi: 10.1038/jid.2009.281. [DOI] [PubMed] [Google Scholar]
- 15.Lanternier F, Cohen-Akenine A, Palmerini F, Feger F, Yang Y, et al. Phenotypic and genotypic characteristics of mastocytosis according to the age of onset. PLoS ONE. 2008;3:e1906. doi: 10.1371/journal.pone.0001906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dubreuil P, Letard S, Ciufolini M, Gros L, Humbert M, et al. Masitinib (AB1010), a potent and selective tyrosine kinase inhibitor targeting KIT. PLoS One. 2009;4:e7258. doi: 10.1371/journal.pone.0007258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horny HP, Valent P. Histopathological and immunohistochemical aspects of mastocytosis. Int Arch Allergy Immunol. 2002;127:115–117. doi: 10.1159/000048180. [DOI] [PubMed] [Google Scholar]
- 18.Boncoraglio GB, Brucato A, Carriero MR, Maccagnano E, Robbiolo L, et al. Systemic mastocytosis: a potential neurologic emergency. Neurology. 2005;65:332–333. doi: 10.1212/01.wnl.0000168897.35545.61. [DOI] [PubMed] [Google Scholar]
- 19.Pehlivanidis C, Fotoulaki M, Boucher W, Kempuraj D, Pang X, et al. Acute stress-induced seizures and loss of consciousness in a ten-year-old boy with cutaneous mastocytosis. J Clin Psychopharmacol. 2002;22:221–224. doi: 10.1097/00004714-200204000-00019. [DOI] [PubMed] [Google Scholar]
- 20.Schramm A, Grunewald S, Lorenz R, Classen J, Naumann M. Parkinsonism due to bilateral basal ganglia lesions following mastocytosis-induced hypoxia. J Neurol. 2004;251:1270–1272. doi: 10.1007/s00415-004-0505-1. [DOI] [PubMed] [Google Scholar]
- 21.Trevisan G, Pauluzzi P, Gatti A, Semeraro A. Familial mastocytosis associated with neurosensory deafness. J Eur Acad Dermatol Venereol. 2000;14:119–122. doi: 10.1046/j.1468-3083.2000.00018.x. [DOI] [PubMed] [Google Scholar]
- 22.Bellamy F, Bader T, Moussy A, Hermine O. Pharmacokinetics of masitinib in cats. Vet Res Commun. 2009;33:831–837. doi: 10.1007/s11259-009-9231-6. [DOI] [PubMed] [Google Scholar]
- 23.Hahn KA, Ogilvie G, Rusk T, Devauchelle P, Leblanc A, et al. Masitinib is safe and effective for the treatment of canine mast cell tumors. J Vet Intern Med. 2008;22:1301–1309. doi: 10.1111/j.1939-1676.2008.0190.x. [DOI] [PubMed] [Google Scholar]
- 24.Paul C, Sans B, Suarez F, Casassus P, Barete S, et al. Masitinib for the treatment of systemic and cutaneous mastocytosis with handicap: a phase 2a study. Am J Hematol. 2010;85:921–925. doi: 10.1002/ajh.21894. [DOI] [PubMed] [Google Scholar]
- 25.Rogers MP, Bloomingdale K, Murawski BJ, Soter NA, Reich P, et al. Mixed organic brain syndrome as a manifestation of systemic mastocytosis. Psychosom Med. 1986;48:437–447. doi: 10.1097/00006842-198607000-00006. [DOI] [PubMed] [Google Scholar]
- 26.Laugsand EA, Sprangers MA, Bjordal K, Skorpen F, Kaasa S, et al. Health care providers underestimate symptom intensities of cancer patients: A multicenter European study. Health Qual Life Outcomes. 2010;8:104. doi: 10.1186/1477-7525-8-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stiefel R, Die Trill M, Berney A, Olarte JM, Razavi A. Depression in palliative care: a pragmatic report from the Expert Working Group of the European Association for Palliative Care. Support Care Cancer. 2001;9:477–488. doi: 10.1007/s005200100244. [DOI] [PubMed] [Google Scholar]
- 28.Brintzenhofe-Szoc KM, Levin TT, Li Y, Kissane DW, Zabora JR. Mixed anxiety/depression symptoms in a large cancer cohort: prevalence by cancer type. Psychosomatics. 2009;50:383–391. doi: 10.1176/appi.psy.50.4.383. [DOI] [PubMed] [Google Scholar]
- 29.Sultan S, Hartemann-Heurtier A, Grimaldi A. [Understanding patients to promote self-regulation in Type 2 diabetes: how to live with an illness beginning before its onset?]. Diabetes Metab. 2003;29:S21–30. [PubMed] [Google Scholar]
- 30.Temur'iants NA, Shekhotkin AV, Martyniuk VS. [A role of various components of the diffuse neuroendocrine system in the realization of magnetobiological effects]. Biofizika. 2001;46:901–904. [PubMed] [Google Scholar]
- 31.Tadic A, Helmreich I, Mergl R, Hautzinger M, Kohnen R, et al. Early improvement is a predictor of treatment outcome in patients with mild major, minor or subsyndromal depression. J Affect Disord. 2010;120:86–93. doi: 10.1016/j.jad.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 32.Moller HJ. Methodological aspects in the assessment of severity of depression by the Hamilton Depression Scale. Eur Arch Psychiatry Clin Neurosci. 2001;251(Suppl 2):II13–20. doi: 10.1007/BF03035121. [DOI] [PubMed] [Google Scholar]
- 33.Lai CH. Major depressive disorder: gender differences in symptoms, life quality, and sexual function. J Clin Psychopharmacol. 2010;31:39–44. doi: 10.1097/JCP.0b013e318205a670. [DOI] [PubMed] [Google Scholar]
- 34.Sultan S, Luminet O, Hartemann A. Cognitive and anxiety symptoms in screening for clinical depression in diabetes: a systematic examination of diagnostic performances of the HADS and BDI-SF. J Affect Disord. 2010;123:332–336. doi: 10.1016/j.jad.2009.09.022. [DOI] [PubMed] [Google Scholar]
- 35.Martens EJ, Hoen PW, Mittelhaeuser M, de Jonge P, Denollet J. Symptom dimensions of post-myocardial infarction depression, disease severity and cardiac prognosis. Psychol Med. 2010;40:807–814. doi: 10.1017/S0033291709990997. [DOI] [PubMed] [Google Scholar]
- 36.Amsterdam JD, Shults J. Efficacy and mood conversion rate of short-term fluoxetine monotherapy of bipolar II major depressive episode. J Clin Psychopharmacol. 2010;30:306–311. doi: 10.1097/JCP.0b013e3181da5300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Addolorato G, Marsigli L, Capristo E, Caputo F, Dall'Aglio C, et al. Anxiety and depression: a common feature of health care seeking patients with irritable bowel syndrome and food allergy. Hepatogastroenterology. 1998;45:1559–1564. [PubMed] [Google Scholar]
- 38.Bienenstock J, Tomioka M, Matsuda H, Stead RH, Quinonez G, et al. The role of mast cells in inflammatory processes: evidence for nerve/mast cell interactions. Int Arch Allergy Appl Immunol. 1987;82:238–243. doi: 10.1159/000234197. [DOI] [PubMed] [Google Scholar]
- 39.Nautiyal KM, Ribeiro AC, Pfaff DW, Silver R. Brain mast cells link the immune system to anxiety-like behavior. Proc Natl Acad Sci U S A. 2008;105:18053–18057. doi: 10.1073/pnas.0809479105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bienenstock J. Relationships between mast cells and the nervous system. Revue Française d'Allergologie et d'Immunologie Clinique. 2002;42:11–15. [Google Scholar]
- 41.Black PH. Stress and the inflammatory response: a review of neurogenic inflammation. Brain Behav Immun. 2002;16:622–653. doi: 10.1016/s0889-1591(02)00021-1. [DOI] [PubMed] [Google Scholar]
- 42.Costa-Pinto FA, Basso AS, Russo M. Role of mast cell degranulation in the neural correlates of the immediate allergic reaction in a murine model of asthma. Brain Behav Immun. 2007;21:783–790. doi: 10.1016/j.bbi.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 43.Esposito P, Chandler N, Kandere K, Basu S, Jacobson S, et al. Corticotropin-releasing hormone and brain mast cells regulate blood-brain-barrier permeability induced by acute stress. J Pharmacol Exp Ther. 2002;303:1061–1066. doi: 10.1124/jpet.102.038497. [DOI] [PubMed] [Google Scholar]
- 44.Esposito P, Gheorghe D, Kandere K, Pang X, Connolly R, et al. Acute stress increases permeability of the blood-brain-barrier through activation of brain mast cells. Brain Res. 2001;888:117–127. doi: 10.1016/s0006-8993(00)03026-2. [DOI] [PubMed] [Google Scholar]
- 45.Theoharides TC, Cochrane DE. Critical role of mast cells in inflammatory diseases and the effect of acute stress. J Neuroimmunol. 2004;146:1–12. doi: 10.1016/j.jneuroim.2003.10.041. [DOI] [PubMed] [Google Scholar]
- 46.Theoharides T. Autism spectrum disorders and mastocytosis. Int J Immunopathol Pharmaco. 2009;22:859–865. doi: 10.1177/039463200902200401. [DOI] [PubMed] [Google Scholar]
- 47.Theoharides TC. Mast cells: the immune gate to the brain. Life Sci. 1990;46:607–617. doi: 10.1016/0024-3205(90)90129-f. [DOI] [PubMed] [Google Scholar]
- 48.Theoharides TC, Spanos C, Pang X, Alferes L, Ligris K, et al. Stress-induced intracranial mast cell degranulation: a corticotropin-releasing hormone-mediated effect. Endocrinology. 1995;136:5745–5750. doi: 10.1210/endo.136.12.7588332. [DOI] [PubMed] [Google Scholar]
- 49.Campbell DJ, Kernan JA. Mast cells in the central nervous system. Nature. 1966;210:756–757. doi: 10.1038/210756b0. [DOI] [PubMed] [Google Scholar]
- 50.Cirulli F, Pistillo L, de Acetis L, Alleva E, Aloe L. Increased number of mast cells in the central nervous system of adult male mice following chronic subordination stress. Brain Behav Immun. 1998;12:123–133. doi: 10.1006/brbi.1998.0505. [DOI] [PubMed] [Google Scholar]
- 51.Matsumoto I, Inoue Y, Shimada T, Aikawa T. Brain mast cells act as an immune gate to the hypothalamic-pituitary-adrenal axis in dogs. J Exp Med. 2001;194:71–78. doi: 10.1084/jem.194.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Paus R, Theoharides TC, Arck PC. Neuroimmunoendocrine circuitry of the ‘brain-skin connection’. Trends Immunol. 2006;27:32–39. doi: 10.1016/j.it.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 53.Bogousslavsky J, Regli F, Delaloye B, Delaloye-Bischof A, Assal G, et al. Loss of psychic self-activation with bithalamic infarction. Neurobehavioural, CT, MRI and SPECT correlates. Acta Neurol Scand. 1991;83:309–316. doi: 10.1111/j.1600-0404.1991.tb04708.x. [DOI] [PubMed] [Google Scholar]
- 54.Eclancher F, Karli P. [Combined lesions of ventro-medial hypothalamus and dorso-medial thalamus: effects on interspecific social behavior, emotional reactivity and feeding behavior in the rat]. C R Seances Soc Biol Fil. 1972;166:439–444. [PubMed] [Google Scholar]
- 55.Ng WX, Lau IY, Graham S, Sim K. Neurobiological evidence for thalamic, hippocampal and related glutamatergic abnormalities in bipolar disorder: a review and synthesis. Neurosci Biobehav Rev. 2009;33:336–354. doi: 10.1016/j.neubiorev.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 56.Krack P, Hariz MI, Baunez C, Guridi J, Obeso JA. Deep brain stimulation: from neurology to psychiatry? Trends Neurosci. 2010;33:474–484. doi: 10.1016/j.tins.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 57.Price JL, Drevets WC. Neurocircuitry of mood disorders. Neuropsychopharmacology. 2010;35:192–216. doi: 10.1038/npp.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dantzer R, Capuron L, Irwin MR, Miller AH, Ollat H, et al. Identification and treatment of symptoms associated with inflammation in medically ill patients. Psychoneuroendocrinology. 2008;33:18–29. doi: 10.1016/j.psyneuen.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dantzer R, Kelley KW. Twenty years of research on cytokine-induced sickness behavior. Brain Behav Immun. 2007;21:153–160. doi: 10.1016/j.bbi.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cowen PJ. Not fade away: the HPA axis and depression. Psychological Medecine. 2010;40:1–4. doi: 10.1017/S0033291709005558. [DOI] [PubMed] [Google Scholar]
- 61.Valent P HH-P, Escribano L, Longley BJ, Li CY, Schwartz LB, et al., editors. World Health Organization (WHO) Classification of tumours. Lyon, france: IARC Press; 2001. Mastocytosis (Mast cell disease). pp. 291–302. [Google Scholar]
- 62.Bech P, Engelhardt N, Evans KR, Gibertini M, Kalali AH, et al. Why the Hamilton Depression Rating Scale endures. Am J Psychiatry. 2005;162:2396; author reply 2397-2398. doi: 10.1176/appi.ajp.162.12.2396. [DOI] [PubMed] [Google Scholar]
- 63.Shafer AB. Meta-analysis of the factor structures of four depression questionnaires: Beck, CES-D, Hamilton, and Zung. J Clin Psychol. 2006;62:123–146. doi: 10.1002/jclp.20213. [DOI] [PubMed] [Google Scholar]
- 64.Guelfi JD, Dreyfus JF, Ruschel S, Blanchard C, Pichot P. [Factorial structure of the Hamilton depression scale. I.]. Ann Med Psychol (Paris) 1981;139:199–214. [PubMed] [Google Scholar]
- 65.Williams JB. Standardizing the Hamilton Depression Rating Scale: past, present, and future. Eur Arch Psychiatry Clin Neurosci. 2001;251(Suppl 2):II6–12. doi: 10.1007/BF03035120. [DOI] [PubMed] [Google Scholar]
- 66.Reynolds WM, Kobak KA. Reliability and validity of the Hamilton Depression Inventory: A paper-pendil version of the Hamilton Depression Rating Scale interview. Psychological Assessment. 1995;7:472–483. [Google Scholar]
- 67.Bjordal K, Kaasa S. Psychometric validation of the EORTC Core Quality of Life Questionnaire, 30-item version and a diagnosis-specific module for head and neck cancer patients. Acta Oncol. 1992;31:311–321. doi: 10.3109/02841869209108178. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Improvements in depression following masitinib therapy according to depression and improvement criteria used.
(DOCX)