Abstract
High-protein diets are effective in achieving weight loss which is mainly explained by increased satiety and thermogenic effects. Recent studies suggest that the effects of protein-rich diets on satiety could be mediated by amino acids like leucine or arginine. Although high-protein diets require increased intestinal amino acid absorption, amino acid and peptide absorption has not yet been considered to contribute to satiety effects. We here demonstrate a novel finding that links intestinal peptide transport processes to food intake, but only when a protein-rich diet is provided. When mice lacking the intestinal peptide transporter PEPT1 were fed diets containing 8 or 21 energy% of protein, no differences in food intake and weight gain were observed. However, upon feeding a high-protein (45 energy%) diet, Pept1−/− mice reduced food intake much more pronounced than control animals. Although there was a regain in food consumption after a few days, no weight gain was observed which was associated with a reduced intestinal energy assimilation and increased fecal energy losses. Pept1−/− mice on high-protein diet displayed markedly reduced plasma leptin levels during the period of very low food intake, suggesting a failure of leptin signaling to increase energy intake. This together with an almost two-fold elevated plasma arginine level in Pept1−/− but not wildtype mice, suggests that a cross-talk of arginine with leptin signaling in brain, as described previously, could cause these striking effects on food intake.
Introduction
Numerous studies have demonstrated that diets with a high protein content provide higher satiety levels (at least short term) than the other macronutrients [1], [2]. In addition, it has been shown that protein-rich diets can promote weight loss and cause changes in body composition [1]. Intake of dietary protein is sensed in the intestine with concomitant secretion of gastrointestinal hormones and activation of visceral processes that alter gastric motility, stimulate pancreatic secretion, mediate peripheral effects and contribute to satiety [3]–[5]. Mainly PYY and CCK but also insulin and leptin are discussed to play a prominent role in satiety control [6]–[8]. Yet, in most cases feeding high-protein diets revealed negligible effects on circulating levels of these hormones [9]–[11]. Recently, neuronal pathways in the brainstem nucleus of the solitary tract and hypothalamic arcuate nucleus were shown to be activated by high-protein diets [12]. Amongst the possible dietary signals for this hypothalamic sensing, the amino acid leucine has received particular attention. It was demonstrated that leucine contributes to food intake control via AMP-activated protein kinase and mammalian target of rapamycin when supplied to hypothalamic centers [13], [14]. Since plasma levels of branched chain amino acids (BCAA), including leucine, increase when dietary protein supply is increased and brain leucine levels follow plasma levels, a role of leucine in central regulation of satiety seems plausible. However conflicting results on the role of leucine were obtained in animal studies in which extra leucine was supplied by the diet to affect food intake and body weight [15], [16]. Additionally high-protein intake causes also major adaptations in metabolic processes in intestine and liver associated with changes in plasma levels of a variety of amino acids [17]. Although protein-rich diets challenge the digestive tract with large quantities of amino acids and short chain peptides for uptake into epithelial cells and into circulation, a contribution of intestinal transport processes to food intake control has never been anticipated.
Intestinal protein digestion delivers short chain peptides and free amino acids to epithelial cells. Amino acids are taken up through numerous amino acid transporters acting as symporters or antiporters [18]. For absorption of di- and tripeptides only one transport system in the intestine, designated as PEPT1 (SLC15A1) is known [19]. PEPT1 is a low-affinity but high-capacity transport system and handles essentially all possible protein-derived di- and tripeptides, but also a variety of peptidomimetics like aminocephalosporins and various prodrugs [20]. Peptide transport is electrogenic by charge movement as it involves the cotransport of protons [21]. PEPT1 in the intestine is subject to regulation by a variety of hormones and cytokines [22], but also by the dietary protein content. As demonstrated by Erickson et al, mRNA expression and transport rate of PEPT1 in rat intestine increases 1.5 to 2-fold when animals received a high-protein (50 energy%) diet as compared to a 4% protein diet [23].
Although the structure and function of PEPT1 has been studied in detail [21], its contribution to overall amino acid absorption is still unknown. The PEPT1-deficient model organism Caenorhabditis elegans showed reduced body size, impaired brood size and a retarded postembryonic development [24]. We recently reported that the lack of intestinal peptide transport in Pept1−/− mice is not compensated by changes in mRNA expression or transport capacity of intestinal amino acid transporters. Phenotyping of Pept1−/− mice did not reveal any impairments in reproduction, body weight or any other anthropometric or clinical chemistry measures when animals were fed a standard high-carbohydrate diet [25]. Yet, plasma concentrations of amino acids were increased in Pept1−/− when compared to Pept1+/+ mice, suggesting an altered systemic amino acid handling. Moreover, administration of an acute high protein load via gastric gavage also caused differences in plasma concentrations of several amino acids such as citrulline and arginine and most prominently of proline [26], [27]. Based on this finding, feeding trials with Pept1−/− animals with variations in dietary protein content were performed and we here report striking diet-dependent effects on food intake and weight gain that were further characterized by biochemical analysis. Taken together our findings suggest that the intestinal peptide transporter PEPT1 is part of a metabolic network that affects food intake particularly when high-protein diets are consumed.
Results
Effect of a high-protein diet on food intake and body weight in Pept1−/− and Pept1+/+ mice
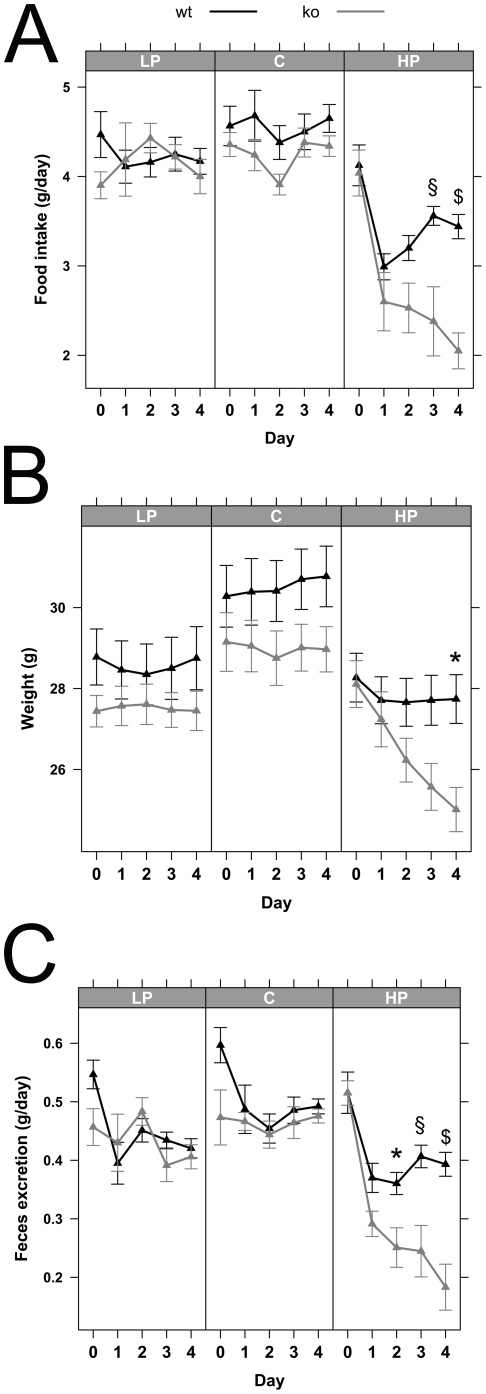
Determination of food intake and body weight changes in Pept1−/− mice fed for 5 days a LP or C diet did not show any significant alterations when compared to wildtype animals. However when animals were provided with a HP diet, food intake rates immediately declined in all animals but more pronounced in Pept1−/− animals (Figure 1). Whereas wildtype animals increased food consumption again after 2 days, Pept1−/− mice reduced food intake even further over 4 days (Figure 1A). This led in Pept1−/− animals also to a decrease in body weight (Figure 1B) and reduced feces excretion (Figure 1C). No differences in water consumption were observed, neither between diets nor genotypes (data not shown). A second feeding trial conducted over a 18 day period revealed a similar initial major reduction in food intake for 4 days in Pept1−/− mice while animals thereafter increased food consumption to reach the same intake rates as observed in wildtype animals (Figure 2A), yet, they failed to show any significant weight gain over the entire feeding trial (Figure 2B).
Figure 1. Pept1−/− animals on high-protein diet show major changes in metabolic parameters.
Metabolic parameters of male mice on low-protein (LP = 8% protein energy), control (C = 21% protein energy) and high-protein (HP = 45% protein energy) diet were determined in Pept1+/+ (wt) and Pept1−/− (ko) animals. During 5 days on the different diets, food intake (A), weight (B), and feces excretion (C) were determined (n = 10). Data are presented as mean±SEM. *P<0.05, $P<0.01, §P<0.001.
Figure 2. Food intake and body weight during feeding Pept1−/− animals a high-protein diet.
Food intake (A) and body weight (B) of male Pept1+/+ (wt) and Pept1−/− (ko) mice on control (C = 21% protein energy) or high-protein (HP = 45% protein energy) diet recorded over the 18 days feeding trial. Data are presented as the mean±SEM. *P<0.05, $P<0.01, §P<0.001.
Analysis of energy content in feces determined by bomb calorimetry revealed significantly higher energy losses in Pept1−/− than in Pept1+/+ animals. At day 5 on the HP diet energy density in feces accounted to 12.20 kJ/g in wildtype animals and 15.23 kJ/g (P<0.001) in Pept1−/− mice and these differences remained to the end of the experiment accounting at day 18 to 12.19 kJ/g feces in Pept1+/+ and 14.68 kJ/g feces (P<0.001) in Pept1−/− mice. In addition, energy balance calculations with the data from the 5 day feeding trial revealed that reduced energy assimilation and increased fecal caloric losses cause a difference of 71 kJ less energy available to PEPT1-deficient animals on the HP diet (Table 1).
Table 1. Energy assimilation in Pept1+/+ and Pept1−/− animals on low-protein (LP = 8% energy from protein), control (C = 21% energy from protein) or high-protein (HP = 45% energy from protein) diet.
| Group | Genotype | Sum of energy intake(kJ) | Sum of energy excretion (kJ) | Sum of energy assimilation (kJ) | Energy difference over 5 days (kJ) |
| LP | Pept1+/+ | 387.23 | 27 | 360.23 | 7.62 |
| Pept1−/− | 379.6 | 26.66 | 352.61 | ||
| C | Pept1+/+ | 409.98 | 29.83 | 380.15 | 28.36 |
| Pept1−/− | 382.08 | 30.29 | 351.79 | ||
| HP | Pept1+/+ | 351.53 | 25.16 | 326.37 | 71.05 |
| Pept1−/− | 276.08 | 20.76 | 255.32 |
The given values are the sum of the 5 day lasting feeding trial. Energy calculations of Pept1+/+ and Pept1−/− animals on low-protein (8% protein energy), control (21% protein energy) and high-protein (45% protein energy) diet for 5 days were analyzed (n = 10). Data are presented as sum of energy intake, energy excretion and energy assimilation over 5 days of feeding the diets.
Changes in plasma parameters in Pept1−/− mice
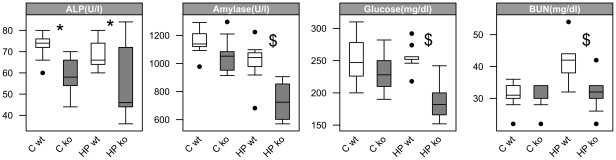
Clinical chemistry data of mouse plasma collected after 5 days revealed lower alkaline phosphatase levels in Pept1−/− animals, both on C and HP diet. In addition Pept1−/− mice, when fed the HP diet displayed also significantly decreased levels of amylase and glucose. In contrast, wildtype animals on HP diet showed higher blood urea nitrogen levels when compared to mice on C diet (HP: 41.8±6.2 mg/dl vs. C: 31±4.1 mg/dl, P<0.001), whereas blood urea nitrogen levels in Pept1−/− animals on HP diet were comparable to those on C diet (HP: 31.8±5.6 mg/dl vs. C: 30.4±3.8 mg/dl; P<0.001). These data are summarized in Figure 3. When blood urea nitrogen levels were determined after 18 days, genotype effects were no longer detectable and enyzme activities did also not show any more differences (data not shown). Yet, independent of genotype, mice on the HP diet displayed significant elevated blood urea nitrogen concentrations (HP: 44±4.3 mg/dl; vs. C: 26±3.6, mg/dl; P<0.001).
Figure 3. Decreased activities of enzymes and glucose in plasma of Pept1−/− animals.
Enzyme and metabolite activities in plasma of Pept1+/+ (wt) and Pept1−/− (ko) animals fed a control (C = 21% protein energy) or high-protein (HP = 45% protein energy) diet were analyzed. Alkaline phosphatase (ALP), amylase, glucose and blood urea nitrogen (BUN) were determined after 5 days on the high-protein diet (n = 10). Data are presented as mean±SD. (•) indicate outlier. *P<0.05, $P<0.01, §P<0.001.
High-protein diet alters plasma levels of amino acids and derivatives
To assess alterations in systemic amino acid levels of mice fed diets with different protein contents, we analyzed plasma samples by LC-MS/MS. As a highly consistent and genotype-specific finding, we observed an almost two-fold increase in plasma levels of arginine in Pept1−/− mice on both, C (ko: 90±29.8 µmol/l vs. wt: 44.8±22.8 µmol/l, P = 0.002) and HP diet (ko: 96.9±42.6 µmol/l vs. wt: 53.1±19.1 µmol/l, P = 0.008) after 5 days (Table S1). Genotype-independent effects of the diets were found for valine, leucine, isoleucine, but also for alpha-aminobutyric acid and glutamine with significantly increased levels in plasma obtained from animals on the HP diet. Decreased plasma levels were found for anserine and hydroxyproline. After 18 days of feeding, alpha-aminobutyric acid levels as well as leucine and isoleucine levels remained increased (Table S2) whereas glutamine and ethanolamine showed higher levels in animals on control than on HP diet irrespective of genotypes. Representative plasma amino acid levels at days 5 and 18 when feeding the HP diet are summarized in Figure 4.
Figure 4. Changed plasma amino acids after 5 and 18 days on high-protein diet in Pept1−/− animals.
By LC-MS/MS plasma amino acid concentrations of Pept1+/+ (wt) and Pept1−/− (ko) animals a high-protein (45% protein energy) diet were analyzed (n = 3–10). Plasma amino acid profiles of arginine (Arg), leucine (Leu) and the sum of all detectable amino acids (Sum) of Pept1−/− (ko) and Pept1+/+ (wt) animals on high-protein (HP) diet for 5 and 18 days are depicted. Data are presented as mean±SD. (•) indicate outlier. *P<0.05, $P<0.01, §P<0.001.
Altered hepatic amino acid levels and enzyme activities in Pept1−/− animals
Amino acid levels and enzyme acitivities in liver tissue samples were determined and revealed that after 18 days of feeding the HP diet, 10 out of 28 quantified metabolites were changed (Table S3) with significantly decreased concentrations of methionine, citrulline and taurine but increased levels of leucine, tyrosine, serine, asparagine, ethanolamine, gamma-aminobutyric acid and the urea cycle intermediate ornithine. However, as shown in Table 2, neither urea levels in liver nor hepatic enzyme activities revealed any genotype- or diet-specific effects.
Table 2. Enzyme activities and urea levels in liver tissue of Pept1+/+ and Pept1−/− animals after feeding a high-protein diet (45% energy from protein) for 18 days.
| Parameter | Control | High-protein | ||
| Pept1+/+ | Pept1−/− | Pept1+/+ | Pept1−/− | |
| GDH (mU/mg) | 752.3±109.8 | 738.0±79.8 | 708.7±82.7 | 955.8±119.2 |
| AST (mU/mg) | 2422.7±338.2 | 2475.1±560.2 | 1965.8±189.9 | 2141.8±351.2 |
| ALT (mU/mg) | 784.3±45.7 | 797.2±18.3 | 623.4±19.4 | 643.2±65.0 |
| Urea (µmol/mg) | 13.9±0.9 | 10.0±0.9 | 16.1±2.1 | 15.0±4.0 |
Enzyme activities of glutmate dehydrogenase (GDH), alanine aminotransferase (AST), alkaline aminotransferase (ALT) as well as urea levels were determined in liver tissue collected from Pept1+/+ and Pept1−/− animals kept on standard diet (21% protein energy) and high-protein (45% protein energy) diet. All data are presented as mean ± SD (n = 3 animals). P-value was obtained by unpaired Student's t-test.
Concentrations of 14N and 15N-lableled amino acids in portal blood after 15N-protein gavage
To determine whether any changes in intestinal amino acid absorption and/or metabolism can be detected, portal vein plasma of Pept1+/+ and Pept1−/− mice was analyzed by LC-MS/MS. Plasma samples were obtained 15 and 30 min after administration of a 15N-labeled yeast protein extract provided by gavage. For 14N-labeled amino acids differences between genotypes were only detectable for lysine after 15 min (ko: 294.8±22.1 µmol/l vs. wt: 197.6±42.2 µmol/l, P = 0.002) and arginine after 30 min (ko: 36.6±4.1 µmol/l vs. wt: 25.1±7.6 µmol/l, P = 0.02). For 15N-labeled amino acids in portal blood only isoleucine showed slightly reduced concentrations with a genotype-specific effect at 15 min (ko: 29.8±4.2 µmol/l vs. wt: 36.6±3.8 µmol/l, P = 0.03) but none of the other amino acids. Taken together, neither levels of 14N nor 15N-labeled amino acids determined in portal blood of Pept1+/+ and Pept1−/− mice revealed remarkable differences (Figure S1) that could have indicated altered amino acid delivery to circulation.
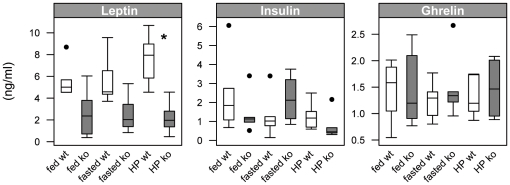
Decreased leptin levels in Pept1−/− animals
To assess whether selected hormones or adipokines revealed changes that could be associated with the differences in food intake, ghrelin, insulin and leptin levels were determined. As shown in Figure 5, neither ghrelin nor insulin levels changed, whereas leptin levels differed significantly between Pept1+/+ and Pept1−/− mice. Plasma leptin levels dropped significantly in PEPT1-deficient animals on the HP diet after 24 h to 2.2±1.4 ng/ml as compared to wildtype animals with 7.6±2.4 ng/ml (P = 0.001). This effect, yet not reaching significance was also observed on control diet in both, the fed (ko: 2.6± 2.1 ng/ml vs. wt: 5.6±1.6 ng/ml) and the fasted state (ko: 2.5±1.7 ng/ml vs. wt: 5.7±2.4 ng/ml).
Figure 5. Analysis of plasma hormone levels reveals lower leptin concentrations in Pept1−/− animals.
Plasma samples of fasted (6 h fasting period) and non-fasted Pept1+/+ (wt) and Pept1−/− (ko) animals fed a control (C = 21% protein energy) diet or a high-protein (HP = 45% protein energy) diet were analysed after 24 h for changes in ghrelin, leptin and insulin levels (n = 6 animals). Data are presented as mean±SD. (•) indicate outliers. *P<0.05, $P<0.01, §P<0.001.
Discussion
Energy intake is strongly affected by diet composition and numerous studies in animals and humans have demonstrated that protein-rich diets initiate higher satiety levels than other macronutrients [1], [28]–[31]. Amongst the various mechanisms proposed to mediate this effect (for review see Thomé [32]) two hypotheses have recently received considerable attention. One involves the hypothalamic sensing of the increased leucine availability on high-protein diets, the other is based on an increased intestinal gluconeogenesis sensed in the portal system and transmitted via afferences of the vagus nerve to brain. We here provide evidence that intestinal transport mechanisms for nutrients involving the intestinal peptide transporter PEPT1 participate in the control of food intake on a high-protein diet. Only in response to a high-protein (45 energy%) diet, Pept1−/− animals showed a much more pronounced reduction in food intake than wildtype animals. Although Pept1−/− mice increased food intake again after 5 days on the HP diet with a similar daily caloric intake as wildtype animals, body weight reduction in Pept1−/− animals sustained, whereas wildtype animals increased body weight after a few days. In parallel to the decline in food intake, body weight decreased with a regain after 4 to 5 days when food intake increased again. Despite very similar food and thus caloric intake after the regain, a significantly increased energy loss in feces on all diets and in particular on the HP diet in Pept1−/− mice was observed. This suggests that energy assimilation in the intestine is modestly impaired and that the lack in weight gain on the high-protein diet, despite similar food intake rates as in control animals after 5 to 6 days is a consequence of this. However, the data also suggest that Pept1−/− mice are unable to regulate energy intake adequately for preventing weight loss or for increasing body weight after the regain of food intake. To assess whether hormones that a part of satiety sensing are altered in PEPT1-deficient mice we analyzed non-fasting and fasting plasma ghrelin and insulin levels but did not observe any differences, neither between diets nor genotypes. In contrast, leptin, a key signaling molecule in energy homeostasis and regulation of food intake [33] displayed significantly decreased plasma levels in Pept1−/− animals on the high-protein diet. Leptin levels generally follow fat mass changes but also decline quickly following food deprivation. Our data suggest that a PEPT1-deficiency could antagonize leptin actions as low plasma leptin levels should lead to an increase in food intake. Most interestingly, leptin was shown to regulate PEPT1 expression and to alter its transport capacity in the intestine [34]–[36] arguing that amino acid absorption in the intestine mediated by PEPT1 could be part of the body's response to changes in leptin levels that participate in control of energy homeostasis.
Various studies have proposed that signalling processes initiated in the portal vein and transmitted from there via afferences of the vagus nerve to brain may provide satiety signals [12], [32], [37]. Such a mechanims was also suggested for the hypophagic effects of high-protein diets via an increased intestinal gluconeogenesis rate and elevated portal glucose concentrations in rats [38]–[40]. However, these findings have been challenged by a lack of evidence for a significant intestinal gluconeogenesis from the stable-isotope labeled glucogenic amino acid glutamine in two strains of fasted rats [41]. This confirmed other studies, that could also not demonstrate a significant intestinal glucose production in rats fed a high-protein diet [42]. We recently reported that there were essentially no differences in the apperance rates of amino acids in the portal vein and peripheral plasma between wildtype and PEPT1-deficient animals [26]. We also administered a 15N-labeled yeast extract to wildtype and PEPT1-deficient animals by gavage and here report the extended analysis of samples for stable isotope labeled amino acids in portal blood with emphasis on glucogenic and non-proteinogenic amino acids. Since there were no detectable differences between genotypes in any of the relevant amino acids we conclude, that despite the questionable role of intestinal gluconeogenesis, this process seems not to account to the satiety effect of the high-protein diet in PEPT1-deficient animals reported here.
In addition to peptide uptake, mediated by PEPT1, a large number of apical and basolateral amino acid transporters contribute to amino acid delivery into systemic circulation (for reviews see [18], [43]). After portal delivery, selective hepatic extraction of amino acids allows only some of the 20 proteinogenic amino acids to change in peripheral blood in the absorptive phase. Amongst them are the BCAA as well as the aromatic amino acids. Especially the BCAA leucine has been proposed to contribute to satiety control. Administration of leucine into hypothalamic regions in rats was shown to increase brain mTOR signaling leading to a decrease in food intake and body weight suggesting that low leucine levels in brain may initiate mechanisms that stimulate food intake whereas high levels can increase satiety [13]. However, this finding contradicts with clinical experiences in various human diseases in which supplementing BCAA in patients generally caused an increase in food intake [44], [45]. Moreover, a supplementation of leucine in a diet with otherwise normal protein content failed to show in mice any effect on food intake or weight gain [46]. When provided in drinking water to mice fed either a control or a high-fat diet, leucine supplementation did also not reveal effects on food intake but improved some metabolic parameters in animals on the high-fat diet [47]. In the present study plasma amino acid profiling in animals on high-protein or control diet after 5 or 18 days revealed that plasma BCAA and in particular leucine levels increased significantly on the high-protein diet (P = 0.02) without differences by genotype. This means that despite almost identical plasma leucine levels, Pept1−/− mice reduced food intake much more pronounced than wildtype animals over the first 5 days of feeding the HP diet, suggesting that increased plasma leucine per se cannot account for the effects of HP diets on satiation.
To assess whether any changes in liver metabolism can be associated with the diet effects in PEPT1-deficient animals we analyzed hepatic amino acid levels and enzyme activities. Protein-rich diets require increased nitrogen elimination from amino acid oxidation which is achieved by an increased delivery of nitrogen to liver via glutamine, glutamate and alanine, an enhanced urea cycle flux and increased renal excretion of urea. Blood urea concentrations increased as expected in animals fed the HP diet but were significantly lower in Pept1−/− animals (Fig. 3), while liver urea levels did not reveal any genotype effect despite the diet-effects. A recent proteome analysis of hepatic proteins in mice fed a normal or a high-protein diet [48] identified carbamoylphosphate synthetase 1 and ornithine aminotransferase with increased protein levels, suggesting that the increased ornithine demand is achieved from proline while ornithine-transcarbamoylase-activity may limit efficient urea cycle flux. We here observed that citrulline levels in liver tissue declined while ornithine levels increased upon high-protein feeding, but without a genotype-specific effect. Although liver arginine was below detection limit, Pept1−/− mice displayed significantly increased plasma arginine levels on both, control as well as high-protein diet at days 5 and 18. Plasma citrulline levels were also significantly higher in Pept1−/− animals at day 5 but no longer at day 18. Although portal blood arginine and citrulline levels did not reveal any genotype-specific differences, arginine concentrations in portal blood were much lower than those in peripheral blood. This suggests that the unusual high plasma levels of arginine in Pept1−/− mice originate most likely from an increased renal arginine production from citrulline. In this respect it is of interest that dietary L-arginine supplementation (but not D-arginine) was shown to reduce food intake in mice and this was attributed to altered nitric oxide (NO) levels in brain [49]. Since brain arginine concentrations were shown to change almost proportional to plasma arginine levels [50], it is suggested that Pept1−/− mice may have also an increased arginine level in brain. Most interestingly, delivery of arginine to brain was shown to antagonize the leptin effects on food intake in mice with a prominent regain of food consumption when co-administered centrally with leptin [51]. Moreover, this effect was abolished in NO-Synthase (nNOS) deficient mice and nNOS is shown to be upregulated by leptin [52] and down-regulated by food deprivation [53]. A similar effect of leptin on brain NO production and food intake has also been shown in chicken [54], [55]. Since we observed low leptin levels in Pept1−/− mice only when animals received the HP diet and when food intake was markedly reduced while animals had almost two-fold higher plasma arginine levels than wildtype controls, it is tempting to speculate that the leptin-NOS axis in brain contributes to the changes in food intake on protein-rich diets. Low leptin levels in PEPT1-deficient mice on the HP diet most likely arise from the very low food intake and the apparent ‘starvation condition’. Plasma leptin levels are known to decrease radiply upon starvation, yet, low leptin levels should increase food intake and this response could be antagonized by the high plasma arginine levels in Pept1−/− mice. Assuming in analogy to previous studies that the low leptin levels in Pept1−/− mice and the food deprivation cause major changes in NOS levels [51], [53], then NO production could in spite of the high arginine levels, be reduced. This would be in line with the reported anorexic effects of inhibitors of NOS [56], [57]. Although we cannot provide conclusive evidence for this hypothesis, our findings call for future studies employing agonist and antagonists to alter brain NO pathways to assess whether high plasma arginine levels in mice lacking PEPT1 indeed prevent proper leptin signalling for an adaptive increase in food intake on protein-rich diets.
In summary, we have demonstrated that Pept1−/− animals on a high-protein diet show a severe reduction in food intake compared to wildtype animals that lasts for 5 to 6 days. Thereafter a regain in food intake is observed but despite almost identical caloric intake rates like the control animals, Pept1−/− mice do not show a weight gain. This is probably a consequence of a yet unexplained reduction in energy assimilation with an increased fecal energy loss most pronounced on a high-protein diet. As a striking finding we observed significantly elevated plasma arginine levels in Pept1−/− mice on both, control and HP diet and a markedly reduced plasma leptin level only on the HP diet. Previous studies suggested a cross-talk of leptin with the arginine-dependent NO system in brain as part of the hypothalamic control loops that affect food intake. Based on our data we hypothesize that during the food intake reduction phase, the orexigenic action of low leptin levels is blunted in PEPT1-deficient animals in association with the constitutively increased plasma and possibly also brain arginine levels. Further studies in Pept1−/− mice with high-protein diets and extra injections of leptin or administration of NOS antagonists could help to identify the mechanims underlying these genotype and diet-specific effects described here.
Materials and Methods
Animals
Mice lacking PEPT1 were created by targeted disruption of the Pept1 gene and obtained from Deltagen (San Mateo, California, USA) [25]. Animals were backcrossed for 10 generations to C57BL/6J background and maintained at 22±2°C and a 12∶12 h light/dark cycle. All procedures were conducted according to the German guidelines for animal care and approved by the state of Bavaria (Regierung von Oberbayern) ethics committee (Reference number: 55.2-1-54-2531-140-08).
Study design of feeding trials for 5 or 18 days
For the feeding studies Pept1+/+ and Pept1−/− animals (n = 10, per diet and genotype) received semi-synthetic purified diets with low (8% of energy; Ssniff E15202, Ssniff, Germany), medium (21% of energy; Ssniff E15000) or high (45% of energy; Ssniff E15209) protein content. Protein was isoenergetically exchanged for starch. Mice were kept individually to allow recording of food intake and water consumption. Throughout the study, mice had access to tap water and food ad libitum. To allow for adaptation, animals received the medium protein diet for 3 days. In the following 5 days, mice had free access to either low-protein (LP), medium = control (C) or high-protein (HP) diet. Body weight, food and water consumption were monitored daily between 8:00 and 9:00 a.m. Feces was collected daily. On the last day, blood was collected into Li-Heparin coated tubes (Sarstedt, Nümbrecht, Germany) by puncturing the retro-orbital sinus under isoflurane anaesthesia. In the second feeding trial, lasting 18 days (n = 3), only the C and HP diet were given and metabolic parameters were measured daily. Every 5 days, fecal samples were collected and on day 18 plasma and tissue samples were taken.
Plasma amino acid analysis
By liquid chromatography-tandem mass spectrometry (LC-MS/MS) (3200QTRAP LC/MS/MS, Applied Biosystems, USA) with iTRAQ labeling for quantification, amino acids and derivatives in plasma using the AA45/32 Kit, according to the manufacturer's instructions (Applied Biosystems, USA), were determined. The data obtained were analyzed using the Analyst® 1.5 Software.
Analysis of portal blood for labeled amino acids derived from an oral 15N-protein administration
Analysis of portal blood and tissue samples from jejunum, duodenum and ileum collected from Pept1+/+ and Pept1−/− mice after administration of a 15N-yeast extract to mice via intragastric gavage have been described previously [26]. Briefly, mice (n = 6, per group and genotype) received 8.83 mg 15N-labeled yeast protein by gavage. After 15 and 30 min blood was collected from portal vein and plasma samples were analyzed for labeled and non-labeled amino acids via LC-MS/MS.
Measurement of AST, ALT and GDH activities and urea
In liver samples of mice receiving the C or the HP diet, enzyme activities of aspartate aminotransferase (AST), alanine aminotransferase (ALT) and glutamate dehydrogenase (GDH) were analyzed. Samples were homogenized with 0.9% NaCl and centrifuged (15 min, 14000 g). Supernatant was diluted 1∶100 and enzyme activities were determined with the GPT (ALT) liquidUVTest #12012 and GOT (AST) liquiUV Test #12011 (both Human Gesellschaft für Biochemica und Diagnostica, Wiesbaden, Germany) and GDH FS #G82100 (Rolf Greiner BioChemica GmbH, Flacht, Germany) according to the manufacturer's instructions. Urea levels were analyzed from supernatant without dilution with the Urea Liquicolor Test #10505 (Human Gesellschaft für Biochemica und Diagnostica, Wiesbaden, Germany) according to the manufacturer's instructions.
Clinical chemistry of plasma samples
A multiple analyte panel by the Piccolo xpress™ Chemistry analyzer (Abaxis, California, USA) was used for quantitative determination of albumin, alkaline phosphatase (ALP), amylase, aspartate aminotransferase (AST), calcium, creatinine, gamma glutamyltransferase (GGT), glucose, total bilirubin, total protein, blood urea nitrogen (BUN), and uric acid in plasma samples.
Hormone levels in plasma
In plasma of Pept1+/+ and Pept1−/− animals (n = 10), basal hormone levels in fed and fasted state (fasting period: 6 h) on C diet and after feeding the HP diet for 24 h were determined. Samples were collected by puncturing the retro-orbital sinus under isoflurane anaesthesia. Blood was collected in either Li-Heparin coated tubes for insulin and leptin measurements, or in EDTA-coated tubes (both Sarstedt, Nümbrecht, Germany) for ghrelin determination. For ghrelin measurement, Pefabloc® SC (Sigma-Aldrich, Steinheim, Germany) was added for a final concentration of 1 mg/ml. Insulin levels were determined using the Ultra Sensitive Mouse Insulin ELISA Kit and leptin levels using the Mouse Leptin ELISA Kit (both Crystal Chem Inc., Illinois, USA). Ghrelin levels were assayed by the Rat/Mouse Ghrelin (active) ELISA Kit (Millipore GmbH, Schwalbach, Germany).
Bomb calorimetry of feces samples
Gross energy content of feces was determined using a bomb calorimeter (Parr 6300 Calorimeter, Parr Instrument Co., Illinois, USA). Due to the low amount of feces in the feeding studies, dried feces samples were pooled (per groups) and grinded with a pebble mill (TissueLyser, Qiagen, Hilden, Germany), pressed to a pellet (∼1 g of feces) and analyzed by a bomb calorimeter.
Statistical analysis
Statistical analysis was performed using R 2.8 (R Foundation of Statistical Computing [58]) and GraphPad Prism 4.01 (GraphPad Software, California, USA). One-way or two-way ANOVA and Tukey-test or unpaired Students t-test were used to test for statistical significance. Data are presented as mean±SD unless stated otherwise.
Supporting Information
Amino acid appearance in plasma after administration of a low dose of 15N-labeled protein. Analysis of 14N and 15N labeled amino acids from plasma of portal vein 15 (A) and 30 min (B) after administration of 15N-labeled protein (8.83 mg) by gavage in 12h-fasted Pept1+/+ (wt) and Pept1 knockout (ko) animals (n = 5 animals per timepoint and genotype). Cit, citrulline; Sum, sum of all measured amino acids. (•) indicate outlier. Data are presented as mean ± SD. *P<0.05, $P<0.01, §P<0.001.
(TIF)
Plasma amino acid concentrations of male Pept1+/+ and Pept1−/− animals after 5 days on control or high-protein diet. By LC-MS/MS plasma amino acid concentrations of Pept1+/+ and Pept1−/− animals on control (21% energy from protein) or high-protein (45% energy from protein) diet for 5 days were analyzed (n = 10). Data shows all analyzed amino acids plus sum of all amino acids.
(DOC)
Plasma amino acid concentrations of male Pept1+/+ and Pept1−/− animals on control or high-protein diet for 18 days. After feeding a control (21% energy from protein) or high-protein (45% energy from protein) diet for 18 days plasma amino acid concentrations of Pept1+/+ and Pept1−/−animals were analyzed by LC-MS/MS (n = 3). Data shows all analyzed amino acids plus sum of all amino acids.
(DOC)
Concentrations of liver amino acids and derivatives of Pept1+/+ and Pept1−/− animals on control or high-protein diet after 18 days of feeding. By LC-MS/MS liver amino acid concentrations of Pept1+/+ and Pept1−/− animals on control (21% energy from protein) or high-protein (45% energy from protein) diet were analyzed. Data shows all analyzed amino acids plus sum of all amino acids. Data are presented as mean±SD. (n = 3).
(DOC)
Acknowledgments
We thank Ronny Scheundel and Johanna Welzhofer for excellent technical assistance.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by a grant (DA 190/8-1) from the Deutsche Forschungsgemeinschaft (DFG). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Bensaid A, Tome D, Gietzen D, Even P, Morens C, et al. Protein is more potent than carbohydrate for reducing appetite in rats. Physiol Behav. 2002;75:577–582. doi: 10.1016/s0031-9384(02)00646-7. [DOI] [PubMed] [Google Scholar]
- 2.Latner JD, Schwartz M. The effects of a high-carbohydrate, high-protein or balanced lunch upon later food intake and hunger ratings. Appetite. 1999;33:119–128. doi: 10.1006/appe.1999.0237. [DOI] [PubMed] [Google Scholar]
- 3.Phillips RJ, Powley TL. Gastric volume rather than nutrient content inhibits food intake. Am J Physiol. 1996;271:R766–769. doi: 10.1152/ajpregu.1996.271.3.R766. [DOI] [PubMed] [Google Scholar]
- 4.Eastwood C, Maubach K, Kirkup AJ, Grundy D. The role of endogenous cholecystokinin in the sensory transduction of luminal nutrient signals in the rat jejunum. Neurosci Lett. 1998;254:145–148. doi: 10.1016/s0304-3940(98)00666-1. [DOI] [PubMed] [Google Scholar]
- 5.Schwartz GJ. The role of gastrointestinal vagal afferents in the control of food intake: current prospects. Nutrition. 2000;16:866–873. doi: 10.1016/s0899-9007(00)00464-0. [DOI] [PubMed] [Google Scholar]
- 6.Wren AM, Bloom SR. Gut hormones and appetite control. Gastroenterology. 2007;132:2116–2130. doi: 10.1053/j.gastro.2007.03.048. [DOI] [PubMed] [Google Scholar]
- 7.Smeets AJ, Soenen S, Luscombe-Marsh ND, Ueland O, Westerterp-Plantenga MS. Energy expenditure, satiety, and plasma ghrelin, glucagon-like peptide 1, and peptide tyrosine-tyrosine concentrations following a single high-protein lunch. J Nutr. 2008;138:698–702. doi: 10.1093/jn/138.4.698. [DOI] [PubMed] [Google Scholar]
- 8.Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, et al. A role for ghrelin in the central regulation of feeding. Nature. 2001;409:194–198. doi: 10.1038/35051587. [DOI] [PubMed] [Google Scholar]
- 9.Jean C, Fromentin G, Tome D, Larue-Achagiotis C. Wistar rats allowed to self-select macronutrients from weaning to maturity choose a high-protein, high-lipid diet. Physiol Behav. 2002;76:65–73. doi: 10.1016/s0031-9384(02)00676-5. [DOI] [PubMed] [Google Scholar]
- 10.Moran TH. Cholecystokinin and satiety: current perspectives. Nutrition. 2000;16:858–865. doi: 10.1016/s0899-9007(00)00419-6. [DOI] [PubMed] [Google Scholar]
- 11.Halatchev IG, Cone RD. Peripheral administration of PYY(3-36) produces conditioned taste aversion in mice. Cell Metab. 2005;1:159–168. doi: 10.1016/j.cmet.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 12.Tome D, Schwarz J, Darcel N, Fromentin G. Protein, amino acids, vagus nerve signaling, and the brain. Am J Clin Nutr. 2009;90:838S–843S. doi: 10.3945/ajcn.2009.27462W. [DOI] [PubMed] [Google Scholar]
- 13.Cota D, Proulx K, Smith KA, Kozma SC, Thomas G, et al. Hypothalamic mTOR signaling regulates food intake. Science. 2006;312:927–930. doi: 10.1126/science.1124147. [DOI] [PubMed] [Google Scholar]
- 14.Lynch CJ, Gern B, Lloyd C, Hutson SM, Eicher R, et al. Leucine in food mediates some of the postprandial rise in plasma leptin concentrations. Am J Physiol Endocrinol Metab. 2006;291:E621–630. doi: 10.1152/ajpendo.00462.2005. [DOI] [PubMed] [Google Scholar]
- 15.Balage M, Dardevet D. Long-term effects of leucine supplementation on body composition. Curr Opin Clin Nutr Metab Care. 2010;13:265–270. doi: 10.1097/MCO.0b013e328336f6b8. [DOI] [PubMed] [Google Scholar]
- 16.Nairizi A, She P, Vary TC, Lynch CJ. Leucine supplementation of drinking water does not alter susceptibility to diet-induced obesity in mice. J Nutr. 2009;139:715–719. doi: 10.3945/jn.108.100081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peters JC, Harper AE. Adaptation of rats to diets containing different levels of protein: effects on food intake, plasma and brain amino acid concentrations and brain neurotransmitter metabolism. J Nutr. 1985;115:382–398. doi: 10.1093/jn/115.3.382. [DOI] [PubMed] [Google Scholar]
- 18.Palacin M, Estevez R, Bertran J, Zorzano A. Molecular biology of mammalian plasma membrane amino acid transporters. Physiol Rev. 1998;78:969–1054. doi: 10.1152/physrev.1998.78.4.969. [DOI] [PubMed] [Google Scholar]
- 19.Adibi SA. The oligopeptide transporter (Pept-1) in human intestine: biology and function. Gastroenterology. 1997;113:332–340. doi: 10.1016/s0016-5085(97)70112-4. [DOI] [PubMed] [Google Scholar]
- 20.Daniel H. Molecular and integrative physiology of intestinal peptide transport. Annu Rev Physiol. 2004;66:361–384. doi: 10.1146/annurev.physiol.66.032102.144149. [DOI] [PubMed] [Google Scholar]
- 21.Daniel H, Kottra G. The proton oligopeptide cotransporter family SLC15 in physiology and pharmacology. Pflugers Arch. 2004;447:610–618. doi: 10.1007/s00424-003-1101-4. [DOI] [PubMed] [Google Scholar]
- 22.Terada T, Inui K. Gene expression and regulation of drug transporters in the intestine and kidney. Biochem Pharmacol. 2007;73:440–449. doi: 10.1016/j.bcp.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 23.Erickson RH, Gum JR, Jr, Lindstrom MM, McKean D, Kim YS. Regional expression and dietary regulation of rat small intestinal peptide and amino acid transporter mRNAs. Biochem Biophys Res Commun. 1995;216:249–257. doi: 10.1006/bbrc.1995.2617. [DOI] [PubMed] [Google Scholar]
- 24.Meissner B, Boll M, Daniel H, Baumeister R. Deletion of the intestinal peptide transporter affects insulin and TOR signaling in Caenorhabditis elegans. J Biol Chem. 2004;279:36739–36745. doi: 10.1074/jbc.M403415200. [DOI] [PubMed] [Google Scholar]
- 25.Hu Y, Smith DE, Ma K, Jappar D, Thomas W, et al. Targeted disruption of peptide transporter Pept1 gene in mice significantly reduces dipeptide absorption in intestine. Mol Pharm. 2008;5:1122–1130. doi: 10.1021/mp8001655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nässl A, Rubio-Aliaga I, Fenselau H, Marth M, Kottra G, et al. Am J Physiol Gastrointest Liver Physiol [Epub ahead of print]; 2011. Amino acid absorption and homeostasis in mice lacking the intestinal peptide transporter PEPT1. [DOI] [PubMed] [Google Scholar]
- 27.Chen M, Singh AK, Xiao F, Dringenberg U, Wang J, et al. Am J Physiol Gastrointest Liver Physiol; 2010. Gene ablation for PEPT1 in mice abolishes the effects of dipeptides on small intestinal fluid absorption, short circuit current and intracellular pH. [DOI] [PubMed] [Google Scholar]
- 28.Bensaid A, Tome D, L'Heureux-Bourdon D, Even P, Gietzen D, et al. A high-protein diet enhances satiety without conditioned taste aversion in the rat. Physiol Behav. 2003;78:311–320. doi: 10.1016/s0031-9384(02)00977-0. [DOI] [PubMed] [Google Scholar]
- 29.Krauss RM, Mayer J. Influence of protein and amino acids on food intake in the rat. Am J Physiol. 1965;209:479–483. doi: 10.1152/ajplegacy.1965.209.3.479. [DOI] [PubMed] [Google Scholar]
- 30.Potier M, Darcel N, Tome D. Protein, amino acids and the control of food intake. Curr Opin Clin Nutr Metab Care. 2009;12:54–58. doi: 10.1097/MCO.0b013e32831b9e01. [DOI] [PubMed] [Google Scholar]
- 31.Weigle DS, Breen PA, Matthys CC, Callahan HS, Meeuws KE, et al. A high-protein diet induces sustained reductions in appetite, ad libitum caloric intake, and body weight despite compensatory changes in diurnal plasma leptin and ghrelin concentrations. Am J Clin Nutr. 2005;82:41–48. doi: 10.1093/ajcn.82.1.41. [DOI] [PubMed] [Google Scholar]
- 32.Tome D. Protein, amino acids and the control of food intake. Br J Nutr. 2004;92(Suppl 1):S27–30. doi: 10.1079/bjn20041138. [DOI] [PubMed] [Google Scholar]
- 33.Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404:661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- 34.Hindlet P, Bado A, Farinotti R, Buyse M. Long-term effect of leptin on H+-coupled peptide cotransporter 1 activity and expression in vivo: evidence in leptin-deficient mice. J Pharmacol Exp Ther. 2007;323:192–201. doi: 10.1124/jpet.107.125799. [DOI] [PubMed] [Google Scholar]
- 35.Buyse M, Berlioz F, Guilmeau S, Tsocas A, Voisin T, et al. PepT1-mediated epithelial transport of dipeptides and cephalexin is enhanced by luminal leptin in the small intestine. J Clin Invest. 2001;108:1483–1494. doi: 10.1172/JCI13219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hindlet P, Bado A, Kamenicky P, Delomenie C, Bourasset F, et al. Reduced intestinal absorption of dipeptides via PepT1 in mice with diet-induced obesity is associated with leptin receptor down-regulation. J Biol Chem. 2009;284:6801–6808. doi: 10.1074/jbc.M805564200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Faipoux R, Tome D, Gougis S, Darcel N, Fromentin G. Proteins activate satiety-related neuronal pathways in the brainstem and hypothalamus of rats. J Nutr. 2008;138:1172–1178. doi: 10.1093/jn/138.6.1172. [DOI] [PubMed] [Google Scholar]
- 38.Mithieux G, Misery P, Magnan C, Pillot B, Gautier-Stein A, et al. Portal sensing of intestinal gluconeogenesis is a mechanistic link in the diminution of food intake induced by diet protein. Cell Metab. 2005;2:321–329. doi: 10.1016/j.cmet.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 39.Mithieux G. A novel function of intestinal gluconeogenesis: central signaling in glucose and energy homeostasis. Nutrition. 2009;25:881–884. doi: 10.1016/j.nut.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 40.Mithieux G, Andreelli F, Magnan C. Intestinal gluconeogenesis: key signal of central control of energy and glucose homeostasis. Curr Opin Clin Nutr Metab Care. 2009;12:419–423. doi: 10.1097/MCO.0b013e32832c4d6a. [DOI] [PubMed] [Google Scholar]
- 41.Martin G, Ferrier B, Conjard A, Martin M, Nazaret R, et al. Glutamine gluconeogenesis in the small intestine of 72 h-fasted adult rats is undetectable. Biochem J. 2007;401:465–473. doi: 10.1042/BJ20061148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Remesey C, Demigne C, Aufrere J. Inter-organ relationships between glucose, lactate and amino acids in rats fed on high-carbohydrate or high-protein diets. Biochem J. 1978;170:321–329. doi: 10.1042/bj1700321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Broer S. Amino acid transport across mammalian intestinal and renal epithelia. Physiol Rev. 2008;88:249–286. doi: 10.1152/physrev.00018.2006. [DOI] [PubMed] [Google Scholar]
- 44.Le Bricon T. Effects of administration of oral branched-chain amino acids on anorexia and caloric intake in cancer patients. Clin Nutr. 1996;15:337. doi: 10.1016/s0261-5614(96)80011-2. [DOI] [PubMed] [Google Scholar]
- 45.Hiroshige K, Sonta T, Suda T, Kanegae K, Ohtani A. Oral supplementation of branched-chain amino acid improves nutritional status in elderly patients on chronic haemodialysis. Nephrol Dial Transplant. 2001;16:1856–1862. doi: 10.1093/ndt/16.9.1856. [DOI] [PubMed] [Google Scholar]
- 46.Noatsch A, Petzke KJ, Millrose MK, Klaus S. Body weight and energy homeostasis was not affected in C57BL/6 mice fed high whey protein or leucine-supplemented low-fat diets. Eur J Nutr. 2011;50:479–488. doi: 10.1007/s00394-010-0155-2. [DOI] [PubMed] [Google Scholar]
- 47.Zhang Y, Guo K, LeBlanc RE, Loh D, Schwartz GJ, et al. Increasing dietary leucine intake reduces diet-induced obesity and improves glucose and cholesterol metabolism in mice via multimechanisms. Diabetes. 2007;56:1647–1654. doi: 10.2337/db07-0123. [DOI] [PubMed] [Google Scholar]
- 48.Kuhla B, Kucia M, Gors S, Albrecht D, Langhammer M, et al. Effect of a high-protein diet on food intake and liver metabolism during pregnancy, lactation and after weaning in mice. Proteomics. 2010;10:2573–2588. doi: 10.1002/pmic.200900789. [DOI] [PubMed] [Google Scholar]
- 49.Morley JE, Flood JF. Evidence that nitric oxide modulates food intake in mice. Life Sci. 1991;49:707–711. doi: 10.1016/0024-3205(91)90102-h. [DOI] [PubMed] [Google Scholar]
- 50.Buchmann I, Milakofsky L, Harris N, Hofford JM, Vogel WH. Effect of arginine administration on plasma and brain levels of arginine and various related amino compounds in the rat. Pharmacology. 1996;53:133–142. doi: 10.1159/000139424. [DOI] [PubMed] [Google Scholar]
- 51.Calapai G, Corica F, Corsonello A, Sautebin L, Di Rosa M, et al. Leptin increases serotonin turnover by inhibition of brain nitric oxide synthesis. J Clin Invest. 1999;104:975–982. doi: 10.1172/JCI5867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van't Hof RJ, Macphee J, Libouban H, Helfrich MH, Ralston SH. Regulation of bone mass and bone turnover by neuronal nitric oxide synthase. Endocrinology. 2004;145:5068–5074. doi: 10.1210/en.2004-0205. [DOI] [PubMed] [Google Scholar]
- 53.Squadrito F, Calapai G, Altavilla D, Cucinotta D, Zingarelli B, et al. Food deprivation increases brain nitric oxide synthase and depresses brain serotonin levels in rats. Neuropharmacology. 1994;33:83–86. doi: 10.1016/0028-3908(94)90100-7. [DOI] [PubMed] [Google Scholar]
- 54.Yang SJ, Denbow DM. Interaction of leptin and nitric oxide on food intake in broilers and Leghorns. Physiol Behav. 2007;92:651–657. doi: 10.1016/j.physbeh.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 55.Denbow DM, Meade S, Robertson A, McMurtry JP, Richards M, et al. Leptin-induced decrease in food intake in chickens. Physiol Behav. 2000;69:359–362. doi: 10.1016/s0031-9384(99)00258-9. [DOI] [PubMed] [Google Scholar]
- 56.Squadrito F, Calapai G, Altavilla D, Cucinotta D, Zingarelli B, et al. Central serotoninergic system involvement in the anorexia induced by NG-nitro-L-arginine, an inhibitor of nitric oxide synthase. Eur J Pharmacol. 1994;255:51–55. doi: 10.1016/0014-2999(94)90081-7. [DOI] [PubMed] [Google Scholar]
- 57.Squadrito F, Calapai G, Cucinotta D, Altavilla D, Zingarelli B, et al. Anorectic activity of NG-nitro-L-arginine, an inhibitor of brain nitric oxide synthase, in obese Zucker rats. Eur J Pharmacol. 1993;230:125–128. doi: 10.1016/0014-2999(93)90422-e. [DOI] [PubMed] [Google Scholar]
- 58.Team RDC. Vienna.Austria: R Foundation for Statistical Computing; 2009. R: A language and environment for statistical computing. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Amino acid appearance in plasma after administration of a low dose of 15N-labeled protein. Analysis of 14N and 15N labeled amino acids from plasma of portal vein 15 (A) and 30 min (B) after administration of 15N-labeled protein (8.83 mg) by gavage in 12h-fasted Pept1+/+ (wt) and Pept1 knockout (ko) animals (n = 5 animals per timepoint and genotype). Cit, citrulline; Sum, sum of all measured amino acids. (•) indicate outlier. Data are presented as mean ± SD. *P<0.05, $P<0.01, §P<0.001.
(TIF)
Plasma amino acid concentrations of male Pept1+/+ and Pept1−/− animals after 5 days on control or high-protein diet. By LC-MS/MS plasma amino acid concentrations of Pept1+/+ and Pept1−/− animals on control (21% energy from protein) or high-protein (45% energy from protein) diet for 5 days were analyzed (n = 10). Data shows all analyzed amino acids plus sum of all amino acids.
(DOC)
Plasma amino acid concentrations of male Pept1+/+ and Pept1−/− animals on control or high-protein diet for 18 days. After feeding a control (21% energy from protein) or high-protein (45% energy from protein) diet for 18 days plasma amino acid concentrations of Pept1+/+ and Pept1−/−animals were analyzed by LC-MS/MS (n = 3). Data shows all analyzed amino acids plus sum of all amino acids.
(DOC)
Concentrations of liver amino acids and derivatives of Pept1+/+ and Pept1−/− animals on control or high-protein diet after 18 days of feeding. By LC-MS/MS liver amino acid concentrations of Pept1+/+ and Pept1−/− animals on control (21% energy from protein) or high-protein (45% energy from protein) diet were analyzed. Data shows all analyzed amino acids plus sum of all amino acids. Data are presented as mean±SD. (n = 3).
(DOC)